Abstract
Aquatic non-targeted organisms are more likely to be exposed to herbicides in multiple pulse events then long continuous exposure. The potential of an organism to recover between exposures has an important role in the overall effects of the toxicant. Common duckweeds show high potential for recovery after a single exposure to isoproturon. To evaluate the growth patterns and recovery potential between multiple exposures, L. minor plants were exposed to isoproturon in three repetitive 7-day treatment cycles in three time-variable exposure scenarios with equivalent time-weighted average concentrations. The growth was significantly inhibited during each exposure phase with significant cumulative effects in every subsequent treatment cycle resulting in a cumulative decrease in biomass production. However, inhibitory effects were reversible upon transferring plants to a herbicide-free nutrient solution. These results indicate that L. minor plants have a high recovery potential even after multiple exposures to isoproturon. Observed cumulative decrease in biomass production, as well as the potential for fast and efficient recovery from repeated herbicide exposure, might affect the competitiveness of L. minor in surface water communities. The observations made during each exposure period, recovery patterns, and the resulting cumulative effects over time may contribute to further development, calibration and validation of mechanistic toxicokinetic/toxicodynamic models for simulating the effects of pesticides on aquatic plants populations in the laboratory and environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic environments are subjected to pesticide contamination due to their worldwide usage and high consumption in modern agriculture and urban landscaping processes. High pesticide concentrations in aquatic ecosystems are mainly correlated with rain events after the application periods and spray drift from agricultural surfaces (Cedergreen and Rasmussen 2017). On the other hand, transformation processes, fast dispersion, phase distribution after the application, degradation by hydrolysis or photolysis or chemical processes such as sorption or evaporation, may result in a rapid reduction of the pesticide concentration in the aquatic environment (Reinert et al. 2002; Rosenkrantz et al. 2013). As a consequence, non-targeted aquatic autotrophic organisms are more likely to be exposed to short pulse events instead of a long term exposure with a constant pesticide concentration, usually represented in the standard laboratory toxicity tests. Pesticide pulses are considered more as a rule rather than the exception in edge-of-field water bodies (Cedergreen et al. 2005; Belgers et al. 2011). Pulse exposure events may last from hours to days with significant differences in effective herbicide concentration depending on the pesticide characteristics as well as characteristics of recipient water body (Leu et al. 2004; Zhao and Newman 2006; Rabiet et al. 2010).
Moreover, aquatic non-targeted organisms are likely to be exposed to multiple pulse events rather than single pulse exposure (Leu et al. 2004; Vallotton et al. 2009). For evaluating the effects of repeated exposures several experimental approaches may be used, including the time-weighted averages (TWA), FOCUS scenarios for predicted environmental concentration (FOCUS 2001), as well as modeling approaches (Schmitt et al. 2013). In the TWA approach, an average concentration is calculated for the exposure period and compared with the results of a standard toxicity test with continuous exposure (Boxall et al. 2013). Results obtained in studies including a single pulse exposure with the TWA concentrations often show that toxicity is of a similar or lesser magnitude for shorter pulses over longer pulses or continuous exposure (Naddy et al. 2000; Belgers et al. 2011; Angel et al. 2015, 2017).
The recovery potential of an exposed organism has an important role in the overall effects of the toxicant in repeated exposures. Recovery potential may be defined as a sum of toxicokinetics and toxicodynamics recovery. Toxicokinetics recovery includes distribution, biotransformation, and excretion of the toxicant from the organism, while toxicodynamics refers to re-establishing the homeostasis (Ashauer et al. 2017). Therefore, recovery depends on the time needed for the affected organism to eliminate the toxicant and to recover from the sustained damage. If the subsequent pulse exposure starts before the organism completed the recovery, the cumulative effects of the pulse exposures are to be expected. On the other hand, acclimatization of the organism to the toxicant can lead to repeating pulse exposures being less toxic in comparison to continuous exposure (Angel et al. 2018).
Among various chemical families of pesticides, phenylurea herbicides, such as isoproturon, have been used worldwide for weed control in several different crops as well as in irrigation channels and drainage ditches (Crépet et al. 2013; Gatidou et al. 2015). Consequently, isoproturon has been frequently detected in soil drainage water and edge-of-the-field aquatic environments (Fairbairn et al. 2018; Casado et al. 2019). Pérès et al. (1996) have reported an average half-life for isoproturon in the water column ranging approximately 50 to 60 days measured for the indoor freshwater microcosm. In natural more diverse aquatic systems, processes such as microbial degradation (Hussain et al. 2011, 2015) and photolytic degradation (López-Muñoz et al. 2013), accumulation in aquatic plants (Böttcher and Schroll 2007) as well as metabolic degradation in cells of dicotyledonous plants like Ceratophyllum demersum (Pietsch et al. 2006) might contribute to faster reduction of isoproturon concentration in water. Once accumulated in the plant cells, isoproturon binds to the D1 protein in the thylakoid membrane and blocks the flow of electrons through photosystem II (PS II), which results in inhibition of photosynthetic efficiency (Laviale et al. 2011). Inhibition of photosynthesis can lead to slow starvation of the plant followed by diminished growth (Varshney et al. 2012) and increased production of reactive oxygen species in affected plants (Bi et al. 2012; Wang et al. 2015). Growth inhibition caused by isoproturon has been reported in studies with photosynthetic non-targeted aquatic organisms (Bi et al. 2012; Tunić et al. 2015; Knežević et al. 2016).
In the previous study, we have observed that a single 2- or 3-days long exposure to isoproturon had a lesser overall effect on the growth of common duckweed compared to continuous treatment with equivalent TWA concentrations. Nevertheless, these short exposures induced significant physiological changes such as the reduced concentration of proteins and photosynthetic pigments, as well as increased damage to the lipids due to significant accumulation of hydrogen peroxide in the tissue (Varga et al. 2019). Moreover, these significant changes were observable even after 4 and 5 days of the recovery period, in which growth endpoints indicated complete recovery. Although plants showed high growth recovery potential after a single exposure, isoproturon induced physiological changes may affect the ability of plants to adequately respond to the next exposure event.
Therefore, the present study aimed to investigate the effects of repeated exposures to isoproturon on growth, as a standard endpoint, and recovery potential of common duckweed, Lemna minor L. Tests were performed as a modified standard growth inhibition test in three repeated treatment cycles with isoproturon in a concentration range below reported 7-day effective concentration (EC50) causing 50% inhibition of frond number growth rate (Knežević et al. (2016): EC50 = 220 µg L−1; Tunić et al. (2015): EC50 = 230 µg L−1). L. minor, a standard photosynthetic non-targeted aquatic organism has been chosen for this study due to favorable traits that enable population-level studies in laboratory conditions and a high potential for recovery from a single exposure to isoproturon (Varga et al. 2019) and other types of herbicides (Wilson and Koch 2013; Burns et al. 2015; Tunić et al. 2015). The specific study objectives were (1) to compare the effects of repeated isoproturon exposure on the growth of L. minor in two time-variable exposure scenarios with the effects observed in a standard growth inhibition test with continuous exposure (to enable direct comparison of effects from different time-variable exposure scenarios TWA approach was used), (2) to assess the overall effects of multiple exposures and expected carry-over effect on plant biomass production and (3) to discuss comparability of standard toxicity endpoints in modified laboratory test simulating repeated exposures with the recovery phase.
