Abstract
Information regarding the safety and environmental risks of pesticides intended for urban use remains limited. This study aimed to assess the effects of four common pesticides on the microalga Raphidocelis subcapitata: DIAZINON® 25% C. E., Roundup®, URBACIN® 20C. E., and VAPODEL® 20% C. E., which are commercial formulations of diazinon, glyphosate, dichlorvos, and cypermethrin, respectively. According to 96-h inhibition of population growth bioassays, the four pesticide toxicities exemplified the following order: DIAZINON® (diazinon) > Roundup® (glyphosate) > VAPODEL® (dichlorvos) > URBACIN® (cypermethrin). Increasing pesticide concentrations elicited alterations in the specific growth rates (µmax). The macromolecule contents and photosynthetic pigments increased in groups exposed to the highest concentrations of DIAZINON® 25%, Roundup®, and URBACIN® 20 compared to the control group, despite these treatments inducing lower population growth rates. VAPODEL® 20% induced higher growth rates and lower macromolecule content compared to the control. Since active ingredients were not quantified, certain comparisons may prove limiting, but it is important to assess the effects of the whole mixtures in the form that they enter the environment, especially for urban-intended applications or generic formulations with higher additive contents. Finally, this study demonstrated that commercial pesticide formulations designed for urban applications might pose a threat to freshwater microalgae due to their underestimated toxic potential, but further studies are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are designed to eliminate undesirable organisms that affect human health or cropland productivity; however, side effects on both terrestrial and aquatic non-target organisms and food webs have been documented, altering the structures of communities (Caihong et al. 2015; Nowell et al. 2018). The United States Environmental Protection Agency (US EPA) defines pesticides as “any substance or mixture of substances intended for preventing, destroying, repelling, or mitigating any pest” (US EPA 2022), while in the European Union (EU), pesticides are defined as those products intended for plant protection and biocides as the products used for non-plant protection purposes (EU 2009/128/EC). Therefore, this study employs the definition of pesticides as stated by the US EPA (2022).
Pesticide use has substantially increased in recent decades; the Food and Agriculture Organization (FAO) of the United Nations registered more than four megatons of pesticide use across 164 countries (FAO 2017). Although the concentration of pesticides per hectare is expected to be higher in rural areas compared to urban industrial zones, misuse of pesticides has increased concentrations per hectare in urban zones (US EPA 2005; Wittmer et al. 2011). Thus, the effects of pesticides in non-agricultural zones should be investigated, especially considering approximately 80% of families in the United States use at least one pesticide per household for fumigation and gardening purposes (Horton et al. 2011; US EPA 2015).
Glyphosate is a frequently applied herbicide in both agricultural and urban areas, and it is approved for use in Australia, the European Union, and the USA (Singh et al. 2020). Insecticides comprise two main groups, organophosphates and pyrethroids. Of the organophosphates, diazinon and dichlorvos are not approved in the European Union, but, in Canada, some exceptions are considered to meet the standards for the protection of human health and environment (Canada Pest Management Regulatory Agency [PMRA] 2017, 2021). Organophosphates, which have been detected in urban dust and waterways, are higher toxicity and are gradually being substituted with pyrethroids (Nowell et al. 2021).
Diverse active ingredients have been quantified in several countries and matrices, including dust, soil, and water, in both rural and urban areas, and it is postulated that there is a “pesticide signature” for every city, dependent on current uses and applications, that varies over the time with changes in regulations and use patterns (Stehle et al. 2019). Environmental pesticide concentrations differ among high and low-income zones; in developed countries, the pesticide concentrations generally range from nanograms per liter occasionally to micrograms per liter (Spahr et al. 2020), and in least-developed countries, the concentration of active ingredients can reach the order of micrograms or per liter or higher.
Pesticide research primarily focuses on the effects of active ingredients. However, these are typically a part of complex mixtures within commercial formulations that include inert compounds and adjuvants. Assessing the effects of these complex mixtures is essential as it represents the true form in which pesticides are released into the environment, and the interactions among these chemicals might result in synergistic effects (Cox and Surgan 2006; Pereira et al. 2009).
Consequently, this study utilized the algal species Raphidocelis subcapitata as a test organism to assess the effects of four commercial pesticide formulations that are commonly used in urban areas of Mexico: DIAZINON® 25% C.E. (diazinon), Roundup® (glyphosate), URBACIN® 20 C.E. (cypermethrin), and VAPODEL® 20% C.E. (dichlorvos). These were selected as representatives of organophosphates still in use in non-developed countries, a pyrethroid insecticide, and an herbicide most commonly used in urban areas. This research aimed to demonstrate that pesticides used for urban applications at low concentrations (0.009–0.201 × 10−3% (v/v) of the commercial formulation) interfere with algal population growth and induce biochemical changes. Finally, we discuss the importance of assessing holistic commercial formulations instead of active ingredients exclusively.
Materials and Methods
Chemicals
The four commercial pesticides were purchased from distributors located in the urban area of Aguascalientes, Mexico. Manufactured in México by Delta S.A. de C.V: DIAZINON® 25% (75% inert ingredients, 236 mg/mL diazinon), URBACIN® 20 (70% inert ingredients, 189.2 mg/mL cypermethrin), and VAPODEL® (80% other ingredients, 226 mg/mL dichlorvos). Manufactured in USA by Monsanto Company, Roundup® (98% other ingredients, 15.22 mg/mL glyphosate).
Pesticides were diluted in deionized water as stock solutions 24 h prior to testing and were subsequently stored at 4 °C for no longer than 60 d. All stock solutions were filtered with 0.45 μm syringe filters to avoid microbial contamination or degradation. Further dilutions were conducted in aseptic conditions. All concentrations are expressed as a percentage of the commercial pesticides and the expected concentration of the active ingredient (nominal concentration according to the manufacturer’s formulations). Stock solutions were prepared as ten-fold dilutions of the commercial pesticides at 1:1000 (Roundup® 2%) and 1:10,000 (DIAZINON® 25%, URBACIN® 20, and VAPODEL® 20%).
Culture conditions
The R. subcapitata strain used in this study was kindly donated by the Laboratory of Experimental Hydrobiology, Escuela Nacional de Ciencias Biológicas of the Instituto Politécnico Nacional and was maintained in Bold’s Basal Medium (BBM) with continuous aeration and a photoperiod of 12 h light and 12 h dark at 25 °C. The inoculum for experiments was collected when the culture reached the exponential growth phase determined by the culture absorbance. The biomass was centrifuged at 5000 rpm for 10 min at room temperature, and the supernatant was discarded. The pellet was washed three times with fresh BBM and resuspended in 10 mL of fresh BBM prior to experimentation. The cell density was estimated using a hemocytometer and adjusted to 104 cells/mL, which was the initial concentration used for all bioassays.
