Abstract
Climate change can alter the toxic effects of pesticides on soil invertebrates. However, the nature and magnitude of the influence of climatic factors on clothianidin impacts in tropical soils are still unknown. The influence of increasing atmospheric temperature and the reduction in soil moisture on the toxicity and risk of clothianidin (seed dressing formulation Inside FS®) were assessed through chronic toxicity tests with collembolans Folsomia candida in a tropical field soil (Entisol). The risk of clothianidin for collembolans was estimated using the Toxicity-Exposure Ratio (TER) approach. Organisms were exposed to increasing clothianidin concentrations at 20, 25 and 27 °C in combination with two soil moisture conditions (30 and 60% of the maximum water holding capacity—WHC). The effect of temperature and soil moisture content on clothianidin toxicity was verified through the number of F. candida juveniles generated after 28 days of exposure to the spiked soil. The toxicities estimated at 25 °C (EC50_30%WHC = 0.014 mg kg−1; EC50_60%WHC = 0.010 mg kg−1) and 27 °C (EC50_30%WHC = 0.006 mg kg−1; EC50_60%WHC = 0.007 mg kg−1) were 2.9–3.0-fold (25 °C) and 4.3–6.7-fold (27 °C) higher than those found at 20 °C (EC50_30%WHC = 0.040 mg kg−1; EC50_60%WHC = 0.030 mg kg−1), indicating that clothianidin toxicity increases with temperature. No clear influence of soil moisture content on clothianidin toxicity could be observed once the EC50 values estimated at 30% and 60% WHC, within the same temperature, did not significantly differ. A significant risk was detected in all temperatures and soil moisture scenarios studied, and the TER values indicate that the risk can increase with increasing temperatures. Our results revealed that temperature could overlap with soil moisture in regulating clothianidin toxicity and reinforce the importance of including climatic factors in the prospective risk assessment of pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global climate changes are expected to cause substantial impacts on the ecology of soil communities (Mekala and Polepongu 2019). For instance, increases in atmospheric temperatures and the frequency of drought events can alter the metabolism and physiology of soil organisms, resulting in impacts on their growth and reproduction (Noyes et al. 2009). Although the evolutionary responses of the species may protect them against changes in the environment, researchers have been suggesting that the ability of some soil organisms to adapt their physiology or morphology does not keep up with the speed of climate changes, increasing the number of species living near their physiological limits (Noyes et al. 2009; Hoffmann, Sgró (2011)).
On the other hand, there is a growing concern about the pollution of agricultural soils by neonicotinoid insecticides (Wood and Goulson 2017; Atwood et al. 2018; Wu et al. 2020; Yu et al. 2021; Bonmatin et al. 2021; Gunstone et al. 2021). In recent years, neonicotinoids have been frequently used to treat agricultural seeds, mainly due to their efficiency in protecting seedlings after germination by offering a broad-spectrum control of chewing and sap-sucking insects (Bonmatin et al. 2015; Goulson 2013). Neonicotinoids are a new class of synthetic insecticides and represent approximately one-third of the global pesticide market (Maino et al. 2021).
Clothianidin, a neonicotinoid that belongs to the N-nitroguanidines group, acts on the nicotinic acetylcholine receptors in the central nervous system of insects, causing paralysis and death of exposed organisms (Fakhereddin and Dogan 2021; Pearsons et al. 2021). Clothianidin is among the three most worldwide used neonicotinoids insecticides, with dominant usages in the United Kingdom and Latin and North America (Bass et al., 2015; Yang et al., 2022). Although some studies have demonstrated the higher insecticidal activity of clothianidin compared to the top-selling neonicotinoid, imidacloprid, its ecotoxicological effects on non-target soil invertebrates are less studied (Ihara et al., 2004; Bandeira et al., 2021). Due to its high persistence in soil, with half-lives ranging from 148 to 6931 days depending on the soil type (Goulson 2013), some authors suggest that >80% of clothianidin applied in seed treatment can remain in the soil once the roots effectively absorb <20% of the active ingredient (a.i.) after seed germination (Sur and Stork 2003; Alford and Krupke 2017). Therefore, soil invertebrates may be chronically exposed to clothianidin residues in agricultural soils, impacting their population and, consequently, their ecosystem services (Wood and Goulson 2017).
Although the ecotoxicological effects of clothianidin both as pure compounds (De Lima e Silva et al. 2019) and as commercial formulations (Ritchie et al. 2019; Bandeira et al. 2021) on soil invertebrates have been reported in the last years, studies on the influence of climate changes on the toxic potential of this insecticide to soil organisms were not found. Increases in the global atmospheric temperature ranging from 1.0 to 5.7 °C are expected until 2100, depending on the greenhouse gas emissions (IPCC—Intergovernmental Panel on Climate Change (2021)). In Latin America, the warming trend is around 0.2 °C per decade, based on the average temperature increase of the last 30 years (WMO—World Meteorological Organization (2022)). Due to these temperature increases, lower precipitation and higher soil evaporation rates are likely in the coming years, resulting in lower soil moisture in some regions (Dai 2013). These changes in climatic factors may be critical in establishing trigger values for the Ecological Risk Assessment (ERA) of pesticides to soil invertebrates. For instance, higher temperatures can modify the pesticide bioavailability in soils (Biswas et al. 2018; Kaka et al. 2021) and their toxicokinetics on invertebrates by increasing the chemicals’ accumulation and their metabolism, resulting in increased toxicities (Noyes et al. 2009; Velki and Ečimović 2015). Decreased soil moisture contents can influence the pesticide sorption-desorption processes in soil and increase the bioavailable concentrations of pesticides for soil invertebrates in the soil solution (Shelton and Parkin 1991; Garcia-Valcarcel and Tadeo 1999). Since interstitial water in the soil is one of the main exposure routes for collembolans, its lack or excess can modulate the toxicity of chemicals to these arthropods in soil (Ogungbemi and van Gestel 2018). Equally, soil species living in dry soils may be subjected to dehydration, which can have an adverse impact on their development and modify their responses to pesticides (Lima et al. 2011; Bandow et al. 2014b; Hennig et al. 2021).