Materials and methods
Culture conditions
L. minor L. plants from the laboratory culture maintained at the Department of Biology, University of Osijek, Croatia (RDSC Clone ID 5574) were used as a test organism in this study. Experimental material was cultured in Erlenmeyer flasks on a modified Steinberg nutrient solution (Steinberg 1946). The nutrient solution was composed of the following compounds: 3.46 mmol L−1 KNO3, 1.25 mmol L−1 Ca(NO3) × 4 H2O, 0.66 mmol L−1 KH2PO4, 0.072 mmol L−1 K2HPO4, 0.41 mmol L−1 MgSO4 × 7 H2O, 1.94 µmol L−1 H3BO3, 0.63 µmol L−1 ZnSO4 × 7 H2O, 0.18 µmol L−1 Na2MoO4 × 2 H2O, 0.91 µmol L−1 MnCl2 × 4 H2O, 2.81 µmol L−1 FeCl3 × 7 H2O and 4.03 µmol L−1 EDTA. The pH was adjusted to 5.5 ± 0.2 by adding 0.1 M KOH. The stock cultures were kept in the growth chamber under a continuous light intensity of 70 µmol m−2 s−1 provided by cool white fluorescent lamps and at a temperature of 25 ± 1 ˚C. A selection of five healthy colonies, each having three fronds, was transferred aseptically to a freshly prepared sterile nutrient solution contained in Erlenmeyer flask every seven days. These conditions enabled frond doubling time less than 2.5 days and corresponding average specific growth rate of 0.275 day−1 in the stock culture as required by the standard Lemna sp. Growth inhibition test (OECD 2006).
Experimental design for repeated exposure
All tests were conducted in the growth chamber at the Department of Biology in the above-described conditions. The experiments were run in six-well transparent sterile plates with covers (Jet Bio-Filtration, China). Test results were considered acceptable if the average growth rate of control plants was ≥0.275 day−1 (OECD 2006) and the coefficient of variation (CV) in the control was <15%.
Due to higher solubility of isoproturon in methanol compared to water, isoproturon stock solution was prepared in analytical grade methanol (Carlo Erba) with analytical standard reagent Isoproturon PESTANAL (Sigma, CAS 34123-59-6). The final methanol concentration in each test solution was kept under 100 µL L−1, as recommended by the standard Lemna sp. Growth inhibition test (OECD 2006).
The experiment included three repetitive 7-day treatment cycles with a total duration of the test being 21 days. A 7-day experimental cycle was selected according to the recommended duration of standard Lemna sp. Growth inhibition test (OECD 2006) for continuous exposure. L. minor plants were treated with isoproturon in two pulsed exposure periods (2 and 3 days) followed by a recovery period (5 and 4 days) in a fresh nutrient solution, as well as a standard semi-renewal continuous toxicity test (7 days, without the recovery period). For continuous toxicity test, plants were treated with 50, 100, 150, and 200 µg L−1 of isoproturon in the nutrient solution. To provide live and growing population during the whole test, selected herbicide levels included a range of concentrations lower than the reported 7-day EC50 value for common duckweed reported in the literature (Knežević et al. (2016): EC50 = 220 µg L−1 and Tunić et al. (2015): EC50 = 230 µg L−1). Duckweed plants were exposed to isoproturon at 8 different intended peak concentrations (ranging from 116.65 to 700 µg L−1) for the pulse exposure scenarios (Supplementary Table 1). These intended pulse concentrations corresponded with the 7-day TWA concentrations of 50, 100, 150, and 200 µg L−1.
A schematic overview of the experiments conducted in the laboratory is given in Fig. 1. The experiment was started by transferring 6 healthy duckweed colonies (with 3-4 visible plants each) from the stock culture to sterile test plates with 10 ml of test solution in each well. For the first pulse scenario (pulse-2d), plants were exposed to isoproturon for the first two days and then transferred to a new sterile Steinberg nutrient solution (with an appropriate volume of the solvent) for the remaining five days of the test cycle. In the second pulse scenario (pulse-3d), the pulse exposure time was three days, and the recovery period lasted for the remaining four days of the cycle. The duration of the exposure phase in pulse exposure scenarios was set at 2 and 3 days respectively so that selected isoproturon concentration range (50–200 µg L−1, below the reported EC50 value) would induce significant and observable changes in growth as a standard endpoint. After the pulse exposure period, plants in each test system were carefully rinsed with sterile nutrient solution and transferred to the new test plate for the recovery. In the continuous treatment, L. minor plants were exposed exclusively to isoproturon containing nutrient solution throughout the entire test cycle (7 days). Ten six-well plates were used for each treatment level: four plates were used for herbicide treatment (total of 24 replicates), two plates were used as control (same initial number of plants in clean nutrient solution), and four plates were used as solvent control (same initial number of plants in the nutrient solution to which we added an equal amount of solvent to that used in the herbicide treatment). Test plates were randomly repositioned each day to eliminate the slight temperature and light variations in the growth chamber. The nutrient solution in controls and continuous tests was renewed in the same dynamics as in the pulse exposure tests. At the end of the treatment cycle, plants in each treatment level were subsampled due to limited surface area of the six-well plates and a rapid multiplication rate of untreated plants (doubling time less than 2.5 days). Subsamples, same as at the beginning of the test, consisted of 6 duckweed colonies (with 2-4 fronds, depending on the treatment), which were reinoculated in the fresh sterile exposure solution, and the next treatment cycle was started. Whenever possible, the most recently developed fronds were selected for the subsample.
Schematic overview of the experiments conducted in the laboratory; (a) procedure for exposing L. minor plants to isoproturon in three subsequent 7-day treatment cycles; (b) differences between three experimental scenarios tested (continuous, pulse-2d and pulse-3d). E corresponds to exposure period (days) and R is the recovery period (days) within each treatment cycles
Chemical analysis of isoproturon
Analytical verification of exposure concentrations was performed with the ultra-performance liquid chromatography-tandem mass spectrometer (UPLC-MS/MS). For the analysis, a 10 µL-aliquot of the sample was injected into an Eksigent expert ultraLC 100 system comprised of degasser, binary pump, autosampler with a 100 µL sample loop and a column oven (ABSciex, Framingham, MA, USA). The analytical column was Kinetex 2.6 µm C18 100 Å (100×2.1 mm) with precolumn SecurityGuard Ultra UHPLC C18 for 2.1 mm id columns, both supplied by Phenomenex (Torrance, CA, USA). The mobile phase consisted of (A) methanol: water (10:90, with 5 mM ammonium acetate) and (B) methanol: water (90:10 with 5 mM ammonium acetate), delivered at a constant flow rate of 0.4 ml min−1. The gradient elution started with 10% mobile phase B, which was ramped within 7 min to 90% mobile phase B, held for 2 min, and then reverted to initial conditions to the end of the analysis. The total analysis time was 12 min and the temperature was kept at 40 °C. Mass analysis was performed with the ABSciex QTRAP 4500 (ABSciex, Framingham, MA, USA). For the quantification of the target analyte, the QTRAP system was operated in multiple reaction monitoring (MRM) acquisition modes (MS/MS) with electrospray ionization (ESI). The analysis was performed in positive ionization mode with following operating conditions: ion spray voltage 5.500 V; curtain gas 30 psi; ion source gases GS1 and GS2 were 50 and 55 psi, respectively; probe temperature was 450 °C. Nitrogen served as nebulizer and collision gas. Declustering potential (DP) and collision energy (CE) of the selected transitions were optimized from a continuous flow of a standard injection (100 μg L−1 solution at 10 μL min−1) to obtain the maximum intensities. The entrance potential (EP) for precursor ion was 10 V, and the collision cell exit potential (CXP) for product ion was 4 V. The declustering potential was 66 V. Two MS/MS transitions were acquired for isoproturon, 207.2/72.1 (CE 29 V), using the intensity ratio as a confirmatory parameter. Using the above conditions, the calibration curve was made by analyzing standard solutions of isoproturon in methanol. Methanol (LC-MS grade) was supplied by Merck (Darmstadt, Germany) and ammonium acetate (LC MC grade) by Sigma-Aldrich (St. Louis, MO, USA). The detection limit was 10 µg L−1.