Population growth inhibition tests
Population growth inhibition assays were performed according to the OECD Guideline 201 (2011). All experiments were conducted aseptically in 24-well microplates. The initial cell density was adjusted to 104 cells/mL with a final volume of 2 mL, and cells were incubated at 25 ± 2 °C with a 12:12 h light:dark photoperiod for 96 h. Each pesticide was assayed at five concentrations, and one control group (BBM) was included in every microplate. The microalgae suspensions were manually homogenized twice a day throughout the bioassay. The range-finding tests included nominal active ingredient concentrations from 0.01−100 mg/L. Subsequent experimental concentrations were selected according to the range in which approximately 50% growth inhibition was observed. Cell counts were performed daily using a hemocytometer, which were used to estimate the median inhibitory concentration (IC50). The IC50 values are reported as mg active ingredient per liter (mg a.i./L).
Biomarker assessments at subinhibitory concentrations
Five concentrations corresponding to fractions (0.01, 0.02, 0.05, 0.10, and 0.20) of each IC50 were selected to assess the effects of pesticides on the photosynthetic pigment and caloric contents of R. subcapitata. Table 1 shows the respective concentrations used for each pesticide. The cell density was adjusted to 4 × 105 cells/mL with a final volume of 100 mL BBM in 350 mL glass bottles. Flasks were incubated at 25 ± 2 °C with a 12:12 h light:dark photoperiod for 144 h. The extended period and inoculum were adjusted to obtain the biomass required to perform the biochemical determinations. Samples were collected daily under aseptic conditions to estimate the cell density. At the end of the exposure period, the biomass was centrifuged at 5000 rpm for 10 min at room temperature, the supernatant was discarded, and the pellet was washed three times with fresh BBM. The pellet was resuspended in 5 mL BBM and stored at −80 °C until further use.
Quantification of photosynthetic pigments
Five mL of methanol (90%, v/v) was added to a 100 µL aliquot of the concentrated biomass. The suspension was heated to 90 °C for 5 min, cooled to room temperature, and stored in the dark at 4 °C overnight. Samples were centrifuged at 5000 rpm for 5 min, and the pellet was discarded. The supernatant was recovered and adjusted to a 5 mL volume with methanol (90%, v/v). The absorbance was measured at 470, 649, and 665 nm. The following equations were used to calculate the pigment contents (Martínez-Ruiz and Martínez-Jerónimo 2018):
The results are expressed as picograms of pigment per algal cell.
Determination of the caloric contents in R. subcapitata
To quantify total carbohydrates, 100 µL of phenol (5%, w/v) was added to 100 µL of sample, followed by the slow addition of 500 µL concentrated sulfuric acid. The mixture was heated to 90 °C for 10 min. After cooling to room temperature, the absorbance was taken at 490 nm against a standard curve generated using dextrose (0, 2.5, 5, 10, 15 and 20 µg). The results are expressed as picograms of carbohydrates per algal cell (Hernández-Zamora and Martínez-Jerónimo 2019).
For the total lipid determination, 500 µL of chloroform:methanol (2:1, v/v) and 500 µL of deionized water were added to 100 µL sample. The mixture was homogenized and centrifuged; the organic phase, containing the lipid extract, was recovered. Extraction was performed three times, and the organic phases of every extraction were combined and heated to dryness. Then, 100 µL of distilled water and 1 mL of concentrated sulfuric acid were added, and the solution was heated to 90 °C for 10 min. After cooling to room temperature, 1000 µL of 9 mM phospho-vanillin reactive was added, and the resulting solution was incubated in the dark for 15 min. The absorbance was read at 525 nm and compared against a canola oil standard curve (0, 0.5, 1, 2, 2.5 and 5 µg) (Mishra et al. 2014). The results are expressed as picograms of lipids per algal cell.
Total proteins were quantified by adding 1 mL Bradford’s reactive to 100 µL sample, which was incubated for 10 min to develop color. The absorbance was measured at 595 nm against a standard curve of bovine serum albumin (0, 2.5, 5, 10, 15 and 20 µg) (Patnaik et al. 2019). The results are expressed as picograms of total protein per algal cell.
Finally, the caloric contents of the microalgae were obtained by multiplying the contents of carbohydrates, lipids, and proteins by their combustion factors: 4.11 cal/mg, 9.45 cal/mg, and 5.65 cal/mg, respectively (Arzate-Cárdenas and Martínez-Jerónimo 2012). The results are expressed as pcal per algal cell.
Statistical analysis
The IC50 of each pesticide was calculated using the log-logistic model in the drc package in the R Studio software. All results were compared through one-way analysis of variance (ANOVA), and multiple comparison tests were performed using Bonferroni’s least significant difference (LSD) with the statistical software R (v.3.5.1). Significance was established at P < 0.05.
Results
The IC50 values were as follows: DIAZINON® 25% 0.13 × 10−3% (0.31 mg a.i./L), Roundup® 8.2 × 10−3% (1.25 mg a.i./L), URBACIN® 209.9 × 10−3% (18.70 mg a.i./L), and VAPODEL® 20% 2.6 × 10−3% (6.03 mg a.i./L). The values of IC1, IC10, and IC50 are shown in Table 2. Based on the percentage of the commercial formulation, the toxicity toward microalgae showed the following order: DIAZINON® > VAPODEL® > Roundup® > URBACIN®. Alternatively, when based on the active ingredient concentration, the toxicity toward R. subcapitata exemplified the following order: DIAZINON® > Roundup® > VAPODEL® > URBACIN®. These results revealed that DIAZINON® was the most toxic formulation, and URBACIN® was the least toxic formulation within the commercial pesticides tested.
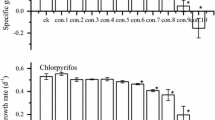
Exposure to fractions of each respective IC50 demonstrated that DIAZINON®, Roundup®, and URBACIN® caused significant decreases in the R. subcapitata maximum population growth rates (µ) after 144 h exposure (p < 0.05) (Fig. 1). In contrast, the groups exposed to VAPODEL® exhibited higher growth rates (p < 0.05) (Table 3). Exposure to increasing concentrations was associated with increased chlorophyll contents, carotenoids, carbohydrates, lipids, and proteins for all pesticides excluding VAPODEL® (p < 0.05) (Figs. 2–6). The effective concentrations (EC50) of the biomarkers assessed in this study are shown in Table 4.