Several studies have demonstrated that the increase in temperature and the reduction in soil moisture content can increase the toxicity of certain pesticides to soil fauna (Bandow et al. 2014a, b; Jegede et al. 2017; Bandeira et al. 2020b; Hennig et al. 2021, 2022), but so far it is still unclear how these factors affect the toxicity of clothianidin. Thus, this study aimed to assess the influence of increased atmospheric temperatures and reduced soil moisture contents (alone and in combination) on clothianidin chronic toxicity for collembolans Folsomia candida in a tropical field soil. The risk of exposure to the predicted environmental concentrations (PEC) also was estimated for the species. We hypothesized that: (1) the increases in atmospheric temperature or reductions in soil moisture contents increase the toxicity of clothianidin to F. candida; (2) the combination of higher temperature and lower soil moisture has an increased influence on clothianidin toxicity to the collembolans when compared to the influence of these climatic factors in isolation (i.e., only higher temperatures or only lower soil moistures); and (3) the risk of exposure to PEC of clothianidin increases with the increase of the temperature and with the reduction of the soil moisture.
Materials and methods
Chronic toxicity assays were performed in a complete factorial design of 6 × 3 × 2, where collembolans were exposed to a tropical field soil spiked with five increasing concentrations of clothianidin plus a control (the same test soil containing only distilled water up to the levels needed to achieve the desired soil moisture), under three different temperatures and two soil moisture levels. The assays were performed with five replicas, and an extra replica (without collembolans) was used to measure the soil moisture and pH at the end of the assays.
Test species
Collembolans F. candida were reared in cylindrical plastic containers with a mixture of powdered plaster of Paris, water and powdered activated charcoal in a proportion of 10:7:1 (w:w:w), following ISO (2014). The containers were closed with perforated lids to allow gas exchange. The culture was kept in a climate-controlled room (20 ± 2 °C) with a photoperiod of 12:12 h light:dark. Twice a week, individuals were fed with granulated dry yeast (Saccharomyces cerevisiae), and the moisture of the culture medium was adjusted with distilled water.
To obtain age-synchronized F. candida juveniles, newly laid eggs were carefully collected from culture containers with a wet brush. The egg clusters were then transferred to a piece of the breeding substrate, which was placed on a new culture container (without organisms). After 48 h of the start of hatching, the piece of the breeding substrate containing the eggs was removed, ensuring that the newborn juveniles were between 0 and 2 days old. Ten days after this procedure, the juveniles of the new culture container were age synchronized (10–12 days old) and appropriate for use in the assays (ISO 2014).
Test soil
The soil used in the chronic toxicity assays with collembolans was an Entisol of sandy texture, sampled in the municipality of Araranguá, State of Santa Catarina, Brazil (29° 00’S, 49° 31’W), in areas with no history of contamination by pesticides. Soil samples were taken from the superficial soil layer (0–20 cm), sieved through a 2.0 mm mesh, air-dried and defaunated through three cycles of freezing and thawing, as described in Alves et al. (2013). Samples were stored in the laboratory, at room temperature, before use in ecotoxicological assays. The pH (4.4) in 1 M KCl and the maximum water-holding capacity (WHC - 31.6%) were determined according to ISO (2014). The soil organic matter (2.2%), sand (93.8%), clay (4.1%) and silt (2.1%) contents, and the cation exchange capacity (CEC - 1.36 cmolc dm−3) were measured following the methods described in Tedesco et al. (1995). The detailed chemical and physical properties of this Entisol were previously reported in Bandeira et al. (2020a).
Test substances
The seed dressing commercial formulation Inside FS, containing 600 g clothianidin L−1, was used to spike the soil samples. The same nominal concentrations (0.011, 0.017, 0.026, 0.039 and 0.060 mg kg−1, plus a negative control containing only distilled water) were used in the different scenarios of temperatures and soil moisture contents. Concentrations were chosen based on preliminary assays and literature data (Bandeira et al. 2021). The range of concentrations used in the assays is environmentally relevant once values of the same order of magnitude (0.012–0.063 mg kg−1; Dankyi et al. 2014; Ramasubramanian 2013) were already found in field soils.
An aqueous stock solution (10 mg a.i. L−1) was prepared at the beginning of the assays and used to obtain the spiking solutions with the abovementioned concentrations of clothianidin. These spiking solutions were prepared in volumes calculated to achieve the desired soil moisture (30% WHC and 60% WHC) in Entisol samples (250 g per treatment).
The PEC of clothianidin 28 days after the sowing of treated seeds was estimated on the software ESCAPE® (EPPO 2003) for the three tested atmospheric temperatures. The lowest manufacturer’s recommended dose to treat corn seeds (2.1 g a.i. per kg of seeds), at a sowing density of 30 kg of corn seeds per hectare and an incorporation depth of 10 cm in the soil profile, were assumed for the calculations. A soil density of 1.5 g cm−3 (Souza et al. 2005) was used to calculate the soil mass in contact with the amount of insecticide applied and an interception of the insecticide by plants of 1.5% (Alford and Krukpe 2017). In addition, a clothianidin half-life (DT50) of 58 days was used for the temperature of 20 °C (De Lima e Silva et al. 2019); the DT50 values estimated using the ESCAPE® for the temperatures of 25 and 27 °C were 39 and 33 days, respectively. Finally, the PEC values estimated via ESCAPE® software for the 20, 25 and 27 °C were 0.035, 0.033 and 0.031 mg kg−1, respectively.
Test environment
The ecotoxicological assays were conducted in climate-controlled chambers with a constant light intensity of 400–800 lux and a photoperiod of 12:12 h light:dark, under six different climate scenarios, which were based on the full factorial combination of three atmospheric temperatures (i.e., 20, 25 and 27 °C) and two soil moisture contents (30% WHC, simulating a scenario of water restriction/drought; and 60% WHC, standard soil moisture). The test temperatures used had different reasons. Thus, 20 °C is the standard condition for laboratory ecotoxicity tests with soil invertebrates, 25 °C is a suitable temperature scenario for tropical regions (ISO (2012); Owojori et al. 2019; Bandeira et al. 2020a, b; Hennig et al. 2022), and 27 °C is a scenario that simulates a 2-degree increase in the atmospheric temperature as a consequence of global warming (IPCC—Intergovernmental Panel on Climate Change (2021)). Then, the six climate scenarios tested were: (a) 20 °C/30% WHC; (b) 20 °C/60% WHC; (c) 25 °C/30% WHC; (d) 25 °C/60% WHC; (e) 27 °C/30% WHC; and (f) 27 °C/60% WHC.