Initial isoproturon concentrations in the test system were analyzed after the preparation of treatment solutions. Samples from the pulse scenarios were also analysed during the recovery period. Chemical analysis was performed in duplicate.
Toxicity endpoints
The average growth rate of L. minor frond number was determined by counting all visible fronds in each separate well of the six-well plates used for controls and isoproturon treatments. Average growth rate per day (RGR) and corresponding doubling time (Td) were calculated according to OECD (2006): \({\mathrm{RGR}}_{{\mathrm{frond}}\;{\mathrm{number}}} = \frac{{{\mathrm{ln}}( {{\mathrm{frond}}\;{\mathrm{number}}_{{\mathrm{t}}_{\mathrm{2}}}} ) - {\mathrm{ln}}( {{\mathrm{frond}}\;{\mathrm{number}}_{{\mathrm{t}}_1}} )}}{{{\mathrm{t}}_2 - {\mathrm{t}}_1}}\), where t1 represents the beginning of the test period (beginning of the cycle), and t2 represents the end of the test period (end of the pulse period and end of the cycle). Corresponding doubling time was calculated using the formula \({\mathrm{T}}_{\mathrm{d}} = \frac{{{\mathrm{ln}}\left( 2 \right)}}{{\mathrm{\mu }}}\).
Daily changes in frond number were expressed as the day-to-day growth rate (µd) and calculated according to the equation: \({\mathrm{\mu }}_{\mathbf{d}} = \frac{{{\mathbf{ln}}\left( {{\mathbf{Frond}}\;{\mathbf{number}}} \right)_{\mathbf{t}} - {\mathbf{ln}}\left( {{\mathbf{Frond}}\;{\mathbf{number}}} \right)_{{\mathbf{t}} - 1}}}{{1{\mathbf{d}}}}\), where t is the time after the initiation of the test expressed in days (Rosenkrantz et al. 2013).
Biomass production was determined at the end of each treatment cycle as the relative growth rate of fresh weight and calculated according to the following formula\({\mathrm{RGR}}_{{\mathrm{fresh}}\;{\mathrm{weight}}} = \frac{{{\mathrm{ln}}( {{\mathrm{fresh}}\;{\mathrm{weight}}_{{\mathrm{d}}_0}} ) -\, {\mathrm{ln}}( {{\mathrm{fresh}}\;{\mathrm{weight}}_{{\mathrm{d}}_7}} )}}{7}\), where d0 represents the beginning of the treatment and d7 represents the end of the treatment cycle. Initial fresh biomass was determined by weighting six samples from the stock culture, and each sample consisted of 6 healthy duckweed colonies with 3-4 fronds. Initial fresh biomass for subsequent cycles was determined by weighting six samples (6 colonies with 2-4 fronds, depending on the treatment) from each test system before subsample was reinoculated. Before weighting, plants were gently blotted between several layers of tissue paper to remove the nutrient solution from the surface of the plants.
The inhibition of growth rate was calculated as \({\mathrm{\% I}}_{{\mathrm{RGR}}} = \frac{{{\mathrm{RGR}}_{\mathrm{c}} - {\mathrm{RGR}}_{\mathrm{t}}}}{{{\mathrm{RGR}}_{\mathrm{c}}}} \times 100\), where RGRc and RGRt represent the measured endpoint (frond number growth rate and fresh weight growth rate) in control and treatment groups, respectively.
Biomass production over the whole duration of the experiment was calculated to evaluate the cumulative effects of sequential exposure cycles. Since the experiment procedure required subsampling of the plants at the end of each treatment cycle, fresh weight yield values from each cycle were summed for each replicate in all treatments and control. The yield was calculated for endpoints fresh weight and frond number by subtracting the measured value at the beginning of the treatment cycle from the value at the end of the treatment cycle.
Data analysis
TWA concentrations were calculated according to the following formula \({\mathrm{P}}_{\mathrm{c}} = \frac{{{\mathrm{TAC}} \times {\mathrm{C}}_{{\mathrm{exp}}}}}{{{\mathrm{t}}_{{\mathrm{pulse}}}}}\) where Pc= intended pulse concentration (µg L−1), TAC = intended time-weighted average concentration (µg L−1), Cexp = cycle duration (7 days) and tpulse = duration of pulse exposure (2 or 3 days).
Coefficient of variation (CV) was calculated for all measured endpoints according to the following formula: \({\mathrm{CV}} = \frac{{{\mathrm{SD}}}}{{{\mathrm{avg}}.}} \times 100\), where SD is the sample standard deviation and avg. is the mean value for a sample.
The concentration-response curves were modeled at the end of each treatment cycle with inhibition of averaged growth rate of frond number and inhibition of average growth rate of fresh weight to calculate isoproturon effective concentration causing 50% inhibition of growth (EC50). Using average specific growth rate in estimating toxicity is based on the assumption that the growth of the plants is constant during the whole test and independent of test duration, the specific growth rate of the control population, and the slope of the concentration-response curve. On the other hand, ECx results based on the yield response are dependent upon all variables mentioned above (OECD 2006). Since the growth rate in the pulse treatment scenarios was not constant during the treatment cycle, it was considered acceptable to use the yield response method to calculate the ECx values to compare the observed effects of pulse experiments with continuous exposure. Due to different mathematical approaches, EC values based on the yield are expected to be lower in comparison to the values based on the average specific growth rate (OECD 2006). EC concentrations were modeled using nonlinear regression and expressed in terms of nominal TWA concentrations.
Results are presented as average values ± standard deviation of twenty-four replicates (n = 24) for frond number endpoints, 12 replicates (n = 12) for fresh weight endpoints, and two replicates (n = 2) for analytical verification of exposure concentrations in the test system. Every replicate represents a sample from a separate well of the used six-well plates.
To compare treatments, assuming normality and equal variance, the factorial analysis of variance followed by the Tukey HSD post-hoc test was performed (P < 0.05) for each exposure scenario separately. Factors tested were isoproturon concentration, treatment cycles and interaction between these two factors. The analysis of variance was also used for the recovery period, where the term recovery was defined as a lack of statistically significant reduction in comparison to the control for the endpoint day-to-day growth rate of frond number at a specific time point (P < 0.05). Cumulative effects of different exposure scenarios were evaluated at the end of the experiment with the factorial analysis of variance followed by the Tukey HSD post-hoc test (P < 0.05) and factors tested were isoproturon concentration and exposure scenario. All statistical analyses were performed in Statistica 13.5 software (2018; TIBCO Software Inc. USA).