Effects of IC50 fractions of DIAZINON® 25% on Raphidocelis subcapitata exposed for 144 h on (a) total carbohydrates; (b) total lipids; (c) total protein; (d) chlorophyll-a; (e) chlorophyll-b; and (f) carotenoids. Different letters indicate significant differences according to one-way ANOVA and Bonferroni’s LSD test (p < 0.05)
Effects of IC50 fractions of Roundup® on Raphidocelis subcapitata exposed for 144 h on (a) total carbohydrates; (b) total lipids; (c) total protein; (d) chlorophyll-a; (e) chlorophyll-b; and (f) carotenoids. Different letters indicate significant differences according to one-way ANOVA and Bonferroni’s LSD test (p < 0.05)
Effects of IC50 fractions of URBACIN® 20 on Raphidocelis subcapitata exposed for 144 h on (a) total carbohydrates; (b) total lipids; (c) total protein; (d) chlorophyll-a; (e) chlorophyll-b; and (f) carotenoids. Different letters indicate significant differences according to one-way ANOVA and Bonferroni’s LSD test (p < 0.05)
Effects of IC50 fractions of VAPODEL® 20% on Raphidocelis subcapitata exposed for 144 h on (a) total carbohydrates; (b) total lipids; (c) total protein; (d) chlorophyll-a; (e) chlorophyll-b; and (f) carotenoids. Different letters indicate significant differences according to one-way ANOVA and Bonferroni’s LSD test (p < 0.05)
DIAZINON® significantly inhibited the growth of R. subcapitata at all concentrations used in bioassays (Fig. 1a). Algae exposed to the highest DIAZINON® concentration exhibited higher contents of carbohydrates (29.05 pg/cell), lipids (6.06 pg/cell), and proteins (62.80 pg/cell) compared to the control group (p < 0.05) (Fig. 2a–c). Chlorophyll-a and chlorophyll-b increased significantly, with the highest contents (11.44 pg/cell and 8.68 pg/cell, respectively) observed in algal cells exposed to 0.0254 × 10−3% (0.062 mg a.i./L) DIAZINON® (p < 0.05) (Fig. 2d−e). No significant differences in carotenoid contents were observed with different levels of pesticide exposure (p > 0.05) (Fig. 2f).
Significant population growth inhibition was observed after 144 h of exposure to Roundup®, even at the lowest concentration (0.079 × 10−3%), which corresponds to 0.012 mg a.i./L (Fig. 1b). The photosynthetic pigments per cell significantly increased in algae exposed to 0.821 × 10−3% (0.125 mg a.i./L) and 0.164 × 10−3% (0.250 mg a.i./L) of Roundup® (p < 0.05) (Fig. 3d–f). The same patterns were observed for the total carbohydrate and protein contents, with significant increases at 0.164 × 10−3% (0.250 mg a.i./L) (p < 0.05) (Figs. 3a, c). The lipid contents were not affected by exposure to Roundup® at the tested concentrations (p > 0.05) (Fig. 3b).
URBACIN® significantly inhibited algal growth (p < 0.05) (Fig. 1c). Exposure to 0.099 × 10−3% and 1.977 × 10−3% (1.87 mg a.i./L and 3.74 mg a.i./L, respectively) of URBACIN® decreased Chl-a contents, and concentrations equal to or greater than 0.494 × 10−3% (0.935 mg a.i./L) decreased the carotenoid contents compared to the control group (p < 0.05) (Fig. 4d, f). All URBACIN® concentrations increased the protein contents, which were 2-fold higher in algal cells exposed to 1.977 × 10−3% (3.74 mg a.i./L) than the control group (Fig. 4c). The carbohydrate contents significantly increased after exposure to 0.494 × 10−3% (0.935 mg a.i./L), 0.988 × 10−3% (1.870 mg a.i./L), and 1.977 × 10−3% (3.740 mg a.i./L) of cypermethrin (URBACIN®; Fig. 4a) (p < 0.05). The lipid contents remained unaltered by all tested cypermethrin concentrations (p > 0.05) (Fig. 4b).
VAPODEL® was the only commercial formulation tested that promoted algal growth (p < 0.05) (Fig. 1d). Carotenoids were significantly reduced in the VAPODEL®-exposed groups (p < 0.05) (Fig. 5f). The carbohydrate, lipid, and protein contents decreased in algae exposed to VAPODEL® at 0.13 × 10−3% (0.301 mg a.i./L), 0.26 × 10−3% (0.603 mg a.i./L), and 0.53 × 10−3% (1.206 mg a.i./L) (p < 0.05) (Fig. 5a−c). Contrasting with the other three commercial formulations used in this study, exposure to VAPODEL® decreased the energy contents per cell in R. subcapitata (p < 0.05) (Fig. 6d).
Discussion
The results of the growth inhibition tests classified the four commercial formulations as follows: URBACIN®, moderately toxic (IC50 10–100 mg a.i./L), Roundup® and VAPODEL®, toxic (IC50 1–10 mg a.i./L), and DIAZINON®, highly toxic (IC50 0.1–1 mg a.i./L) according to US EPA guidelines (US EPA 2015). These classifications are based on the toxicity of the active ingredients, without accounting for the occurrence of pesticides in the environment as mixtures, combined with “other” or “inert” ingredients, which are also responsible for deleterious effects (Gonçalves et al. 2019).
RoundUp® is a systemic herbicide used to control weeds and grass. The commercial formulation available corresponds to the “ready to use” category, which includes polyoxyethylene tallow amine as a surfactant that increases the penetration on plant cells and, thus, its toxicity. Although glyphosate-based herbicides are among the most studied plant protection products, their exact formulation is not fully known as it remains as confidential information. Nonetheless, the noxious and synergistic effects of glyphosate and tallow amine surfactant are well described in the current literature (Martins-Gomes et al. 2022).
Contact insecticides like DIAZION®, URBACIN®, and VAPODEL®, produce deleterious effects on insects due to penetration via the integument and trachea; they eventually reach all organs, affecting the central nervous system. To facilitate their transport, commercial formulations require carrier solvents and surfactants (Castro et al. 2014). The commercial insecticides studied here were purchased as emulsifiable concentrates (EC), which have a general formula resembling: active ingredient (20%), emulsifier blend (5–10%), and solvent(s) (up to 100%) (Knowles 2008). However, the current composition of these insecticides remains confidential. Water-insoluble insecticides are dissolved in organic solvents, such as xylene, petroleum oils, and cyclohexanone. Then, the insecticide-solvent mixture is emulsified by the addition of surfactants, such as organic alcohols or any substance that lowers superficial tension. Some solvents evaporate rapidly and allow the deposition of active ingredients onto surfaces; petroleum oils are preferred due to improved residual effects, despite some additives exerting negative effects on non-target organisms, like phytotoxicity on the plants being treated (Pascual-Villalobos et al. 2019).