Collembola chronic toxicity assays
Chronic toxicity assays were performed according to ISO 11267 (2014). Collembolans were exposed to Entisol samples spiked with the five increasing concentrations of clothianidin (and the control) in glass vessels (4 cm diameter, 9 cm height) containing 30 g of soil (spiked or control) with moisture adjusted to the desired percentage of maximum WHC. Then, ten 10–12 days old individuals were inserted into each container. S. cerevisiae (≅2 mg) was given as food on the first and 14th days of the assays. Five replicates were performed for each treatment, besides an extra replica, without organisms, which was used to measure the soil moisture and pH at the end of the assays. Containers were closed with pressure lids, and the assays were kept in climate-controlled chambers with a temperature of 20, 25 or 27 °C. Twice a week, containers were opened to allow gas exchanges and soil moisture adjustment (weight-based). On the 28th day, the content of each replica (soil + organisms) was submerged in water with five drops of black ink to favor the observation of surviving adults and juveniles that floated. Subsequently, the replicates were photographed in high resolution using a digital camera, and the images were analyzed through the software ImageJ® version 1.47, when the number of juveniles was counted.
Data analysis
The normality and homoscedasticity of the data were checked through the Kolmogorov-Smirnov and Bartlett tests, respectively. When necessary, logarithmic transformations were applied to the data to meet the ANOVA assumptions. Significant differences between the number of juveniles generated in treatments and the respective control (in each tested scenario) were tested through a one-way ANOVA followed by a Dunnett post-hoc test (p < 0.05). These results supported the determination of the no observed effect concentration (NOEC) and the lowest observed effect concentration (LOEC). Simultaneously, significant interactions between clothianidin concentrations, soil moisture, and temperatures on collembolans reproduction were checked using a factorial ANOVA. The 50% lethal concentrations (LC50) for 28-d adult survival were estimated by a variable slope sigmoidal function, following Gainer et al. (2018). The a.i. concentration that decreased the reproduction by 10% and 50% (EC10 and EC50, respectively) were estimated by fitting non-linear regressions to the data (Environmental Canada 2007). The significant differences between the EC50 values from the six distinct exposure scenarios (temperatures × soil moistures) were tested via a generalized likelihood ratio test (p < 0.05), as described in Natal-da-luz et al. (2011). All statistical analyses were performed in the software Statistica® version 13.5.0.17.
The risk of clothianidin on F. candida was estimated following the Toxicity-Exposure Ratio (TER) approach (EC 2003), which was calculated by dividing the EC10 value obtained in each one of the six tested scenarios (temperatures × soil moistures) by the PEC estimated for the same temperature level (TER = EC10/PEC). A significant risk of clothianidin was assumed when the value of TER was lower than 5 (EC 2003).
Results
All the validity criteria for the ecotoxicological assays (ISO, 2014) performed at 20 °C were met (Table S1). Although assays performed at 25 and 27 °C did not fully meet the validity criteria of the guideline ISO 11267 (Table S1), their results were considered because the validity criteria established in ISO (2014) were designed to check the F. candida reproductive performance at the standard temperature (20 °C). Also, the pH of the soil samples did not significantly change with clothianidin application and throughout time (Table S2).
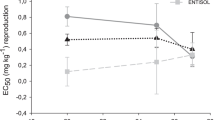
The inhibitory effect of clothianidin on collembolans reproduction started (LOEC values) at the same concentration for both soil moisture contents in tests conducted at 20 and 25 °C (Table 1 and Fig. 1). At 27 °C, the LOEC was lower in the soil with 30% WHC (Table 1). However, no significant differences (p > 0.05) were found between the EC50 values estimated from distinct soil moistures within the same temperature based on the generalized likelihood ratio test (Table 1).
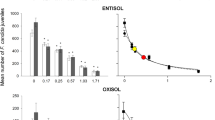
Mean number (± standard deviation; n = 5) of Folsomia candida juveniles (y-axis) found in Entisol after 28 days of exposure to increasing concentrations of clothianidin (x-axis) under two soil moisture contents (30 and 60% of the soil water-holding capacity—WHC) and three atmospheric temperatures (20, 25 and 27 °C). Dark gray bars represent the juveniles in the assays at 30% WHC, while light gray bars represent the juveniles in the assays at 60% WHC. Asterisks (*) indicate a significant reduction in the number of juveniles compared to the control treatment (Dunnett’s test, p < 0.05)
Clothianidin toxicity to Folsomia candida increased with increasing atmospheric temperatures, as evidenced by the decrease in the EC50 and LC50 values with the increase in the temperature (Table 1 and Figs. 2 and 3). In soil samples with 30% WHC, the EC50 values at 25 and 27 °C were 2.9 and 6.7-fold lower (p < 0.05) than the value at 20 °C, respectively (Table 1). The toxicity (EC50-based) was also significantly higher (p < 0.05) at 27 °C compared to 25 °C at 30% WHC. In samples with 60% WHC, EC50 values were significantly lower (p < 0.05) than those obtained at 20 °C, but the toxicity (EC50-based) did not differ between 25 and 27 °C (p > 0.05; Table 1).
Mean number (± standard deviation; n = 5) and dose-response curves of Folsomia candida adults found in Entisol after 28 days of exposure to increasing concentrations of clothianidin, under two soil moisture contents (30 and 60% of the soil water-holding capacity—WHC) and three atmospheric temperatures (20, 25 and 27 °C). The LC50 values were represented by squares (■)
The factorial ANOVA showed that the studied factors, in isolation (concentrations, temperatures or soil moisture), could significantly influence (p < 0.001) F. candida reproduction (Table S3). In addition, significant interactions (p < 0.001) were observed between clothianidin concentrations and temperatures, concentrations and soil moisture, temperatures and soil moisture contents, and between the three factors (Table S3).
TER values indicated a significant risk of clothianidin toward F. candida in all scenarios tested, regardless of temperature or soil moisture (Table 2). However, the TER values decreased with increasing temperature at both 30 and 60% WHC, revealing a higher risk at 25 and 27 °C.