Results
Chemical analysis
Mean measured exposure peak concentrations at the beginning of the test for the different exposure scenarios are summarized in supplementary Table 1. A comparison of mean measured concentrations in test solution with nominal concentrations showed that actual exposure concentrations ranged from 89.5% to 98.1% of intended isoproturon concentrations. Hence, the results are reported as nominal concentrations for the continuous test scenario, and the pulse scenarios in terms of their 7-day TWA concentrations. After transferring the plants in a clean nutrient solution for the recovery period, herbicide concentration remained below the detection limit.
Treatment-related effects on frond number endpoint
Control growth rates of frond number ranged from 0.286 ± 0.03 day−1 to 0.289 ± 0.01 day−1 (with a corresponding average doubling time of 2.4 ± 0.01 days), which is above the OECD (2006) validity criteria indicating that L. minor performance in the test system was acceptable. During the experiment, there were no significant differences in the frond number growth rates between the plants grown in clean nutrient solution and different solvent controls spiked with methanol (F = 1.4, P = 0.24). Therefore, the growth of control plants is reported as an average value of all tested solvent controls.
Relative growth rates for frond number endpoint at the end of treatment cycles with isoproturon in three exposure scenarios are summarized in supplementary Table 2. The inhibition of average growth rates of L. minor frond number at the end of three subsequent treatment cycles with isoproturon is given in Fig. 2. In the continuous test growth of L. minor plants was significantly influenced by the applied herbicide concentration and the cycle of the treatment (Fig. 2a). At the end of each cycle, a concentration-depended inhibition of average growth rate was observed (cycle 1: r2 = 0.800; cycle 2: r2 = 0.975; cycle 3: r2 = 0.964; P < 0.01). Also, every subsequent treatment cycle resulted in significantly higher inhibition of growth. At the end of the first treatment cycle inhibition of growth ranged from 15 to 43%, at the end of the second cycle from 21 to 58%, and at the end of the third cycle from 28 to 63% in treatments with isoproturon in concentrations from 50 to 200 µg L−1. Therefore calculated EC50 values for endpoint average growth rate of frond number in continuous treatment scenario were >200 µg L−1 at the end of the first cycle, 168.6 ± 6.0 µg L−1 at the end of the second cycle and, 127.9 ± 2.8 µg L−1 at the end of the last treatment cycle.
Inhibition (%) of L. minor frond number relative growth rate (RGRfrond number) at the end of three subsequent treatment cycles with 50, 100, 150 and 200 µg L−1 of isoproturon in exposure scenario (a) continuous, (b) pulse-2d and (c) pulse-3d. Columns represent an average value and error bars indicate standard deviation (n = 24). Different uppercase letters indicate significantly different values across four treatment concentrations and three treatment cycles separately for each exposure scenarios, while lowercase letters indicate significantly different values across three exposure scenarios for each isoproturon concentration within each treatment cycle separately (HSD, P ≤ 0.05)
When plants were treated in pulse-2d scenario, at the end of the pulse exposure period isoproturon inhibited the growth of the duckweed cultures in a concentration-depended manner (Table 1: cycle 1: r2 = 0.972; cycle 2: r2 = 0.942; cycle 3: r2 = 0.977; P < 0.01). Generally, the average growth rate of the cultures exposed to 50, 100, 150 and 200 µg L−1 of isoproturon in pulse-2d treatment scenario was 0.190–0.198, 0.146–0.152, 0.095–0.108, and 0.044–0.055 day−1, respectively. Compared to the growth of the control plants, the growth of treated plants was inhibited from 31% at 50 µg L−1 up to 84% at the highest isoproturon concentration. According to the statistical analysis, the interaction of isoproturon concentration and treatment cycle in the pulse-2d scenario was not significant (P > 0.05), meaning that inhibition of the average growth rate at the end of the first cycle’s exposure period was not significantly different from the inhibition at the end of subsequent pulses in the cycles 2 and 3.
At the end of the treatment cycles, after the recovery period in treatment scenario pulse-2d, the growth of treated plants was significantly inhibited in comparison to the controls (Fig. 2b). The inhibition of growth at the end of the cycle showed a significant correlation to the applied isoproturon concentrations (correlation coefficients were 0.985, 0.942, and 0.978 for the three subsequent cycles, respectively; P < 0.05). According to the statistical analysis, the interaction of isoproturon concentration and treatment cycle had a significant effect (F = 27.22, P > 0.01) on the average growth rate of plants at the end of the recovery period. In treatment with 50 and 100 µg L−1, growth rates were lower at the end of the second cycle, while the two higher isoproturon concentrations caused a significant decrease in the average growth rate in each subsequent treatment cycle (Fig. 2b).
When plants were treated in pulse-3d treatment scenario, a concentration-depended inhibition of growth was evident at the end of the pulse exposure periods (Table 2; cycle 1: r2 = 0.923; cycle 2: r2 = 0.950; cycle 3: r2 = 0.962; P < 0.01). Compared to the growth of the control plants, the growth of treated plants was inhibited from 24% at 50 µg L−1 up to 73% in treatment with 200 µg L−1 of isoproturon. Interaction of isoproturon concentration and treatment cycle had a significant effect on the average growth rate of L. minor at the end of the exposure period (F = 14.01, P > 0.01). Isoproturon in concentrations 100, 150, and 200 µg L−1 caused significantly higher inhibition of growth with every subsequent exposure period of the treatment cycle (Table 2).
Growth of isoproturon treated plants at the end of treatment cycles in pulse-3d treatment scenario, after the recovery period, was also significantly inhibited in comparison to controls (Fig. 2c). Concentration-dependent inhibition of growth was evident in every treatments cycles (cycle 1: r2 = 0.910; cycle 2: r2 = 0.969; cycle 1: r2 = 0.960; P < 0.05). The average growth rate of plants at the end of the treatment cycles was significantly influenced by the interaction of isoproturon concentration and treatment cycle (F = 55.58, P > 0.01). Isoproturon concentration above 50 µg L−1 caused significantly higher inhibition of growth with every subsequent treatment cycle (Fig. 2c).
Generally, inhibition of frond number growth rate in the continuous exposure scenario was higher in comparison to the inhibition caused by the same isoproturon concentration in pulse exposure scenarios, especially at the end of the second and third treatment cycle (Fig. 2).
The EC50 values for relative growth rate of frond number in pulse exposure scenarios could not be estimated since they were significantly above 200 µg L−1, that is the highest applied isoproturon concertation. Therefore, in order to compare the impact of isoproturon between treatment scenarios EC50, EC25 and EC10 values for frond number yield were estimated for each treatment cycle and summarized in Table 3. Generally, the impact of isoproturon exposure scenario on the frond number yield could be described as continuous > pulse-3d > pulse-2d for all three treatment cycles. In continuous treatment EC50 values were 150 ± 8 µg L−1 at the end of the first cycle, 83 ± 4 µg L−1 at the end of the second cycle and, 57 ± 4 µg L−1 at the end of the last treatment cycle. A comparison of ECx values from pulse exposure scenarios with values for continuous treatment show a factor 1.2–3.4 higher EC values for pulse scenarios (Table 3).