Inert ingredients used in the commercial formulations can also increase the mobility of the active ingredients, thus facilitating their movement in aquatic environments (Beggel et al. 2010). Therefore, low concentrations of active ingredients in aquatic environments might be highly toxic resulting from interactions with inert ingredients; for instance, the four pesticides assessed in this study presented high toxicity towards R. subcapitata at relatively low concentrations of active ingredients – emphasizing that these commercial products were diluted more than 104-fold to elicit negative effects on algae. Moreover, commercial formulations of glyphosate have been shown to be 10−100 times more toxic than the active ingredient alone (Cox and Surgan 2006; Svartz and Pérez-Coll 2013). Therefore, the assessment of active ingredients alone underestimates the effects on aquatic biota, and it is critical to evaluate the effect of the commercial formulations as that is the condition in which they are administered into the environment.
For DIAZINON®, the IC50 1.3 × 10−4% (0.3 mg a.i./L) was at least 10-fold lower and up to 150-fold more toxic than other commercial formulations featuring these organophosphate-based pesticides (Ma et al. 2005). For glyphosate-based pesticides, such as Roundup®, several authors have described the increased toxicity associated with the use of adjuvants, particularly tallow amines, which continue to be used in formulations despite their documented toxicity (Gonçalves et al. 2019). Results reported here showed that higher concentrations of adjuvants (approximately 88% of “other ingredients”) promoted lower IC50 values for microalgae compared with the formulations used for land crops (Table 5).
Sáenz et al. (2012) tested a commercial formulation of cypermethrin with 10% active ingredient that caused significant inhibition of four microalgae species; the IC50 values (0.20 mg a.i./L) for R. subcapitata were lower than those of URBACIN® (9.9 × 10−3%, 18.77 mg a.i/L), which contains 20% active ingredient and 80% of “other ingredients”. These IC50 values indicated that higher concentrations of adjuvants within the commercial formulations increased the toxicity of cypermethrin (Svartz and Pérez-Coll 2013). In contrast, cypermethrin alone has low toxicity to microalgae, according to its IC50 of 112.45 mg/L (Li et al. 2005).
VAPODEL® is one of several available commercial pesticide formulations containing dichlorvos, in addition to VAPONA® and Nuván®, which remain available although this pesticide has been banned in several countries due to its high toxicity (Okoroiwu and Iwara 2018). Contrasting to the other tested pesticides, this study revealed that the dichlorvos commercial formulation (VAPODEL®) presented reduced toxicity for microalgae compared to the active ingredient (Table 5). These results disagree with other studies that reported micrograms per liter concentrations of the active ingredient inhibited algal growth (Agirman et al. 2014). Thus, this apparent reduction in toxicity observed in the present study might be related to either the intrinsic tolerance of the algal strain used or to the interaction of the “other ingredients” included in the commercial formulation; however, this formulation cannot be considered as non-toxic because environmental risk assessments require the assessment against various non-target organisms in addition to this algal strain.
Although DIAZINON®, Roundup®, and URBACIN® were assessed at concentrations below their respective IC50 values, the significant inhibition of the algal growth was observed in response to exposure to these pesticides (Fig. 1). The modes of action for diazinon and cypermethrin in algal species are not well-understood because algae are not the target organisms for these pesticides. Pesticides can alter antioxidant mechanisms, enzymatic pathways, and energy allocation; moreover, pesticides can induce vacuolation, the disruption of mitochondrial membranes, and the impairment of photosynthetic pathways, among other outcomes (Sun et al. 2015; Mansano et al. 2017).
Baruah and Chaurasia (2020) reported the enhanced activity of reactive oxygen species (ROS) and the accumulation of lipids in Chlorella after exposure to cypermethrin, the active ingredient of URBACIN®; thus, the commercial formulation of cypermethrin increased the intracellular contents of lipids and photosynthetic pigments as mechanisms to counteract the effects of the pesticide. As observed in R. subcapitata, the energy content and photosynthetic pigments increased, but the growth performance was low in comparison to non-exposed microalgae.
Kurade et al. (2016) described several alterations in Chlorella vulgaris following the exposure to diazinon, including low growth rates, low dry weight, low chlorophyll and carotenoid contents, and diminished activity of antioxidant enzymes, including superoxide dismutase and catalase. Nevertheless, such results were observed in analysis of the active ingredient, but commercial formulations, like DIAZINON®, might represent a higher concern as the “other ingredients” significantly increased the effect of the active ingredient. This requires lower amounts of the commercial pesticide, which diluted at one million times elicited the aforementioned effects on R. subcapitata.
Pedrosa-Gomes and Juneau (2016) reported that glyphosate induced oxidative stress in Lemna minor, and despite that it is an aquatic plant, the mechanisms underlying the toxicity of glyphosate-based pesticides could be applicable to other photosynthetic, non-target species like microalgae. Therefore, glyphosate can alter energy metabolism by disrupting biochemical pathways within either mitochondria or chloroplasts, increasing the available energy in terms of total carbohydrates, lipids, and protein, as observed in the present work. Besides higher energy levels, microalgae can also exhibit higher energy demands to manage the pesticide-promoted stress, likely through the production of ROS and through the impairment of mitochondrial electron transport chain and thylakoids photosystems. Furthermore, pesticides like glyphosate have been shown to inhibit the activity of enzymes directly involved in the metabolism of carbohydrates, such as hexokinase, a key step in glycolysis (Panetto et al. 2019).
In contrast, VAPODEL® stimulated the population growth of R. subcapitata but significantly diminished algal energy contents compared to the control group (Fig. 6). This effect might be attributed to the increased population growth rate. This behavior was different in inhibitory bioassays, because at higher VAPODEL® concentrations of 1.70 × 10−3–4.40 × 10−3% (4–10 mg/L), population growth was inhibited similar to the other three commercial formulations; however, at lower subinhibitory concentrations from 0.133 × 10−3–0.534 × 10−3% (0.301–1.206 mg/L), a growth-stimulating behavior was observed (Fig. 1). Some authors have described this phenomenon as hormesis, in which there is a stimulation effect within a certain interval of concentrations. Alternatively, concentrations outside this interval, both below and above, elicit negative impacts on the same parameters (Cedergreen et al. 2006; Mansano et al. 2017).
Dichlorvos, at concentrations below 1 mg/L, was reported to cause hormetic responses on the growth rate of the cyanobacteria Microcystis wesenbergii (Sun et al. 2015) and the alga Heterosigma akashiwo (Fang and Zhang 2010). In the present study, concentrations of dichlorvos for the standard inhibition test (96 h) were higher, which indicates that the commercial formulation is less toxic than the active ingredient alone. In fact, the formulation toxicity might be underestimated if one endpoint is exclusively assessed, such as the population growth. As in this study, the evaluation of biomarkers at concentrations below the IC50 showed significant alterations on the content of photosynthetic pigments and energy availability or macromolecules content. For such effects, concentrations are as low as 0.160 mg a.i./L (0.266 mg a.i./L, on average), which corresponds to the 0.071 × 10−3% of the commercial formulation (Table 4). Thus, it is relevant to assess the effects of the whole formula in its entirety, especially for those intended for urban applications, which seem to be more toxic than agrochemicals.