Discussion
Collembolans are poikilothermic ectotherms, which means their body temperature is subject to variations with changes in environmental temperature (Everatt et al. 2013). The increase of offspring production in the control treatment at 25 °C (60% WHC) compared to 20 °C (Fig. 1) was also observed by Hennig et al. (2022) in Entisol and can be a strategic response to the low-level stress exposure. When experiencing temperatures slightly above the ideal range for species reproduction (Fountain and Hopkin, 2005), collembolans may have increased egg production as a natural effort to guarantee the species’ perpetuation (i.e., a hormetic stimulation; Rix and Cutler 2022; Cutler et al. 2022). On the other hand, when the heat stress is accentuated, collembolans may suffer alterations in gene and protein expression and disturbances in their metabolism (Waagner et al. 2010). In these situations, they can modify their energy partitioning by designating more resources to mechanisms that alleviate heat stress, such as the induction of antioxidant enzymes (Hackenberger et al. 2018) and, consequently, less energy to reproductive outputs. This is probably the reason for the lower reproductive performance observed in the control treatments at 27 °C, compared to 25 and 20 °C. According to Fountain and Hopkin (2005), at 27 °C, F. candida adults lay substantially fewer eggs than at 20 °C, and at the lower temperature, the hatching success is also more significant when compared to temperatures above 27 °C.
Once collembolans have a permeable integument (Bahrndorff et al. 2007), they have a limited capacity to regulate water exchange between their body and the external medium (Everts et al. 1991; Morgado et al. 2016). Consequently, drought scenarios can disturb their water balance, leading to metabolic disorders and energy reallocation, ultimately impacting their reproductive performance (Capela 2015). Recently, Wang et al. (2022) verified that although collembolans can survive in extreme drought scenarios, small decreases in soil water potential are sufficient to impact their reproductive output negatively. This could be the reason for the lower reproduction in control treatments at the drier condition since the number of juveniles in the controls at 30% WHC represented 69%, 33% and 50% of that produced at 60% WHC, at 20, 25 and 27 °C, respectively (Fig. 1). The drier condition is probably out of the suitable soil moisture range for F. candida reproduction, which is close to 60% WHC (van Gestel and Van Diepen 1997; Crouau et al. 1999).
The deleterious effects of clothianidin on collembolans reproduction, observed in all assays performed regardless of soil moisture content or temperature (Fig. 1), are probably related to its action on the nicotinic acetylcholine receptors in the central nervous system of insects (Pearsons et al. 2021), and are in line with previous literature reports. For instance, Bandeira et al. (2021) reported the clothianidin toxicity (EC50 = 0.15 [0.12–0.18] mg kg−1) to F. candida after the exposure to Inside FS (600 g clothianidin L−1) in a tropical artificial soil (TAS; Sandy Loam; 1.4% OM, CEC = 3.3 cmolc dm−3). Ritchie et al. (2019) obtained an EC50 value of 0.069 (0.039–0.12) mg kg−1 after the exposure of F. candida to the commercial formulation Titan (600 g L−1 clothianidin) in a Sandy Loam field soil (2.5% OM, CEC = 13 cmolc dm−3). De Lima e Silva et al. (2019) found an EC50 of 0.05 (0.05–0.06) mg kg−1 for clothianidin (pure a.i.) in a LUFA 2.2 soil (Loamy Sand, OM ≈ 4%, CEC ≈ 10 cmolc dm−3). In our study, at the standard condition (i.e., 20 °C and 60% WHC), the EC50 values (Table 1) were lower than the ones found in the literature, indicating that the toxicity observed in this work was even higher. This is certainly due to the differences in the soil properties, namely the clay, sand and silt contents. CEC also showed differences between the sandy soil used in our assays (Entisol—see section Test soil) and those from the cited studies, which probably led to a higher amount of clothianidin bioavailable for collembolans in the soil solution (van Gestel 2012; Ogungbemi and van Gestel 2018; Bandeira et al. 2020a).
When we add increased temperatures to the directly impacted life traits of collembolans, they also influenced the toxicity of clothianidin to F. candida, since lower EC50 values were observed in our experiments at 25 and 27 °C, when compared to 20 °C, under both soil moisture contents (Fig. 2 and Table 1). This confirms the first hypothesis of this study and agrees with other studies that observed the same trend of increased pesticide toxicity to soil invertebrates with increasing temperatures (Lima et al. 2015; Velki and Ečimović 2015; Bandeira et al. 2020b; Hennig et al. 2022). This can be due to the decrease in pesticide sorption onto the soil particles with increasing temperature, which results in increased bioavailability in soil pore water (Broznić and Milin 2012). Exposure to temperatures above optimum may have increased the vulnerability of soil organisms to the toxic effects of pesticides once heat stress can alter the expression of genes (Nota et al. 2010). They induce the generation of reactive oxygen species (Tumminello and Fuller-Espie 2013), impair the protein-synthesizing capacity (Tripathi et al. 2011) and reduce the activity of enzymes involved in detoxification processes of soil invertebrates (Ferreira et al. 2016). In addition, when exposed to pesticides at temperatures above their optimal range, soil invertebrates may experience alterations in their homeostasis and metabolism rates, resulting in enhanced uptake of chemicals (Römbke et al. 2007; Lima et al. 2015; Hackenberger et al. 2018). In these circumstances, organisms may allocate more energy to self-defense mechanisms induced by the combined chemical and heat stress, reducing the resources destined to growth and reproduction (Ferreira et al. 2016).
Our second hypothesis could not be confirmed, once our experiments showed no clear influences of soil moisture content on the toxicity of clothianidin (Table 1). Although a decrease in soil moisture did not affect the toxicity of clothianidin in the sandy Entisol, higher toxicities at the lower soil moisture content (30% WHC) were observed for F. candida, when testing the fungicide pyrimethanil in a clayey OECD artificial soil (Bandow et al. 2014b). The same was true when the neonicotinoid imidacloprid in Oxisol (a clayey soil) and TAS (Hennig et al. 2020), as well as for the phenylpyrazole fipronil when tested in Oxisol (Hennig et al. 2022). This indicates that the influence of soil moisture on pesticide toxicity may be soil-type dependent and vary with the molecule. On the other hand, in agreement with our findings, Hennig et al. (2020) also did not observe an evident influence of soil moisture on the toxicity of imidacloprid for F. candida in Entisol. A possible explanation for the absence of any impact of soil moisture on clothianidin toxicity in Entisol is that the reduction in soil moisture tested in our experiment was not great enough to be critical. It did not cause significant increases in the bioavailability of CLO in soil solution, once the partition of hydrophilic pesticides between the solid phase and the soil solution does not seem to change with soil contents in predominantly sandy soils (Ochsner et al., 2006). At extreme drought scenarios, collembolans can maintain hydration by absorbing water from surfaces under relatively dry conditions (Eisenbeis 1982; Bayley and Holmstrup 1999). As sandy-textured soils generally have thicker water films surrounding soil particles than fine-textured soils (Saarenketo 1998; Demeure et al. 1979), the exposure to Entisol probably allowed the water uptake by collembolans even in scenarios of reduced soil moisture content.