Treatment-related effects on biomass production endpoint
Relative growth rates for fresh biomass production endpoint at the end of treatment cycles with isoproturon in three exposure scenarios are summarized in supplementary table 3. Similarly to the observed inhibition of the average growth rate of frond number, isoproturon exposure also inhibited the average growth rate of fresh weight (Fig. 3). Isoproturon had a significant effect on the average growth rate of fresh weight for plants treated in the continuous exposure scenario (Fig. 3a) with significant interaction of applied herbicide concentration and the cycle of the treatment (F = 66.8, P < 0.01). Isoproturon treatment resulted in significantly lower fresh biomass accumulation with every subsequent treatment cycle. Therefore calculated EC50 concentrations were 209.6 ± 11.7 µg L−1 at the end of the first cycle, 172.9 ± 3.9 µg L−1 at the end of the second cycle and, 142.8 ± 4.0 µg L−1 at the end of the last treatment cycle.
Inhibition (%) of L. minor fresh weight relative growth rate (RGRfresh weight) at the end of three subsequent treatment cycles with 50, 100, 150, and 200 µg L−1 of isoproturon in exposure scenario (a) continuous, (b) pulse-2d, and (c) pulse-3d. Columns represent an average value and error bars indicate standard deviation (n = 12). Different uppercase letters indicate significantly different values across four treatment concentrations and three treatment cycles separately for each exposure scenarios, while lowercase letters indicate significantly different values across three exposure scenarios for each isoproturon concentration within each treatment cycle separately (HSD, P ≤ 0.05)
When plants were treated with isoproturon in the pulse-2d scenario, no significant effects on the biomass production were observed at the end of the first treatment cycle (Fig. 3b). Values recorded for plants treated with 50, 100, and 150 µg L−1 were even higher than control values, but this increase was not statistically significant. Significant inhibition of fresh biomass production was observed at the end of the second cycle in treatments with 100, 150, and 200 µg L−1 where biomass production was inhibited 9%, 14% and, 21% respectively. On the other hand, at the end of the third cycle, all applied isoproturon concentrations caused significant inhibition of fresh weight accumulation ranging from 9% in treatment with 50 µg L−1 to 29% in the treatment with 200 µg L−1.
In the pulse-3d exposure scenario, all applied isoproturon concentrations caused significant inhibition of biomass production (Fig. 3c). Fresh biomass production was progressively reduced at the end of each subsequent treatment cycle with 50 and 100 µg L−1 of isoproturon. Treatments with 150 and 200 µg L−1 caused similar inhibition of fresh biomass production at the end of the second (27% and 44% respectively) and third cycles (29% and 47% respectively).
Comparing the effects of the same isoproturon concentration between different exposure scenarios revealed that continuous treatment caused the most pronounced inhibition of biomass production during the whole test. Generally, the effect of the exposure scenario on the biomass production at the end of all three treatment cycles could be described as continuous > pulse-3d > pulse-2d for all tested isoproturon concentrations (Fig. 3).
The values of EC50 for relative growth rate of fresh weight in pulse exposure scenarios could not be estimated (>200 µg L−1). Isoproturon effects between treatment scenarios were compared with EC50, EC25 and EC10 values for fresh weight yield (Table 3). Same as for the frond number yield, the impact of isoproturon exposure scenario on the fresh weight yield could be described as continuous > pulse-3d > pulse-2d for all three treatment cycles. In continuous treatment EC50 values were 126 ± 2 µg L−1 at the end of the first cycle, 106 ± 3 µg L−1 at the end of the second cycle and, 75 ± 6 µg L−1 at the end of the last treatment cycle. Values from pulse exposure scenarios were a factor 1.1–2.9 higher (Table 3).
Cumulative treatment-related effects on biomass production
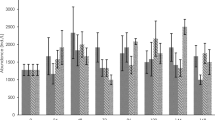
Inhibition of fresh biomass production during sequential pulses resulted in a concentration-dependent cumulative decrease in biomass yield at the end of the experiment (Fig. 4). According to the results of ANOVA, the impact of the same isoproturon concentration across the tested exposure scenarios could be described as continuous > pulse-3d > pulse-2d.
Cumulative biomass production at the end of the experiment (sum of three sequential treatment cycles) for control and cultures exposed to 50, 100, 150, and 200 µg L−1 of isoproturon in continuous, pulse-2d and pulse-3d exposure scenario. Columns represent an average value and error bars indicate standard deviation (n=12). Biomass production for the control is reported as an average value of all tested solvent controls. Different letters indicate significantly different values across five treatment levels (control, 50, 100, 150 and 200 µg L−1) and three exposure scenarios (HSD, P < 0.05)
Calculated ECx values for cumulative biomass production at the end of the whole experiment with continuous exposure were EC50 = 103 µg L−1, EC25 = 55 µg L−1, and EC10 = 37 µg L−1. In the pulse-2d exposure scenario, treatment with the highest isoproturon concentration resulted in a 31% cumulative decrease of fresh weight yield in comparison to the control. Therefore, the EC25 value was 164 µg L−1, and EC10 was 72 µg L−1. These EC25 and EC10 were three- and two-fold higher in the pulse-2d exposure scenario when compared to the respective EC values from the continuous treatment. The inhibition of fresh biomass production of plants in pulse-3d exposure scenario ranged from 21% in treatment with 50 µg L−1 to 59% in treatment with 200 µg L−1. Corresponding ECx values for cumulative biomass production were EC50 = 166 µg L−1, EC25=71 µg L−1, and EC10 = 43 µg L−1.
Recovery patterns of L. minor after isoproturon exposure in pulse treatment scenarios
The recovery patterns are visualized in Fig. 5, which shows the day-to-day growth rates of the frond number. Control day-to-day frond number growth rates ranged from 0.286 ± 0.033 day−1 to 0.291 ± 0.030 day−1. Isoproturon caused a concentration-depended and time-depended reduction of day-to-day growth rates during the exposure periods of all three treatment scenarios.
Day-to-day growth rate (µd,) expressed as a percent of control during the three subsequent treatment cycles with 50, 100, 150 and 200 µg L−1 of isoproturon in exposure scenario (a) continuous, (b) pulse-2d and (c) pulse-3d. Values are average from 24 replicates (n = 24). Gray columns represent the exposure period of the treatment cycle (continuous 7 days, first 2 days for pulse-2d, and first 3 days for pulse-3d). The coefficient of variation range in control was 5.9–11.7%, in continuous test 1.0–14.1%, in pulse-2d treatment scenario 1.0–13.5%, and 1.0–14.9% in pulse-3d treatment scenario
Plants treated with the lowest isoproturon concentration in the pulse-2d scenario (Fig. 5b), immediately after the end of exposure period in all treatment cycles, showed complete recovery of day-to-day growth rates. When treated with 100 µg L−1 of isoproturon, in each subsequent treatment cycle, complete recovery of growth was observed on the fifth day. The day-to-day growth rate of plants treated with 150 and 200 µg L−1 of isoproturon completely recovered on the fifth day of the first treatment cycle. On the other hand, second and third subsequent pulse exposure resulted in growth that was significantly lower at the end of the recovery period in comparison to the control.
Growth inhibition resulting from pulse-3d exposure to 50 µg L−1 of isoproturon was completely reversible in all three subsequent treatment cycles (Fig. 5c). The day-to-day growth rate reached the control levels on the fifth day of the treatment cycles. When plants were treated with 100 and 150 µg L−1 of isoproturon, growth recovery was observed on the fifth and sixth day of the first treatment cycle, respectively. However, when plants were treated with 100 and 150 µg L−1 in the subsequent second and third treatment cycles, day-to-day growth rates never reached the growth rate of the untreated controls. Moreover, treatment with 200 µg L−1 of isoproturon caused significant inhibition of growth from which plants did not recover even after the first treatment cycle.