Another explanation of diminished energy contents in algae exposed VAPODEL® relies upon the mechanisms described for dichlorvos, which are known to inhibit the synthesis of ATP by decreasing the electron transport complexes within mitochondria and affect their membrane integrity, ultimately promoting the formation of ROS and oxidative stress (Binukumar et al. 2010). Moreover, dichlorvos as active ingredient is known to alter biochemical pathways like those involved in the metabolism of carbohydrates and lipids (Biu-Nguyen et al. 2015). Therefore, commercial formulations of dichlorvos may result in algae with lower energy content as observed with these results.
The tested commercial formulations DIAZINON®, Roundup®, and URBACIN® increased the content of photosynthetic pigments of R. subcapitata. Higher pigment contents have been associated with mechanisms activated to prevent stress related to ROS, which might interfere with photosynthesis and prevent these oxidant species from damaging thylakoids and chloroplasts (Chen et al. 2020). Some antioxidant enzymes are co-regulated with the biosynthetic pathways for carotenoids; thus, the increased activity of certain enzymes, such as glutathione reductase or glutathione transferase, might be associated with the increased carotenoid contents in algae exposed to pesticides (Gomes et al. 2017).
Commercial formulations of insecticides include substances like butoxypolypropylene glycol, dichlorobenzene (isomers ortho and para), and xylene, which differ toxicologically. Xylene is a toxic additive carrier used in insecticides formulations (Maliszewska and Tęgowska 2018). Dichlorobenzene is an aromatic compound used in insecticides formulations that produces several deleterious effects on exposed organisms (Linde 2005). EC formulations can include: (a) linear alkylbenzene sulfonates (LAS) that are used as anionic surfactants and adjuvants; (b) non-ionic surfactants that are synthesized from alkylphenols, fatty alcohols, fatty acids, or fatty amines, by the addition of ethylene or propylene oxides, and are mainly used as emulsifiers; or (c) amphoteric surfactants, contained in some agrochemicals but their use is a minority in comparison to anionic and non-ionic surfactants (Castro et al. 2014).
Liner alkylbenzene sulfonates (LAS) are toxic compounds for a range of organisms like fish and crustaceans. For instance, sodium dodecylbenzene sulfonate (SDS) alters the life cycle of the cladocerans Ceridaphnia dubia (da Silva Coelho and Rocha 2010) and Daphnia magna (de Lima e Silva et al. 2022); moreover, SDS elicits deleterious effects in a variety of diverse fish species (Gouda et al. 2022; Shukla and Trivedi 2018). In microalgae, some authors considered that the contribution of LAS to toxicity is not significant (Tamura et al. 2017) and that these surfactants can even be used in mass cultivation of algae at concentrations up to 10 mg/L (Zhang et al. 2021). Nevertheless, it has been demonstrated that LAS altered the normal growth and colony formation in algae (Oda et al. 2022).
Polyoxyethylene amine (POEA) has been used for glyphosate-based herbicides. Mesnage et al. (2019) documented the erroneous use of the term “POEA” in publications concerning glyphosate formulations that have gradually changed the polyethoxylated surfactants for propoxylated quaternary ammonium surfactants, which are less toxic to non-target organisms. The European Commission banned the use of POEA in commercial formulations of glyphosate, and glyphosate-based formulations received an approval for five years, until December 2022 (Kudsk and Mathiassen 2020). Perhaps, as leading-economy countries avoid glyphosate-based products, their use in low-income countries might persist as other chemicals, like diazinon and dichlorvos, phase out.
Defarge et al. (2018) evaluated the toxicity of glyphosate-based herbicides and co-formulants in Solanum lycopersicum (tomato plants) and described that toxicity toward plants was caused by adjuvants rather than the active ingredient singularly. Moreover, chemical analysis of herbicides and co-formulants revealed the presence of metals like chromium, cobalt, lead, and nickel, as well as the metalloid arsenic, which might be involved in the toxic effects of pesticide formulations. Commercial pesticides are complex mixtures that must be assessed as a whole as toxicity is beyond the effects of the recognized active ingredients.
Surfactants play a complex role in the fate and toxicity of pesticides by modifying their deposition and availability on the surfaces to be protected and by facilitating their transport through water run-off. Jorgenson and Young (2010) tested the influence of LAS as emulsifiers for pyrethroids, suggesting that the deposition of mixtures of active ingredients and surfactants on concrete (the main surface type in urban areas) facilitate re-wetting of the active ingredients and contribute to their wash-off. As a consequence, pesticide components migrate from the site of application and infiltrate into the soil or discharge in streams, water bodies, or drain inlets. Therefore, products like DIAZINON®, RoundUp®, URBACIN®, and VAPODEL®, among others used for urban applications, require a re-evaluation of their potential toxicity since only the active ingredient was initially assessed, while the “other ingredients”, many times considered to be non-toxic, elicit negative effects in the alga R. subcapitata and could be toxic to other organisms.
Despite efforts to control the amount of pesticides used in rural and urban areas, issues, like illegal commerce, fraudulent formulations, reuse of plastic containers, high cost of low toxicity formulations, and low cost of generic products, pose a threat to human and environmental health. In this regard, generic alternatives are formulated with higher additive contents and similar active ingredients, but they are not focused on lowering toxicity to non-target organisms (Sarkar et al. 2021). Therefore, toxic effects of generic, low-cost pesticide formulations must be assessed as they are dispensed into the environment as complex mixtures of active ingredients and additives. It is understood that the present study exhibits some limitations as the active ingredients were not quantified and the concentration of ingredients in pesticide formulations could differ from references on labels. However, these formulations represent higher risk as their content might exhibit higher toxicity in relation to additives. This issue becomes a matter of concern, not only in developing countries that are more susceptible to acquiring low-cost, high-toxicity products as well as illegal pesticides that represent approximately 20−30% of the plant protection market (Płonka et al. 2016), but also in the EU, where more than 1000 tons of illegal pesticides have been seized (European Anti-Fraud Office [OLAF] 2021).
As observed in this study, the commercial formulation of pesticides elicited negative effects on microalgae after dilution at several orders of magnitude; for instance, dilution from one to ten parts per million of any of the four commercial pesticides from this research significantly altered: (a) algal growth rates; (b) carbohydrate, lipid, and protein contents; and (c) the content of photosynthetic pigments of R. subcapitata. Some factors, like the misuse of pesticides by the end users who exceed regulation concentrations and residues, the continued use in both indoor and outdoor applications, the facilitated transport due to the physicochemical properties of surfaces in urban environments, and the complexity of commercial formulations, make urban-intended pesticides an important issue for further studies, monitoring programs, and environmental regulations.