Despite the significant three-way interaction between clothianidin concentration, temperature and soil moisture (Table S3), our data suggest that reducing soil moisture in scenarios of heat (i.e., 25 and 27 °C) did not promote significant increments in the clothianidin toxicity (EC50-based) compared to the standard soil moisture content. Therefore, the second work hypothesis cannot be confirmed. However, for pyrimethanil, Bandow et al. (2014b) observed that in a warm scenario (26 °C), the reduction of soil moisture from 70% WHC to 30% WHC resulted in a 1.5-fold reduction in the EC50 value for F. candida in an OECD artificial soil. These results suggest that the combined effect of heat and drought on the toxic effects can depend on the tested pesticide and soil type and indicates that the number of existing studies performed to understand the influences of climate changes on pesticide toxicity are still incipient to explain completely the complex relationships between pesticides, environmental matrixes and climate.
Our PEC values estimated for the different temperature scenarios (Table 2) are compatible with residual concentrations already found in agricultural soils, indicating that the estimate is realistic. For instance, Jones et al. (2014) reported clothianidin values ranging from <0.0002 to 0.013 mg kg−1 in field soils from eastern England that received treated seeds in the previous 3 years. In soils from cocoa farmings in Ghana, Dankyi et al. (2014) found residual levels of clothianidin ranging from 0.012 to 0.023 mg kg−1. Ramasubramanian (2013) found clothianidin concentrations of 0.031 (±0.003) and 0.063 (±0.003) mg kg−1 in soil 60 days after spray application in sugarcane at doses of 50 and 100 g a.i. ha−1, respectively. Based on our LOEC values, these clothianidin residual levels are expected to impact the F. candida reproduction if the contamination occurs in Entisol at 25 and 27 °C, and high levels of contamination, such as those reported by Zhang et al. (2016) (2.06 mg kg−1), could cease the reproduction of F. candida.
The significant risk found in all tested scenarios (Table 2) reveals that the exposure of collembolans to clothianidin in Entisol can be harmful regardless of the climatic factors. The TER values showed that the risk is higher at 25 and 27 °C than at 20 °C, confirming our third working hypothesis. The increase in the risk with increasing temperature (from 20 to 25/27 °C) was confirmed by the 2.8–6.7-fold decrease in the EC10 values (Table 2). A higher risk of chlorpyrifos (Jegede et al. 2017) and imidacloprid (Bandeira et al. 2020b) to F. candida was also detected at 28 °C when compared to 20 °C, which corroborates our findings and reinforces the importance of pesticide risk assessment approaches that take into account the scenarios of climate change.
Conclusion
Climate change induced by anthropogenic actions will exert uncertain consequences on the bioavailability and toxicity of pesticides in soil. Our results demonstrated that the toxic effects of clothianidin toward F. candida reproduction in Entisol are increased by increases in atmospheric temperature but not by reductions in soil moisture. Higher toxicities (EC50-based) were observed at the increased temperatures (25 and 27 °C) compared to the standard one (20 °C). On the other hand, the reduction in soil moisture (from 60 to 30% WHC) content caused no influence on insecticide toxicity, regardless of the temperature tested. Significant risk (TER-based) of clothianidin toward F. candida was detected at all temperature and soil moisture scenarios tested, suggesting that the exposure of collembolans to clothianidin in Entisol is harmful regardless of the altered environmental conditions. However, the TER values also showed that the risk increased with temperature. Our data suggest that clothianidin toxicity may vary under different climatic conditions in sandy soils and reinforces the importance of including climatic factors in the prospective risk assessment of pesticides.
Data availability
The data that support the findings of this study are available and should be requested by e-mail.
Code availability
ImageJ, RRID: SCR_003070
STATISTICA, RRID: SCR_014213
References
Alford A, Krupke CH (2017) Translocation of the neonicotinoid seed treatment clothianidin in maize. PLOS One 12(3):0173836. https://doi.org/10.1371/journal.pone.0173836
Alves PRL, Cardoso EJBN, Martines AM, Sousa JP, Pasini A (2013) Earthworm ecotoxicological assessments of pesticides used to treat seeds under tropical conditions. Chemosphere 90:2674–2682. https://doi.org/10.1016/j.chemosphere.2012.11.046
Atwood LW, Mortensen DA, Koide RT, Smith RG (2018) Evidence for multi-trophic effects of pesticide seed treatments on non-targeted soil fauna. Soil Biol Biochem 125:144–155. https://doi.org/10.1016/j.soilbio.2018.07.007
Bahrndorff S, Petersen SO, Loeschcke V, Overgaard J, Holmstrup M (2007) Differences in cold and drought tolerance of high arctic and sub-arctic populations of Megaphorura arctica Tullberg 1876 (Onychiuridae: Collembola). Cryobiology 55:315–323. https://doi.org/10.1016/j.cryobiol.2007.09.001
Bandeira FO, Alves PRL, Hennig TB, Schiehl A, Cardoso EJBN, Baretta D (2020a) Toxicity of imidacloprid to the earthworm Eisenia andrei and collembolan Folsomia candida in three contrasting tropical soils. J Soils Sediments 20:1997–2007. https://doi.org/10.1007/s11368-019-02538-6
Bandeira FO, Alves PRL, Hennig TB, Toniolo T, Natal-da-Luz T, Baretta D (2020b) Effect of temperature on the toxicity of imidacloprid to Eisenia andrei and Folsomia candida in tropical soils. Environ Pollut 267:115565. https://doi.org/10.1016/j.envpol.2020.115565
