Abstract
Excessive nutrient loading may cause a shift from submerged macrophyte dominance to free-floating macrophyte dominance. Tolerance and persistence of submerged plants in response to shade may be key characteristics in determining when/if such a shift occurs in shallow eutrophic lakes. This study examines how the cover of floating macrophyte (Lemna minor) and shade of dark mesh affect the growth and photosynthetic efficiency of two submerged plants (Vallisneria natans and Myriophyllum spicatum) on different nutrient substrates. We found that low- and mid-cover intensities generally enhanced the leaf/shoot growth of both submerged plants under all cover and substrate types. The relative growth rates (RGR) were slightly enhanced under the treatment of Lemna with low- and mid-intensity cover on both nutrient-rich substrates. The leaf/shoot growth and RGR of both submerged macrophytes generally increased more under Lemna cover than mesh cover. The photosynthetic efficiency (F v/F m value) typically increased with the duration of treatment and the cover densities. In addition, these two macrophytes with contrasting growth forms have markedly different growth and survival strategies in response to covers. These results strengthen the hypothesis that submerged plants can successfully develop under a low-intensity cover of floating vegetation on nutrient-rich substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shallow lakes often possess two alternative stable states, a turbid and devoid of submerged macrophytes or clear and vegetated (Scheffer et al., 1993). Excessive nutrient loading has caused most lakes to shift to a turbid state dominated by phytoplankton as the main primary producers (Scheffer et al., 1993). However, nutrient-rich shallow lakes may also be dominated alternatively by free-floating macrophytes in stable states (Morris et al., 2003; Scheffer et al., 2003; Scheffer and Van Nes 2007), especially in subtropical and tropical shallow lakes, where free-floating plant dominant states may occur frequently. Several recent papers have supported that free-floating macrophytes will profit most from enhanced eutrophication and future climate change (Feuchtmayr et al., 2009; Netten et al., 2010, 2011).
Free-floating macrophyte dominance creates dark and anoxic underwater conditions that leave little opportunity for animal or plant life in shallow lakes (Janes et al., 1996; Scheffer et al., 2003; Morris et al., 2004; Abdel-Tawwab, 2006; Meerhoff et al., 2006, 2007). However, the environmental conditions created by free-floating macrophyte mats are not at all deleterious to submerged plant growth. For instances, a high-intensity cover of free-floating macrophytes may lead to strong oxygen depletion (Caraco et al., 2006), and may benefit photosynthesis and reduce photorespiration (Simpson et al., 1980); free-floating macrophyte mats can successfully reduce the biomass of phytoplankton, periphyton and filamentous algae (Parr et al., 2002; Bicudo et al., 2007; O’Farrell et al., 2009) and subsequently improving water transparency. These suggest that the effect of free-floating macrophyte mats on the growth of submerged plants may depend on the cover intensity of free-floating macrophytes.
Besides light and oxygen, nutrient competition between submerged and floating macrophytes may largely determine the shift between floating and submerged plants. Floating macrophytes can acquire inorganic nutrients from water column only, whereas submerged rooted macrophytes can take up nutrients effectively from both substrate (Chambers et al., 1989) and water column (Carignan & Kallf, 1980; Madsen & Cedergreen, 2002). Despite a previous report pointed out that nutrient absorbed from water column was less effective (Barko & Smart, 1986), manipulative experiments on four submerged species have demonstrated that all species were able to satisfy their demand for mineral nutrients by leaf nutrient uptake alone (Madsen & Cedergreen, 2002). Moreover, periphytons attached to submerged plant may also take up nutrients and further reduce water nutrient concentrations (Eriksson & Weisner, 1997). Low nutrient availability of water column might be expected to prevent the growth of floating plants (Szabo et al., 2010). Thus, the competitive advantage of submerged macrophytes relative to floating plants will depend on the nutrient conditions of substrate. Nutrient-rich substrate may favour the persistence of submerged macrophyte dominance. In contrast, in nutrient-poor substrate submerged macrophytes will compete more strongly with floating plants for nutrient from water column. Furthermore, considering into nutrient consumption, the cover effect of floating macrophytes appear to be more complex compared to simple physical shading, such as cover with dark mesh. However, the effects substrate- and cover types on the interactions between submerged macrophytes and free-floating plants have been scarcely studied.