Conclusions
The results presented in this study highlight the importance of assessing the toxicity of commercial pesticide formulations in the form that they are introduced into water systems, as a complex mixture of active ingredients and “other ingredients” which modify their toxicity. In some cases, these mixtures have been demonstrated to exert higher toxicity against aquatic biota than the single active ingredients. Moreover, those products intended for urban applications appear to be more toxic than those used in croplands, which might be related to the concentration of adjuvants used in urban formulations.
Our results highlight the susceptibility of R. subcapitata to commercial formulations of pesticides that are intended for use in urban areas. Population growth inhibition and subinhibitory effects, including altered carbohydrate, lipid, protein, and pigment contents, were documented at environmentally concerning concentrations, which were lower than those concentrations reported for the active ingredients for commercial formulations used in croplands. Thus, the safety of urban-intended formulations should be revised since the four commercial formulations used in this research can be classified as toxic or highly toxic to algae.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files. Raw data are available from the corresponding author upon request.
References
Agirman N, Kendirlioğlu Şimşek G, Cetin A (2014) The effects of four pesticides on the growth of Chlorella vulgaris. Fresenius Environ Bull 23:1418–1422
Arzate-Cárdenas MA, Martínez-Jerónimo F (2012) Energy reserve modification in different age groups of Daphnia schoedleri (Anomopoda: Daphniidae) exposed to hexavalent chromium. Environ Toxicol Pharmacol 34:106–116. https://doi.org/10.1016/j.etap.2012.03.003
Baruah P, Chaurasia N (2020) Ecotoxicological effects of alpha-cypermethrin on freshwater alga Chlorella sp.: Growth inhibition and oxidative stress studies. Environ Toxicol Pharmacol 76:103347. https://doi.org/10.1016/j.etap.2020.103347
Beggel S, Werner I, Connon RE, Geist JP (2010) Sublethal toxicity of commercial insecticide formulations and their active ingredients to larval fathead minnow (Pimephales promelas). Sci Total Environ 408:3169–3175. https://doi.org/10.1016/j.scitotenv.2010.04.004
Binukumar BK, Bal A, Kandimalla R, Sunkaria A, Gill KD (2010) Mitochondrial energy metabolism impairment and liver dysfunction following chronic exposure to dichlorvos. Toxicology 270:77–84. https://doi.org/10.1016/j.tox.2010.01.017
Bui-Nguyen TM, Baer CE, Lewis JA, Yang D, Lein PJ, Jackson DA (2015) Dichlorvos exposure results in large scale disruption of energy metabolism in the liver of the zebrafish, Danio rerio. BMC Genomics 16:853. https://doi.org/10.1186/s12864-015-1941-2
Caihong Y, Chunyan L, Ronghua L, et al. (2015) Eco-toxicity and Risk Assessment of Pesticide on Terrestrial Organisms. Asian J Ecotoxicol 21–28. https://doi.org/10.7524/AJE.1673-5897.20150324013
Canada H (2017) Re-evaluation Note REV2017-13, Special Review Decision: Diazinon -Subsection 17(2) of Pest Control Products Act. https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-note/2017/diazinon-rev-2017-13.html. Accessed 3 Mar 2022
Canada H (2021) Re-evaluation Decision RVD2020-08, Dichlorvos and Its Associated End-use Products. https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-decision/2020/dichlorvos.html. Accessed 3 Mar 2022
Castro MJL, Ojeda C, Cirelli AF (2014) Advances in surfactants for agrochemicals. Environ Chem Lett 12:85–95. https://doi.org/10.1007/s10311-013-0432-4
Cedergreen N, Streibig JC, Kudsk P, Mathiassen SK, Duke SO (2006) The occurrence of hormesis in plants and algae. Dose Response 5:150–162. https://doi.org/10.2203/dose-response.06-008
Chen S, Wang L, Feng W, Yuan M, Li J, Xu H, Zheng X, Zhang W (2020) Sulfonamides-induced oxidative stress in freshwater microalga Chlorella vulgaris: evaluation of growth, photosynthesis, antioxidants, ultrastructure, and nucleic acids. Sci Rep 10:8243. https://doi.org/10.1038/s41598-020-65219-2
Cox C, Surgan M (2006) Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ Health Perspect 114:1803–1806. https://doi.org/10.1289/ehp.9374
da Silva Coelho K, Rocha O (2010) Assessment of the potential toxicity of a linear alkylbenzene sulfonate (LAS) to freshwater animal life by means of cladoceran bioassays. Ecotoxicology 19:812–818. https://doi.org/10.1007/s10646-009-0458-3
de Lima e Silva MR, Bernegossi AC, Castro GB, Ogura AP, Corbi JJ, Felipe MC (2022) Assessing Caffeine and Linear Alkylbenzene Sulfonate Effects on Molting and Reproduction of Daphnia magna by Quantitative and Qualitative Approaches. Water Air Soil Pollut 233:98. https://doi.org/10.1007/s11270-022-05554-4
Defarge N, Spiroux de Vendômois J, Séralini GE (2018) Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol Rep 5:156–163. https://doi.org/10.1016/j.toxrep.2017.12.025
Essumang DK, Ttogoh GK, Chokky L (2009) Pesticide residues in the water and fish (lagoon tilapia) samples from Lagoons in Ghana. Bull Chem Soc Ethiop 23:19–27. https://doi.org/10.4314/bcse.v23i1.21294
EU 2009/128/EC (2009). Framework for Community action to achieve the sustainable use of pesticides. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02009L0128-20190726. Accessed 03 Mar 2022
European Anti-Fraud Office [OLAF] (2021 June 17). OLAF teams up with Europol against illegal pesticides [PDF]. https://ec.europa.eu/anti-fraud/system/files/2021-07/pr_17062021_op_silver_axe_en.pdf. Accessed 3 Mar 2022
Fang H, Zhang L (2010) Hormesis effect of organophosphorus pesticide dichlorvos on harmful algal bloom specie Heterosigma akashiwo. Ecol Environ Scie 19:1025–1029
FAOSTAT (2017) Pesticides use database. http://www.fao.org/faostat/en/#data/RP/visualize. Accessed 15 July 2020
Gomes MP, Le Manac’h SG, Hénault-Ethier L, Labrecque M, Lucotte M, Juneau P (2017) Glyphosate-dependent inhibition of photosynthesis in willow. Front Plant Sci 8:1–32. https://doi.org/10.3389/fpls.2017.00207