Bandeira FO, Alves PRL, Hennig TB, Brancalione J, Nogueira DJ, Matias WG (2021) Chronic effects of clothianidin to non-target soil invertebrates: Ecological risk assessment using the species sensitivity distribution (SSD) approach. J Hazard Mater 419:126491. https://doi.org/10.1016/j.jhazmat.2021.126491
Bandow C, Coors A, Karau N, Römbke J (2014a) Interactive effects of lambda-cyhalothrin, soil moisture and temperature on Folsomia candida and Sinella curviseta (Collembola). Environ Toxicol Chem 33:654–661. https://doi.org/10.1002/etc.2479
Bandow C, Karau N, Römbke J (2014b) Interactive effects of pyrimethanil, soil moisture and temperature on Folsomia candida and Sinella curviseta (Collembola). Appl Soil Ecol 81:22–29. https://doi.org/10.1016/j.apsoil.2014.04.010
Bass C, Denholm I, Williamson MS, Nauen R (2015) The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol 121:78–87. https://doi.org/10.1016/j.pestbp.2015.04.004
Bayley M, Holmstrup M (1999) Water vapor absorption in arthropods by accumulation of myoinositol and glucose. Science 285:1909–1911
Biswas B, Qi F, Biswas JK, Wijayawardena A, Khan MAI, Naidu R (2018) The fate of chemical pollutants with soil properties and processes in the climate change paradigm - a review. Soil Syst 2:1–20. https://doi.org/10.3390/soilsystems2030051
Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EA, Noome DA, Simon-Delso N, Tapparo A (2015) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22:35–67. https://doi.org/10.1007/s11356-014-3332-7
Bonmatin JM, Mitchell EAD, Glauser G, Lumawig-Heitzman E, Claveria F, Bijleveld van Lexmond M, Taira K, Sánchez-Bayo F (2021) Residues of neonicotinoids in soil, water and people’s hair: A case study from three agricultural regions of the Philippines. Sci Total Environ 757:143822. https://doi.org/10.1016/j.scitotenv.2020.143822
Broznić D, Milin Č (2012) Effects of temperature on sorption-desorption processes of imidacloprid in soils of Croatian coastal regions. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes 47:779–794. https://doi.org/10.1080/03601234.2012.676413
Capela NXJ (2015) Climate change effects on Porcellionides sexfasciatus (Isopoda) inhabiting metal-contaminated sites. Master’s Thesis, University of Coimbra, Coimbra, Portugal
Crouau Y, Chenon P, Gisclard C (1999) The use of Folsomia candida (Collembola, Isotomidae) for the bioassay of xenobiotic substances and soil pollutants. Appl Soil Ecol 12:103–111. https://doi.org/10.1016/S0929-1393(99)00002-5
Cutler GC, Amichot M, Benelli G, Guedes RNC, Qu Y, Rix R, Ullah F, Desneux N (2022) Hormesis and insects: Effects and interactions in agroecosystems. Sci Total Environ 825:153899. https://doi.org/10.1016/j.scitotenv.2022.153899
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3:52–58. https://doi.org/10.1038/nclimate1633
Dankyi E, Gordon C, Carboo D, Fomsgaard IS (2014) Quantification of neonicotinoid insecticide residues in soils from cocoa plantations using a QuEChERS extraction procedure and LC-MS/MS. Sci Total Environ 499:276–283. https://doi.org/10.1016/j.scitotenv.2014.08.051
De Lima e Silva C, de Rooij W, Verweij RA, van Gestel CAM (2019) Toxicity in Neonicotinoids to Folsomia candida and Eisenia andrei. Environ Toxicol Chem 39(3):548–555. https://doi.org/10.1002/etc.4634
Demeure Y, Freckman DW, Van Gundy SD (1979) Anhydrobiotic Coiling of Nematodes in Soil. J Nematol 11:189–195
EC - European Commission (2003) Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC for new notified substances and Commission Regulation (EC) No 1488/94 on Risk assessment for existing substances and Commission Directive (EC) 98/8 on Biocides. Part 1, 2 and 3. European Commission - Joint Research Center, Luxembourg
Eisenbeis G (1982) Physiological absorption of liquid water by Collembola: absorption by the ventral tube at different salinities. J Insect Physiol 28:11–20
Environmental Canada (2007) Guidance Document on Statistical Methods for Environmental Toxicity Test. Environmental Protection Series, EPS 1/RM/46, 2005 with 2007 Updates. Environmental Canada, Ottawa
EPPO (2003) Environmental risk assessment scheme for plant protection products (Chapter 4): Soil. OEPP/EPP0 Bull 33:151–162
Everatt MJ, Convey P, Worland MR, Bale JS, Hayward SAL (2013) Heat tolerance and physiological plasticity in the Antarctic collembolan, Cryptopygus antarcticus, and mite, Alaskozetes antarcticus. J Therm Biol 38:264–271. https://doi.org/10.1016/j.jtherbio.2013.02.006
Everts JW, Willemsen I, Stulp M, Simons L, Aukema B, Kammenga J (1991) The toxic effect of deltamethrin on linyphiid and erigonid spiders in connection with ambient temperature, humidity, and predation. Arch Environ Contam Toxicol 20:20–24. https://doi.org/10.1007/BF01065323
Fakhereddin T, Dogan D (2021) Pro-oxidant potency of clothianidin in rainbow trout. Arh Hig Rada Toksikol 72:164–172. https://doi.org/10.2478/aiht-2021-72-3522
Ferreira NGC, Morgado RG, Amaro A, Machado AL, Soares AMVM, Loureiro S (2016) The effects of temperature, soil moisture and UV radiation on biomarkers and energy reserves of the isopod Porcellionides pruinosus. Appl Soil Ecol 107:224–236. https://doi.org/10.1016/j.apsoil.2016.06.007
Fountain MT, Hopkin SP (2005) Folsomia candida (COLLEMBOLA): a “standard” soil arthropod. Annu Rev Entomol 50:201–222. https://doi.org/10.1146/annurev.ento.50.071803.130331
Gainer A, Cousins M, Hogan N, Siciliano SD (2018) Petroleum hydrocarbon mixture toxicity and a trait-based approach to soil invertebrate species for site-specific risk assessments. Environ Toxicol Chem 37:2222–2234. https://doi.org/10.1002/etc.4164
Garcia-Valcarcel AI, Tadeo JL (1999) Influence of soil moisture on sorption and degradation of hexazinone and simazine in soil. J Agric Food Chem 47:3895–3900. https://doi.org/10.1021/jf981326i