Tolerance and growth strategy of species are also important factors in determining the survival of submerged macrophytes below floating mats. High-growing species, for instance, Myriophyllum spicatum, may respond to shading by accelerating elongation, so that they rapidly colonise the subsurface layer where the greatest amount of remaining light is available. In contrast, rosette-forming species can seldom grow to subsurface layer and may be highly tolerant to low-light intensities. For example, species of Vallisneria is a submerged aquatic plant with basal rosettes of flexible ribbonlike leaves that can form an underwater meadow. Vallisneria has been reported to be efficient carbon fixers at low-light intensities (Titus & Adams, 1979) and have a very low-light-compensation point (Blanch et al., 1998, Morris et al., 2004). Here, we studied the effects of cover types, cover intensities and substrate types on the growth of two submerged plants with contrasting growth forms. We hypothesized that the (i) submerged plants can successfully develop under a low-intensity cover of free-floating macrophytes and in a nutrient-rich substrate; (ii) cover effects between free-floating plant and mesh on the growth of submerged macrophytes are different; and (iii) submerged macrophytes with contrasting growth forms have clearly different growth and survival strategies in response to covers.
Materials and methods
Species
Vallisneria natans and M. spicatum were selected for the experiment as the two species are known to persist in very nutrient-rich lakes still supporting submerged vegetation (Sand-Jensen et al. 2008). They have different light-compensation points with 9.4 μmol m−2 s−1 in V. natans and 27.5 μmol m−2 s−1 in M. spicatum at 20°C in studied region (Su et al., 2004), which might imply that they have different responses to cover densities of free-floating plants. Seedlings of V. natans, apical shoots of M. spicatum and fronds of Lemna minor were collected from ditches near Lake Dianchi (24°29′N to 25°28′N, 102°29′E to 103°01′E), a shallow, eutrophic plateau lake located at Kunming, China. The plants were pre-incubated in experimental ponds (2 m × 2 m) filled with tap water (total nitrogen 0.61 ± 0.19, total phosphorus 0.59 ± 0.06 mg l−1; n = 5) for 7 days.
Experimental set-up
A mesocosm experiment was performed in 274 l polyethylene tanks (height: 0.8 m; diameter: 0.66 m) from 10th July to 10th August 2010 in an unheated glasshouse at a site near Lake Dianchi (24°44′32′′N, 102°35′24′′E). Seedlings of V. natans (~8 cm length for the longest leaf) and apical shoots of M. spicatum (8 cm length, without lateral shoots) were weighed and planted in individual plastic pots (8 cm diameter and 13 cm high) that were filled with 10 cm of substrate. Three types of substrate (sand, clay and a 50:50 v/v mixture of the two) were used. Clay was collected from deposits in Lake Dianchi. The six pots (three substrates × two species) were then placed into a tank (Fig. 1). The sides of the tanks were covered by black foil to prevent light penetration from the sides. The initial dry weight:fresh weight ratio was determined on plants identical to the experimental material. A total of 35 tanks were designed and filled with the same tap water as above to 70 cm high.
The experiments were then designed further with a completely randomised block design of 3 × 2 factorial and a control treatment (CK) and replicated five times (Fig. 1). Treatments and levels were as follows: cover type (cover 1—free-floating plant L. minor and cover 2—dark mesh) and cover intensity (three densities of low, medium and high were created by adding 30, 60 and 180 g fresh biomass for L. minor; similar shading effects were simulated by placing a fine dark mesh on the water surface and adjusting number of layers). To maintain the similar cover intensity during whole experiment, the fresh biomasses of floating plants were weekly weighted and adjusted to its initial design value. The CK was designed without any coverage. Given that our main objective in the present study was to elucidate the growth responses of submerged macroptytes on shading under a eutrophic condition, we prioritized low-light levels by shading the experimental glasshouse with two layers of fine dark mesh.