Gonçalves BB, Giaquinto PC, Silva DdS, de Melo e Silva Neto C, de Lima AA, Darosci AAB, Portinho JL, Carvalho WF, Rocha TL (2019) Ecotoxicology of Glyphosate-Based Herbicides on Aquatic Environment. In: Ince M, InceOK, Ondrasek G (Eds.), Biochemical Toxicology-Heavy Metals and Nanomaterials. IntechOpen. https://doi.org/10.5772/intechopen.85157
Gouda AMR, Hagras AE, Okbah MA, El-Gammal MI (2022) Influence of the Linear Alkylbenzene Sulfonate (LAS) on hematological and biochemical parameters of Nile Tilapia, Oreochromis niloticus. Saudi J Biol Sci 29:1006–1013. https://doi.org/10.1016/j.sjbs.2021.09.074
Hernández-Zamora M, Martínez-Jerónimo F (2019) Exposure to the azo dye Direct blue 15 produces toxic effects on microalgae, cladocerans, and zebrafish embryos. Ecotoxicology 28:890–902. https://doi.org/10.1007/s10646-019-02087-1
Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, Diaz BP, Shabbazz F, Shepard P, Rundle A, Whyatt RM (2011) Characterization of residential pest control products used in inner city communities in New York City. J Expo Sci Environ Epidemiol 21:291–301. https://doi.org/10.1038/jes.2010.18
Jorgenson BC, Young TM (2010) Formulation Effects and the Off-Target Transport of Pyrethroid Insecticides from Urban Hard Surfaces. Environ Sci Technol 44:4951–4957. https://doi.org/10.1021/es100094f
Knowles A (2008) Recent developments of safer formulations of agrochemicals. The Environmentalist 28:35–44. https://doi.org/10.1007/s10669-007-9045-4
Kudsk P, Mathiassen SK (2020) Pesticide Regulation in the European Union and the Glyphosate Controversy. Weed Sci 68:214–222. https://doi.org/10.1017/wsc.2019.59
Kurade MB, Kim JR, Govindwar SP, Jeon BH (2016) Insights into microalgae mediated biodegradation of diazinon by Chlorella vulgaris: Microalgal tolerance to xenobiotic pollutants and metabolism. Algal Res 20:126–134. https://doi.org/10.1016/j.algal.2016.10.003
Li X, Ping X, Xiumei S, Zhenbin W, Liqiang X (2005) Toxicity of cypermethrin on growth, pigments, and superoxide dismutase of Scenedesmus obliquus. Ecotoxicol Environ Saf 60:188–192. https://doi.org/10.1016/j.ecoenv.2004.01.012
Linde N (2005) Consumer Products. In: Wexler P (ed) Encyclopedia of Toxicology, Second Edition. Elsevier, New York, p 661–665
Ma J, Wang P, Huang C, Lu N, Qin W, Wang Y (2005) Toxicity of organophosphorous insecticides to three cyanobacterial and five green algal species. Bull Environ Contam Toxicol 75:490–496. https://doi.org/10.1007/s00128-005-0779-8
Maliszewska J, Tęgowska E (2018) Toxicity of insecticide carrier solvent: effect of xylene on hemolymph biochemical parameters in Blaberus giganteus L. Pol J Environ Stud 27:2385–2390. https://doi.org/10.15244/pjoes/77608
Mansano AS, Moreira RA, Dornfeld HC, Freitas EC, Vieira EM, Sarmento H, Rocha O, Seleghim MHR (2017) Effects of diuron and carbofuran and their mixtures on the microalgae Raphidocelis subcapitata. Ecotoxicol Environ Saf 142:312–321. https://doi.org/10.1016/j.ecoenv.2017.04.024
Martínez-Ruiz EB, Martínez-Jerónimo F (2018) Exposure to the herbicide 2,4-D produces different toxic effects in two different phytoplankters: a green microalga (Ankistrodesmus falcatus) and a toxigenic cyanobacterium (Microcystis aeruginosa). Sci Total Environ 619-620:1566–1578. https://doi.org/10.1016/j.scitotenv.2017.10.145
Martins-Gomes C, Silva TL, Andreani T, Silva AM (2022) Glyphosate vs. glyphosate-based herbicides exposure: a review on their toxicity. J Xenobiotics 12:21–40. https://doi.org/10.3390/jox12010003
Mesnage R, Benbrook C, Antoniou MN (2019) Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem Toxicol 128:137–145. https://doi.org/10.1016/j.fct.2019.03.053
Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang JW (2014) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155:330–333. https://doi.org/10.1016/j.biortech.2013.12.077
Moreno-Villa ED, Aldana-Madrid ML, Silveira-Gramont MI et al. (2012) Análisis de piretroides en suelo y agua de zonas agrícolas y urbanas de los Valles del Yaqui y Mayo. Rev Int Contam Ambient 28:303–310
Nowell LH, Moran PW, Bexfield LM et al. (2021) Is there an urban pesticide signature? Urban streams in five U.S. regions share common dissolved-phase pesticides but differ in predicted aquatic toxicity. Sci Total Environ 793:148453. https://doi.org/10.1016/j.scitotenv.2021.148453
Nowell LH, Moran PW, Schmidt TS et al. (2018) Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern U.S. streams. Sci Total Environ 613–614:1469–1488. https://doi.org/10.1016/j.scitotenv.2017.06.156
Oda Y, Sakamoto M, Miyabara Y (2022) Colony formation in three species of the family Scenedesmaceae (Desmodesmus subspicatus, Scenedesmus Acutus, Tetradesmus Dimorphus) exposed to sodium dodecyl sulfate and its interference with grazing of Daphnia galeata. Arch Environ Contam Toxicol 82:37–47. https://doi.org/10.1007/s00244-021-00890-8
OECD (2011) Test No. 201: Freshwater alga and cyanobacteria, growth inhibition test. https://doi.org/10.1787/9789264069923-en
Okoroiwu HU, Iwara IA (2018) Dichlorvos toxicity: a public health perspective. Interdiscip Toxicol 11:129–137. https://doi.org/10.2478/intox-2018-0009
Panetto OS, Gomes HF, Fraga-Gomes DS, Campos E, Romeiro NC, Costa EP, do Carmo PRL, Feitosa NM, Moraes J (2019) The effects of Roundup® in embryo development and energy metabolism of the zebrafish (Danio rerio). Comp Biochem Physiol Part C Toxicol Pharmacol 222:74–81. https://doi.org/10.1016/j.cbpc.2019.04.007
Pascual-Villalobos MJ, Guirao P, Díaz-Baños FG, Cantó-Tejero M, Villora G (2019) Chapter 9 - Oil in water nanoemulsion formulations of botanical active substances. In: Koul O (Ed), Nano-Biopesticides Today and Future Perspectives. Academic Press, p 223–247 https://doi.org/10.1016/B978-0-12-815829-6.00009-7
Patnaik R, Singh NK, Bagchi SK, Rao PS, Mallick N (2019) Utilization of Scenedesmus obliquus protein as a replacement of the commercially available fish meal under an algal refinery approach. Front Microbiol 10:2114. https://doi.org/10.3389/fmicb.2019.02114
Pedrosa-Gomes M, Juneau P (2016) Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: is the mitochondrial electron transport chain a target of this herbicide? Environ Pollut 218:402–409. https://doi.org/10.1016/j.envpol.2016.07.019