Goulson D (2013) REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987. https://doi.org/10.1111/1365-2664.12111
Gunstone T, Cornelisse T, Klein K, Dubey A, Donley N (2021) Pesticides and soil invertebrates: a hazard assessment. Front Environ Sci 9:643847. https://doi.org/10.3389/fenvs.2021.643847
Hackenberger DK, Palijan G, Lončarić Ž, Jovanović Glavaš O, Hackenberger BK (2018) Influence of soil temperature and moisture on biochemical biomarkers in earthworm and microbial activity after exposure to propiconazole and chlorantraniliprole. Ecotoxicol Environ Saf 148:480–489. https://doi.org/10.1016/j.ecoenv.2017.10.072
Hennig TB, Alves PRL, Schiehl A, Araújo RS, Cabrera LC, Morelato RR, Baretta D (2022) Can the increase in atmospheric temperature enhance the toxicity and risk of fipronil for collembolans in tropical soils. Environ Sci Pollut Res 29:27104–27114. https://doi.org/10.1007/s11356-021-18349-7
Hennig TB, Alves PRL, Toniolo T, Bandeira FO, Santos WE, Cabrera LC, Gilson IK, Baretta D (2021) Toxicity of fipronil to Folsomia candida in contrasting tropical soils and soil moisture contents: effects on the reproduction and growth. Ecotoxicology 31:64–74. https://doi.org/10.1007/s10646-021-02490-7
Hennig TB, Bandeira FO, Dalpasquale AJ, Cardoso EJBN, Baretta D, Alves PRL (2020) Toxicity of imidacloprid to collembolans in two tropical soils under different soil moisture. J Environ Qual 49:1491–1501. https://doi.org/10.1002/jeq2.20143
Hoffmann AA, Sgró CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485. https://doi.org/10.1038/nature09670
Ihara M, Matsuda K, Shimomura M, Sattelle DB, Komai K (2004) Super agonist actions of clothianidin and related compounds on the SADβ2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Biosci Biotechnol Biochem 68:761–763. https://doi.org/10.1271/bbb.68.761
IPCC - Intergovernmental Panel on Climate Change (2021) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R and Zhou B (eds)]. Cambridge University Press, Cambridge and New York
ISO (2012) International Standardization Organization - 11268-2. Soil Quality – Effects of Pollutants on Earthworms – Part 2: Determination of Effects on Reproduction of Eisenia fetida/Eisenia andrei. Genève, Switzerland
ISO (2014) International Standardization Organization - 11267. Soil Quality – Inhibition of Reproduction of Collembola (Folsomia candida) by Soil Contaminants. Genève, Switzerland
Jegede OO, Owojori OJ, Römbke J (2017) Temperature influences the toxicity of deltamethrin, chlorpyrifos and dimethoate to the predatory mite Hypoaspis aculeifer (Acari) and the springtail Folsomia candida (Collembola). Ecotoxicol Environ Saf 140:214–221. https://doi.org/10.1016/j.ecoenv.2017.02.046
Jones A, Harrington P, Turnbull G (2014) Neonicotinoid concentrations in arable soils after seed treatment applications in preceding years. Pest Manag Sci 70:1780–1784. https://doi.org/10.1002/ps.3836
Kaka H, Opute PA, Maboeta MS (2021) Potential Impacts of Climate Change on the Toxicity of Pesticides towards Earthworms. J Toxicol 2021. https://doi.org/10.1155/2021/8527991
Lima MPR, Cardoso DN, Soares AMVM, Loureiro S (2015) Carbaryl toxicity prediction to soil organisms under high and low temperature regimes. Ecotoxicol Environ Saf 114:263–272. https://doi.org/10.1016/j.ecoenv.2014.04.004
Lima MPR, Soares AMVM, Loureiro S (2011) Combined effects of soil moisture and carbaryl to earthworms and plants: Simulation of flood and drought scenarios. Environ Pollut 159:1844–1851. https://doi.org/10.1016/j.envpol.2011.03.029
Maino JL, Cushen A, Valavi R, Umina PA (2021) Spatial Variation in Australian Neonicotinoid Usage and Priorities for Resistance Monitoring. J Econ Entomol 114:2524–2533. https://doi.org/10.1093/jee/toab192
Mekala S, Polepongu S (2019) Impact of Climate Change on Soil Microbial Community. In: Plant Biotic Interactions. Springer International Publishing, Cham, p 31–41. https://doi.org/10.1007/978-3-030-26657-8_3.
Morgado RG, Gomes PAD, Ferreira NGC, Cardoso DN, Santos MJG, Soares AMVM, Loureiro S (2016) Toxicity interaction between chlorpyrifos, mancozeb and soil moisture to the terrestrial isopod Porcellionides pruinosus. Chemosphere 144:1845–1853. https://doi.org/10.1016/j.chemosphere.2015.10.034
Natal-da-luz T, Ojeda G, Pratas J, van Gestel CAM, Sousa JP (2011) Toxicity to Eisenia andrei and Folsomia candida of a metal mixture applied to soil directly or via an organic matrix. Ecotoxicol Environ Saf 74(6):1715–1720. https://doi.org/10.1016/j.ecoenv.2011.05.017
Nota B, Van Straalen NM, Ylstra B, Roelofs D (2010) Gene expression microarray analysis of heat stress in the soil invertebrate Folsomia candida. Insect Mol Biol 19:315–322. https://doi.org/10.1111/j.1365-2583.2009.00990.x
Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: Environmental contaminants in a warming world. Environ Int 35:971–986. https://doi.org/10.1016/j.envint.2009.02.006
Ochsner TE, Stephens BM, Koskinen WC, Kookana RS (2006) Sorption of a Hydrophilic Pesticide: Effects of Soil Water Content. Soil Sci Soc Am J 70:1991–1997. https://doi.org/10.2136/sssaj2006.0091
Ogungbemi AO, van Gestel CAM (2018) Extrapolation of imidacloprid toxicity between soils by exposing Folsomia candida in soil pore water. Ecotoxicology 27:1107–1115. https://doi.org/10.1007/s10646-018-1965-x
Owojori OJ, Ademosu OT, Jegede OO, Fajana HO, Kehinde TO, Badejo MA (2019) Tropical oribatid mites in soil toxicity testing: Optimization of test protocol and the effect of two model chemicals (cadmium and dimethoate) on Muliercula inexpectata. Chemosphere 218:948–954. https://doi.org/10.1016/j.chemosphere.2018.11.173
Pearsons KA, Lower SE, Tooker JF (2021) Toxicity of clothianidin to common Eastern North American fireflies. PeerJ 9:e12495. https://doi.org/10.7717/peerj.12495