Discussion
L. minor growth was inhibited in a concentration-depended manner in all three exposure scenarios with repeated exposures to isoproturon (Figs. 2, 3). Estimated EC50 values in continuous exposure scenario based on standard endpoint average growth rate of frond number are comparable to values reported by Teodorović et al. (2012), Knežević et al. (2016) and Tunić et al. (2015). Reduction of biomass, frond number, and growth rates in comparison to the controls indicated that inhibition of photosynthesis occurred at all tested exposure concentrations, but not to the extent that would result in plant death. Giving the mode of action, inhibition of photosynthesis is expected in treatment with isoproturon. However, treated cultures remained viable and growing only at a slower rate in comparison to the control even after prolonged chronic exposure in continuous treatment scenarios (21 days). Similar observations were reported for Lemna gibba and Scenedesmus vacuolatus exposed to atrazine, another PS II inhibiting herbicide (Vallotton et al. 2008; Brain et al. 2012).
In both pulse exposure scenarios, we found that duckweed growth was significantly inhibited during each exposure phase (Tables 1, 2) but that effects were reversible upon transferring plants to the herbicide-free nutrient solution (Fig. 5). L. minor accumulates isoproturon (Böttcher and Schroll 2007; Dosnon-Olette et al. 2011) but duckweed plants are considered not capable to metabolise the aromatic ring of the isoproturon molecule (Böttcher and Schroll 2007). Recovery of growth indicates that the effects of isoproturon on photosynthesis are not permanent possible due to non-covalent binding of phenylurea herbicides to D1 protein and fast elimination from the affected cells (Burns et al. 2015) and the effective repair mechanisms of oxidative damage to PS II (Cedergreen et al. 2005). Restoration of photosystem activity enables recovery of L minor biomass accumulation and production of new daughter fronds. Duckweeds show a high potential of recovery upon single herbicide exposure even at exposure concentration that resulted in a complete arrest of the growth (Mohammad et al. 2010; Teodorović et al. 2012; Rosenkrantz et al. 2013; Burns et al. 2015; Knežević et al. 2016).
In the previous study, we have demonstrated that although L. minor shows high potential for growth recovery after single pulse exposure to isoproturon, significant physiological changes were detected in plants even at the end of the recovery phase such as significantly reduced concentration of proteins and photosynthetic pigments, as well as significantly induced levels of biomarkers for oxidative stress and activities of antioxidative enzymes (Varga et al. 2019). These findings suggest that although growth has recovered, the overall physiological recovery process has not been completed even five days after single exposure to isoproturon and increased toxic effects are expected upon subsequent exposure events. The results of this study show an increase in toxicity with every subsequent treatment cycle for different exposure scenarios, indicating a carry-over effect from the previous cycle regardless of the recovery trend in the pulse treatment scenarios (Table 3). Macinnis-Ng and Ralph (2004) showed that multiple pulses of herbicide and metals, despite the four day recovery period between exposures, had a greater impact on Zostera capricorni than a single toxicant pulse. Moreover, increased toxicity in subsequent pulses was reported when the organism is exposed to different toxicants with a similar mode of action (Ashauer et al. 2007) or even toxicants that act on different targets (Ashauer et al. 2017).
Increased isoproturon toxicity and prolonged recovery time of L. minor plants in every subsequent treatment cycle resulted in a cumulative decrease in biomass production at the end of the experiment (Fig. 4). A similar observation was reported for S. vacuolatus treated with isoproturon (Vallotton et al. 2009). Gustavson et al. (2003) reported that short term pulse exposure to herbicides results in loss of algae biomass as well as notable changes in species composition. The loss of biomass production might have a significant effect on the overall competitiveness of L. minor in surface water communities impacted by the repetitive herbicide pollution. For macrophytes, a loss of biomass production or growth could cause changes in the ecosystem composition as reduction or loss of sensitive species could give room for more resistant and invasive species (Rosenkrantz et al. 2013). On the other hand, the rapid recovery of growth rate or even a recovery trend observed between exposure phases in each treatment cycle may be an advantage for L. minor in comparison to slower-growing macrophyte species. Tunić et al. (2015) reported that L. minor was more sensitive towards isoproturon when compared to the sensitivity of submerged macrophytes Myriophyllum aquaticum and Myriophyllum spicatum. They have reported a 7-day EC50 value based on fresh weight relative growth rate for L. minor to be 200 µg L−1 and value above 1000 µg L−1 for both Myriophyllum species. Also, L. minor was more sensitive in comparison to M. aquaticum in treatment with another PSII inhibiting herbicide atrazine in the concentration range from 40 to 1280 µg L−1 and exposure duration of 3 and 7 days (Teodorović et al. 2012). However, significantly different growth patterns for the two macrophyte species were reported in the recovery period following exposure. While L. minor showed fast and efficient recovery even after prolonged exposure (7 days), recovery of M. aquaticum was rather slow and less efficient even after short exposure (3 days). Therefore, if the total duration of the experiment is considered (exposure + recovery) slow-growing submerged species are more sensitive in contrast to fast-growing free-floating macrophyte (Teodorović et al. 2012). Fast and efficient recovery of L. minor from herbicide exposure may contribute to the development of dense mat of free-floating macrophytes and result in light depletion as well as anoxic conditions under the math, thus affecting submerged autotrophic organisms in the ecosystem and change the ecosystem composition (De Tezanos Pinto et al. 2007; Netten et al. 2011; Lu et al. 2013). However, the question remains whether such rapid recovery would be possible in a native more diverse systems with other factors affecting L. minor such as animal feeding, shading, fluctuation in nutrient supply and presence of other toxicants.
According to standard toxicity protocols (OECD 2006), toxic effects of the tested compound should be evaluated only at the end of the test, or in the case of this study end of the treatment cycles. However, if standard laboratory tests are modified to include a recovery period, cumulative inhibition of growth might overestimate the effects of the toxicant. According to the inhibition of growth calculated for standard endpoints frond number and fresh weight growth rate (Figs. 2, 3) at the end of each treatment cycle in pulse exposure scenarios, it could be concluded that isoproturon was toxic to L. minor even after five or four days long recovery period in all three treatment cycles. On the other hand, changes reported for the day-to-day growth rate indicated that plants either show complete recovery or a recovery trend in each treatment cycle since the day-to-day growth rate values were increasing towards the end of the recovery period (Fig. 5) so based on this endpoint it could be concluded that plants have overcome negative effects of isoproturon.
Moreover, if standard laboratory growth inhibition tests are modified to include a recovery period, such as pulse exposure scenarios presented in this study, growth in pulse exposure scenarios cannot be considered constant due to inhibition during the exposure phase and recovery after transferring the plants to clean nutrient solution. Therefore, EC values calculated with the yield method were used to compare the toxicity of isoproturon between three time-variable exposure scenarios in this study. As expected, based on calculated EC values at the end of treatment cycles isoproturon in pulse exposure scenario had an effect on L. minor growth of a similar or lesser magnitude when compared to continuous treatment with equivalent TWA concentration (Table 3). However, evaluating toxicity with yield as an endpoint made a comparison of our results with results from other studies somewhat difficult, as yield is rarely reported in the literature.