Pereira JL, Antunes SC, Castro BB, Marques CR, Gonçalves AMM, Gonçalves F, Pereira R (2009) Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicology 18:455–463. https://doi.org/10.1007/s10646-009-0300-y
Płonka M, Walorczyk S, Miszczyk M (2016) Chromatographic methods for the determination of active substances and characterization of their impurities in pesticide formulations. TrAC Trends Anal Chem 85:67–80. https://doi.org/10.1016/j.trac.2016.03.011
Sáenz ME, di Marzio WD, Alberdi JL (2012) Effects of a commercial formulation of cypermethrin used in biotech soybean crops on growth and antioxidant enzymes of freshwater algae. J Environ Prot 2:15–22. https://doi.org/10.5963/IJEP0201003
Sarkar S, Gil JDB, Keeley J, Möhring N, Jansen K (2021) The use of pesticides in developing countries and their impact on health and the right to food. QA-03-20-879-EN-N. European Parliament’s Committee on Development https://doi.org/10.2861/28995
Satyavani G, Chandrasehar G, Varma KK, Goparaju A, Ayyappan S, Reddy PN, Murthy PB (2012) Toxicity assessment of expired pesticides to green algae Pseudokirchneriella subcapitata. ISRN Toxicol 2012:247072. https://doi.org/10.5402/2012/247072
Shukla A, Trivedi SP (2018) Anionic surfactant, linear alkyl benzene sulphonate induced oxidative stress and hepatic impairments in fish Channa punctatus. Proc Zool Soc 71:382–389. https://doi.org/10.1007/s12595-017-0223-1
Singh S, Kumar V, Datta S et al. (2020) Glyphosate uptake, translocation, resistance emergence in crops, analytical monitoring, toxicity and degradation: a review. Environ Chem Lett 18:663–702. https://doi.org/10.1007/s10311-020-00969-z
Spahr S, Teixidó M, Sedlak DL, Luthy RG (2020) Hydrophilic trace organic contaminants in urban stormwater: occurrence, toxicological relevance, and the need to enhance green stormwater infrastructure. Environ Sci Water Res Technol 6:15–44. https://doi.org/10.1039/C9EW00674E
Stehle S, Bline A, Bub S et al. (2019) Aquatic pesticide exposure in the U.S. as a result of non-agricultural uses. Environ Int 133:105234. https://doi.org/10.1016/j.envint.2019.105234
Sun KF, Xu XR, Duan SS, Wang YS, Cheng H, Zhang ZW, Zhou GJ, Hong YG (2015) Ecotoxicity of two organophosphate pesticides chlorpyrifos and dichlorvos on non-targeting cyanobacteria Microcystis wesenbergii. Ecotoxicology 24:1498–1507. https://doi.org/10.1007/s10646-015-1458-0
Svartz G, Pérez-Coll C (2013) Comparative toxicity of cypermethrin and a commercial formulation on Rhinella arenarum larval development (Anura: Bufonidae). Int J Environ Health 6:320–329. https://doi.org/10.1504/IJENVH.2013.056973
Tamura I, Yasuda Y, Kagota K, Yoneda S, Nakada N, Kumar V, Kameda Y, Kimura K, Tatarazako N, Yamamoto H (2017) Contribution of pharmaceuticals and personal care products (PPCPs) to whole toxicity of water samples collected in effluent-dominated urban streams. Ecotoxicol Environ Saf 144:338–350. https://doi.org/10.1016/j.ecoenv.2017.06.032
U. S. Environmental Protection Agency (2002) Method 1003.0: green alga, Selenastrum capricornutum, growth test; chronic toxicity 35. EPA-821-R-02-013
U. S. Environmental Protection Agency (2005) Aquatic life ambient water quality criteria: diazinon, final. EPA-822-R-05-006
U. S. Environmental Protection Agency (2015) Technical overview of ecological risk assessment analysis phase: ecological effects characterization. US EPA. URL https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-0. Accessed 14 May 2020
U. S. Environmental Protection Agency (2022) What is a Pesticide? https://www.epa.gov/minimum-risk-pesticides/whatpesticide. Accessed 29 Aug 2022
Wittmer IK, Scheidegger R, Bader HP, Singer H, Stamm C (2011) Loss rates of urban biocides can exceed those of agricultural pesticides. Sci Total Environ 409:920–932. https://doi.org/10.1016/j.scitotenv.2010.11.031
Yeh HJ, Chen CY (2006) Toxicity assessment of pesticides to Pseudokirchneriella subcapitata under air-tight test environment. J. Hazard. Mater 131:6–12. https://doi.org/10.1016/j.jhazmat.2005.09.009
Zhang A, Wen X, Wang K, Huo Y, Geng Y, Ding Y, Li Y (2021) Using surfactants for controlling rotifer contamination in mass cultivation of Chlorella pyrenoidosa. Algal Res 53:102166. https://doi.org/10.1016/j.algal.2020.102166
Acknowledgements
ALCH is grateful for the postdoctoral scholarship received from the Consejo Nacional de Ciencia y Tecnología and thanks the Universidad Autónoma de Aguascalientes for providing support and facilities for conducting the present work. MAAC (CVU 205163) thanks the program Investigadores por México-CONACYT. All authors thank the Sistema Nacional de Investigadores (SNI).
Author contributions
All authors contributed to the study conception and design. ALCH: conceptualization; methodology; formal analysis; investigation; writing original draft. RCVG: investigation; formal analysis. FMJ: supervision, critical review of the final manuscript. MAAC: resources, writing, review and editing; supervision; funding acquisition
Funding
This work was supported by the Universidad Autónoma de Aguascalientes. ALCH received a postdoctoral fellowship from CONACYT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Responsibilities of Authors
The authors certify that this manuscript is our original unpublished work, has not been published elsewhere, and is not under consideration by another journal. Statistical analysis was performed in free-license software. All authors have approved the manuscript and agreed with its submission.
Informed consent
This research did not involve human subjects, so inform consent is not applicable.
Research involving Human Participants and/or Animals
This research did not involve human subjects nor animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carbajal-Hernández, A.L., Arzate-Cárdenas, M.A., Valerio-García, R.C. et al. Commercial pesticides for urban applications induced population growth and sub-cellular alterations in Raphidocelis subcapitata (Chlorophyceae) at concerning environmental concentrations. Ecotoxicology 31, 1462–1476 (2022). https://doi.org/10.1007/s10646-022-02596-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-022-02596-6