Ramasubramanian T (2013) Persistence and dissipation kinetics of clothianidin in the soil of tropical sugarcane ecosystem. Water Air Soil Pollut 224:1468. https://doi.org/10.1007/s11270-013-1468-6
Ritchie EE, Maisonneuve F, Scroggins RP, Princz JI (2019) Lethal and sublethal toxicity of thiamethoxam and clothianidin commercial formulations to soil invertebrates in a natural soil. Environ Toxicol Chem 38(10):2111–2120. https://doi.org/10.1002/etc.4521
Rix RR, Cutler GC (2022) Review of molecular and biochemical responses during stress induced stimulation and hormesis in insects. Sci Total Environ 827:154085. https://doi.org/10.1016/j.scitotenv.2022.154085
Römbke J, Garcia MV, Scheffczyk A (2007) Effects of the fungicide benomyl on earthworms in laboratory tests under tropical and temperate conditions. Arch Environ Contam Toxicol 53:590–598. https://doi.org/10.1007/s00244-006-0219-8
Saarenketo T (1998) Electrical properties of water in clay and silty soils. J Appl Geophy 40:73–88
Shelton DR, Parkin TB (1991) Effect of Moisture on Sorption and Biodegradation of Carbofuran in Soil. J Agric Food Chem 39:2063–2068. https://doi.org/10.1021/jf00011a036
Souza ED, Carneiro MAC, Paulino HB (2005) Physical attributes of a typic quartzipsamment and a rhodic hapludox under different management systems. Pesqui Agropecu Bras 40:1135–1139. https://doi.org/10.1590/S0100-204X2005001100012
Sur R, Stork A (2003) Uptake, translocation and metabolism of imidacloprid in plants. Bull Insectol 56:35–40
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais, 2nd edn. Universidade Federal do Rio Grande do Sul, Porto Alegre, p 147 (Boletim Técnico, 5)
Tripathi G, Kachhwaha N, Dabi I, Bandooni N (2011) Temperature-dependent alterations in metabolic enzymes and proteins of three ecophysiologically different species of earthworms. Brazilian Arch Biol Technol 54:769–776. https://doi.org/10.1590/S1516-89132011000400017
Tumminello RA, Fuller-Espie SL (2013) Heat stress induces ROS production and histone phosphorylation in celomocytes of Eisenia hortensis. Invertebr Surviv J 10:50–57
van Gestel CAM (2012) Soil ecotoxicology: State of the art and future directions. Zookeys 176:275–296. https://doi.org/10.3897/zookeys.176.2275
van Gestel CAM, Van Diepen AMF (1997) The influence of soil moisture content on the bioavailability and toxicity of cadmium for Folsomia candida Willem (Collembola: Isotomidae). Ecotoxicol Environ Saf 36:123–132. https://doi.org/10.1006/eesa.1996.1493
Velki M, Ečimović S (2015) Changes in exposure temperature lead to changes in pesticide toxicity to earthworms: A preliminary study. Environ Toxicol Pharmacol 40:774–784. https://doi.org/10.1016/j.etap.2015.09.009
Waagner D, Heckmann LH, Malmendal A, Nielsen NC, Holmstrup M, Bayley M (2010) Hsp70 expression and metabolite composition in response to short-term thermal changes in Folsomia candida (Collembola). Comp Biochem Physiol - A Mol Integr Physiol 157:177–183. https://doi.org/10.1016/j.cbpa.2010.06.171
Wang Y, Slotsbo S, Holmstrup M (2022) Soil dwelling springtails are resilient to extreme drought in soil, but their reproduction is highly sensitive to small decreases in soil water potential. Geoderma 421:115913. https://doi.org/10.1016/j.geoderma.2022.115913
WMO - World Meteorological Organization (2022) State of the Climate in Latin America and the Caribbean: 2021. Geneva, Switzerland: World Meteorological Organization, 2022. https://library.wmo.int/doc_num.php?explnum_id=11270. Accessed 03 Oct 2022
Wood TJ, Goulson D (2017) The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res 24:17285–17325. https://doi.org/10.1007/s11356-017-9240-x
Wu R, He W, Li Y, Li Y, Qin Y, Meng F, Wang L, Xu F (2020) Residual concentrations and ecological risks of neonicotinoid insecticides in the soils of tomato and cucumber greenhouses in Shouguang, Shandong Province, East China. Sci Total Environ 738:140248. https://doi.org/10.1016/j.scitotenv.2020.140248
Yang Y, Su L, Huang Y, Zhang X, Li C, Wang J, Fan L, Wang S, Zhao YH (2022) Bio-uptake, tissue distribution and metabolism of a neonicotinoid insecticide clothianidin in zebrafish. Environ Pollut 292:118317. https://doi.org/10.1016/j.envpol.2021.118317
Yu Z, Li XF, Wang S, Liu LY, Zeng EY (2021) The human and ecological risks of neonicotinoid insecticides in soils of an agricultural zone within the Pearl River Delta, South China. Environ Pollut 284:117358. https://doi.org/10.1016/j.envpol.2021.117358
Zhang P, Jin F, Yang LL, Wu RN, Zhang YX, Wang J (2016) Residue and dissipation of clothianidin in tomatoes and soil. Chin J Pestic Sci 18(4):490–496. (Chinese)
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (CNPq) for the research grant (Project 407170/2016-2).
Funding
This study was supported by the National Council for Scientific and Technological Development (CNPq) (Research Grant - Project 407170/2016-2).
Author information
Authors and Affiliations
Contributions
TSG: Conceptualization, Data curation, Formal analysis, Investigation, Writing—original draft. FOB: Investigation, Data curation, Formal analysis, Writing—original draft. EJBNC: Supervision, Writing—review & editing. PRLA: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Brazilian regimentation for the scientific use of animals – Law no. 11.794). Consent to participate and publication is not applicable once this study does not involve humans.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Graciani, T.S., Bandeira, F.O., Cardoso, E.J.B.N. et al. Influence of temperature and soil moisture on the toxic potential of clothianidin to collembolan Folsomia candida in a tropical field soil. Ecotoxicology 32, 82–92 (2023). https://doi.org/10.1007/s10646-023-02621-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02621-2