Comparison of the isoproturon effects between three exposure scenarios reported in this study was enabled with the TWA approach since the dose per treatment cycle was equal in all three scenarios (7-day TWA concentration). However, when the results from this study were compared to the literature a number of issues occurred since effects across studies were reported for different endpoints and different time intervals as well as different life forms and growth patterns. Since there is no standardised protocol for assessing the effects of time-variable exposures and recovery potential more studies investigating the effects of repeated exposures to toxicants with a focus on the frequency of the pulses as well as the interval between pulses are necessary to suggest the most appropriate experimental design and acceptable endpoints. Also, studies investigating the recovery of aquatic plants after repeated time-variable exposures to toxicants may contribute to further development, calibration and validation of mechanistic toxicokinetic/toxicodynamic models simulating the effects of pesticides on aquatic plants populations in the laboratory and environmental conditions, like the model described by Schmitt et al. (2013).
Conclusion
L. minor growth was significantly inhibited during each exposure phase with significant cumulative effects in every subsequent treatment cycle resulting in a cumulative decrease in biomass production. Nevertheless, the inhibitory effects were reversible upon transferring plants to herbicide-free nutrient solution indicating that L. minor plants have a high recovery potential even after multiple exposures to isoproturon. Observed cumulative decrease in biomass production, as well as the potential for fast and efficient recovery from repeated herbicide exposure, might have an effect on the competitiveness of L. minor in surface water communities. The observations made during each exposure period, recovery patterns, and the resulting cumulative effects over time may contribute to further development, calibration and validation of mechanistic toxicokinetic/toxicodynamic models for simulating the effects of pesticides on aquatic plants populations in the laboratory and environmental conditions. To suggest the most appropriate experimental design and acceptable endpoints for assessing the effects of herbicides on aquatic primary producers in time-variable exposure regimes, the results from this study should be complemented with data from exposure studies with different intervals between exposures and changes in plant tissue herbicide concentration, especially during the recovery phase.
References
Angel BM, Goodwyn K, Jolley DF, Simpson SL (2018) The use of time-averaged concentrations of metals to predict the toxicity of pulsed complex effluent exposures to a freshwater alga. Environ Pollut 238:607–616. https://doi.org/10.1016/j.envpol.2018.03.095
Angel BM, Simpson SL, Chariton AA et al. (2015) Time-averaged copper concentrations from continuous exposures predicts pulsed exposure toxicity to the marine diatom, Phaeodactylum tricornutum: Importance of uptake and elimination. Aquat Toxicol 164:1–9. https://doi.org/10.1016/j.aquatox.2015.04.008
Angel BM, Simpson SL, Granger E et al. (2017) Time-averaged concentrations are effective for predicting chronic toxicity of varying copper pulse exposures for two freshwater green algae species. Environ Pollut 230:787–797. https://doi.org/10.1016/j.envpol.2017.07.013
Ashauer R, Boxall ABA, Brown CD (2007) Modeling combined effects of pulsed exposure to carbaryl and chlorpyrifos on Gammarus pulex. Environ Sci Technol 41:5535–5541. https://doi.org/10.1021/es070283w
Ashauer R, O’Connor I, Escher BI (2017) Toxic mixtures in time - the sequence makes the poison. Environ Sci Technol 51:3084–3092. https://doi.org/10.1021/acs.est.6b06163
Belgers JDM, Aalderink GH, Arts GHP, Brock TCM (2011) Can time-weighted average concentrations be used to assess the risks of metsulfuron-methyl to Myriophyllum spicatum under different time-variable exposure regimes? Chemosphere 85:1017–1025. https://doi.org/10.1016/j.chemosphere.2011.07.025
Bi YF, Miao SS, Lu YC et al. (2012) Phytotoxicity, bioaccumulation and degradation of isoproturon in green algae. J Hazard Mater 243:242–249. https://doi.org/10.1016/j.jhazmat.2012.10.021
Böttcher T, Schroll R (2007) The fate of isoproturon in a freshwater microcosm with Lemna minor as a model organism. Chemosphere 66:684–689. https://doi.org/10.1016/j.chemosphere.2006.07.087
Boxall ABA, Fogg LA, Ashauer R et al. (2013) Effects of repeated pulsed herbicide exposures on the growth of aquatic macrophytes. Environ Toxicol Chem 32:193–200. https://doi.org/10.1002/etc.2040
Brain RA, Hosmer AJ, Desjardins D et al. (2012) Recovery of duckweed from time-varying exposure to atrazine. Environ Toxicol Chem 31:1121–1128. https://doi.org/10.1002/etc.1806
Burns M, Hanson ML, Prosser RS et al. (2015) Growth recovery of Lemna gibba and Lemna minor following a 7-day exposure to the herbicide diuron. Bull Environ Contam Toxicol 95:150–156. https://doi.org/10.1007/s00128-015-1575-8
Casado J, Brigden K, Santillo D, Johnston P (2019) Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high resolution mass spectrometry. Sci Total Environ 670:1204–1225. https://doi.org/10.1016/J.SCITOTENV.2019.03.207
Cedergreen N, Andersen L, Olesen CF et al. (2005) Does the effect of herbicide pulse exposure on aquatic plants depend on Kow or mode of action? Aquat Toxicol 71:261–271. https://doi.org/10.1016/J.AQUATOX.2004.11.010
Cedergreen N, Rasmussen JJ (2017) Low dose effects of pesticides in the aquatic environment. In: Duke SO, Kudsk P and Solomon K (Eds), Pesticide dose: effects on the environment and target and non-target organisms. American Chemical Society, Washington, DC, pp 167–187
Crépet A, Tressou J, Graillot V et al. (2013) Identification of the main pesticide residue mixtures to which the French population is exposed. Environ Res 126:125–133. https://doi.org/10.1016/J.ENVRES.2013.03.008
De Tezanos Pinto P, Allende L, O’Farrell I (2007) Influence of free-floating plants on the structure of a natural phytoplankton assemblage: An experimental approach. J Plankton Res 29:47–56. https://doi.org/10.1093/plankt/fbl056
Dosnon-Olette R, Couderchet M, Oturan MA et al. (2011) Potential use of Lemna minor for the phytoremediation of isoproturon and glyphosate. Int J Phytoremediation 13:601–612. https://doi.org/10.1080/15226514.2010.525549
Fairbairn DJ, Elliott SM, Kiesling RL et al. (2018) Contaminants of emerging concern in urban stormwater: Spatiotemporal patterns and removal by iron-enhanced sand filters (IESFs). Water Res 145:332–345. https://doi.org/10.1016/J.WATRES.2018.08.020
FOCUS (2001) FOCUS surface water scenarios in the EU evaluation process under 91/414/ EEC. Report of the FOCUS working group on surface water scenarios. EC Doc Ref SANCO/4802/2001‐rev2
Gatidou G, Stasinakis AS, Iatrou EI (2015) Assessing single and joint toxicity of three phenylurea herbicides using Lemna minor and Vibrio fischeri bioassays. Chemosphere 119:69–74. https://doi.org/10.1016/j.chemosphere.2014.04.030
Gustavson K, Møhlenberg F, Schlüter L (2003) Effects of exposure duration of herbicides on natural stream periphyton communities and recovery. Arch Environ Contam Toxicol 45:48–58. https://doi.org/10.1007/s00244-002-0079-9
Hussain S, Arshad M, Springael D et al. (2015) Abiotic and biotic processes governing the fate of phenylurea herbicides in soils: a review. Crit Rev Environ Sci Technol 45:1947–1998. https://doi.org/10.1080/10643389.2014.1001141
Hussain S, Devers-Lamrani M, El Azhari N, Martin-Laurent F (2011) Isolation and characterization of an isoproturon mineralizing Sphingomonas sp. strain SH from a French agricultural soil. Biodegradation 22:637–650. https://doi.org/10.1007/s10532-010-9437-x
Knežević V, Tunić T, Gajić P et al. (2016) Getting more ecologically relevant information from laboratory tests: recovery of Lemna minor after exposure to herbicides and their mixtures. Arch Environ Contam Toxicol 71:572–588. https://doi.org/10.1007/s00244-016-0321-5
Laviale M, Morin S, Créach A (2011) Short term recovery of periphyton photosynthesis after pulse exposition to the photosystem II inhibitors atrazine and isoproturon. Chemosphere 84:731–734. https://doi.org/10.1016/j.chemosphere.2011.03.035
Leu C, Singer H, Stamm C et al. (2004) Simultaneous assessment of sources, processes, and factors influenicing herbicide losses to surface waters in a small agricultural catchment. Environ Sci Technol 38:3827–3834. https://doi.org/10.1021/es0499602
López-Muñoz MJJ, Revilla A, Aguado J (2013) Heterogeneous photocatalytic degradation of isoproturon in aqueous solution: experimental design and intermediate products analysis. Catal Today 209:99–107. https://doi.org/10.1016/j.cattod.2012.11.017
Lu J, Wang Z, Xing W, Liu G (2013) Effects of substrate and shading on the growth of two submerged macrophytes. Hydrobiologia 700:157–167. https://doi.org/10.1007/s10750-012-1227-5
Macinnis-Ng CMO, Ralph PJ (2004) In situ impact of multiple pulses of metal and herbicide on the seagrass, Zostera capricorni. Aquat Toxicol 67:227–237. https://doi.org/10.1016/j.aquatox.2004.01.012
Mohammad M, Itoh K, Suyama K (2010) Effects of herbicides on Lemna gibba and recovery from damage after prolonged exposure. Arch Environ Contam Toxicol 58:605–612. https://doi.org/10.1007/s00244-010-9466-9
Naddy RB, Johnson KA, Klaine SJ (2000) Response of Daphnia magna to pulsed exposures of chlorpyrifos. Environ Toxicol Chem 19:423–431. https://doi.org/10.1002/etc.5620190223
Netten JJC, van Zuidam J, Kosten S, Peeters ETHM (2011) Differential response to climatic variation of free-floating and submerged macrophytes in ditches. Freshw Biol 56:1761–1768. https://doi.org/10.1111/j.1365-2427.2011.02611.x
OECD (2006) Test No. 221: Lemna sp. Growth Inhibition Test. OECD
Pérès F, Florin D, Grollier T et al. (1996) Effects of the phenylurea herbicide isoproturon on periphytic diatom communities in freshwater indoor microcosms. Environ Pollut 94:141–152. https://doi.org/10.1016/S0269-7491(96)00080-2
Pietsch C, Krause E, Burnison BK et al. (2006) Effects and metabolism of the phenylurea herbicide isoproturon in the submerged macrophyte Ceratophyllum demersum L. Association for Applied Botany e. V., German Society for Quality Research on Plant Foods (DGQ)
Rabiet M, Margoum C, Gouy V et al. (2010) Assessing pesticide concentrations and fluxes in the stream of a small vineyard catchment - Effect of sampling frequency. Environ Pollut 158:737–748. https://doi.org/10.1016/j.envpol.2009.10.014
Reinert KH, Giddings JM, Judd L (2002) Effects analysis of time-varying or repeated exposures in aquatic ecological risk assessment of agrochemicals. Environ Toxicol Chem 21:1977–1992. https://doi.org/10.1002/etc.5620210928
Rosenkrantz RT, Baun A, Kusk KO (2013) Growth inhibition and recovery of Lemna gibba after pulse exposure to sulfonylurea herbicides. Ecotoxicol Environ Saf 89:89–94. https://doi.org/10.1016/j.ecoenv.2012.11.017
Schmitt W, Bruns E, Dollinger M, Sowig P (2013) Mechanistic TK/TD-model simulating the effect of growth inhibitors on Lemna populations. Ecol Modell 255:1–10. https://doi.org/10.1016/j.ecolmodel.2013.01.017
Steinberg RA (1946) Mineral requirements of Lemna minor. Plant Physiol 21:42–48. https://doi.org/10.1104/pp.21.1.42
Teodorović I, Knežević V, Tunić T et al. (2012) Myriophyllum aquaticum versus Lemna minor: Sensitivity and recovery potential after exposure to atrazine. Environ Toxicol Chem 31:417–426. https://doi.org/10.1002/etc.748
Tunić T, Knežević V, Kerkez D et al. (2015) Some arguments in favor of a Myriophyllum aquaticum growth inhibition test in a water-sediment system as an additional test in risk assessment of herbicides. Environ Toxicol Chem 34:2104–2115. https://doi.org/10.1002/etc.3034
Vallotton N, Eggen RIL, Chèvre N (2009) Effect of sequential isoproturon pulse exposure on Scenedesmus vacuolatus. Arch Environ Contam Toxicol 56:442–449. https://doi.org/10.1007/s00244-008-9200-z
Vallotton N, Eggen RIL, Escher BI et al. (2008) Effect of pulse herbicidal exposure on Scenedesmus vacuolatus: a comparison of two photosystem II inhibitors. Environ Toxicol Chem 27:1399–1407. https://doi.org/10.1897/07-197.1
Varga M, Horvatić J, Žurga P et al. (2019) Phytotoxicity assessment of isoproturon on growth and physiology of non-targeted aquatic plant Lemna minor L. - A comparison of continuous and pulsed exposure with equivalent time-averaged concentrations. Aquat Toxicol 213:105225. https://doi.org/10.1016/J.AQUATOX.2019.105225
Varshney S, Hayat S, Alyemeni MN, Ahmad A (2012) Effects of herbicide applications in wheat fields: is phytohormones application a remedy? Plant Signal Behav 7:570–575. https://doi.org/10.4161/psb.19689
Wang Q, Que X, Zheng R et al. (2015) Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ Sci Pollut Res 22:9646–9657. https://doi.org/10.1007/s11356-015-4104-8
Wilson PC, Koch R (2013) Influence of exposure concentration and duration on effects and recovery of Lemna minor exposed to the herbicide norflurazon. Arch Environ Contam Toxicol 64:228–234. https://doi.org/10.1007/s00244-012-9834-8
Zhao Y, Newman MC (2006) Effects of exposure duration and recovery time during pulsed exposures. Environ Toxicol Chem 25:1298–1304. https://doi.org/10.1897/05-341R.1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Varga, M., Žurga, P., Brusić, I. et al. Growth inhibition and recovery patterns of common duckweed Lemna minor L. after repeated exposure to isoproturon. Ecotoxicology 29, 1538–1551 (2020). https://doi.org/10.1007/s10646-020-02262-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02262-9