Data collection
To evaluate shading validity, we measured the light intensities at a superficial 5 cm depth for each tank at 4:00 PM–5:00 PM after 2 days of treatment, using a submersible digital illuminometer (ZDS-10W; Shanghai, China). During the experiment, light intensity in glasshouse was measured every 4 days from 8:30 AM to 11:30 AM, every 10 min. Chlorophyll fluorescence, a non-destructive assay used to estimate the intrinsic capacity of photosynthesis in green plants, was measured randomly on fully expanded leaves for each individual plant using a portable plant efficiency analyser (Handy PEA 2919; Hansatech Instruments Ltd., UK) after 10, 20 and 30 days of treatment. Variable fluorescence (F v = F m − F 0) and maximum quantum efficiency of PSII photochemistry (F v/F m) were calculated from F 0 and F m, where F v is variable fluorescence rise, F 0 is the initial minimal fluorescence level and F m is the maximum rise fluorescence.
At the end of the experiment and before the covers were disturbed, dissolved oxygen (DO) concentration at the superficial 5 cm depth was measured using a portable DO analyser (JPBJ-609; Shanghai, China). Water was sampled to measure concentrations of total nitrogen (TN) and total phosphorus (TP) using standard methods (Huang et al., 1999). Then all the individuals were harvested. Unexpectedly, filamentous green algae had emerged around the experimental macrophytes and they were also carefully collected with tweezers and a fine mesh sieve. The longest shoot/leaf length and the number of ramets/lateral shoots were measured for each individual of V. natans and M. spicatum. All experimental individuals of both macrophytes and the total filamentous green algae in each tank were dried at 80°C for 48 h and weighed. Dry mass was used to calculate the relative growth rate of submerged plants during 31 days of incubation (relative growth rates, RGR): RGR = (lnDW t − lnDW0)/t in which DW t and DW0 are the dry masses at time t and time 0 respectively.
Statistical analyses
The significance of cover type and cover intensity on the light intensity, DO, TN and TP was tested by two-way analysis of variance (ANOVA) using SPSS 16.0 software. The significance of cover type, cover intensity and substrate type on the relative growth (final length minus initial length) of longest leaf/shoot, the number of ramets/lateral shoots, RGR and F v/F m value of both submerged macrophytes were evaluated by three-way ANOVA. To further test for significant differences between Lemna coverage and mesh coverage at a specific treatment level, comparisons were made using a t test. The normal distribution of the variables was checked by the Kolmogorov–Smirnov test. When the data did not meet assumptions of homogeneity of variance, log10, cube- or square-root arcsine transformations were performed depending on the type of variables. The total biomass of filamentous green algae per cover intensity was compared between Lemna cover and mesh cover using a non-parametric Kruskal–Wallis test.
Results
Environmental variables
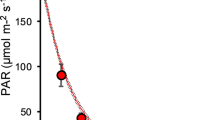
Daily irradiance from 8:30 AM to 11:30 AM in the glasshouse varied from 80 to 944 μmol m−2 s−1, which was above the light-compensation point (Fig. 2). Significant differences of the mean irradiance (E mean) were observed between the three cover densities (F 3,32 = 470.77, P < 0.01, ANOVA) but not between the two cover types (F 1,32 = 3.52, P > 0.05, ANOVA), suggesting that the mesh cover closely mimics the Lemna cover (Fig. 3a).
Light intensity and average for every 10 min in experimental glasshouse from 8:30 AM to 11:30 AM for every 4 days over the experimental period. The light-compensation points are 9.4 μmol m−2 s−1 in V. natans and 27.5 μmol m−2 s−1 in M. spicatum at 20°C in studied region (Su et al., 2004)
Variations in a mean irradiances, b subsuperficial (5 cm depth) dissolved oxygen (DO), c total biomass of filamentous green algae (FGA), d total nitrogen (TN), and e total phosphorus (TP) under different treatments at the end of experiment. Values represent mean ± SE, n = 5. * and ** indicate significant differences between the two cover types at 0.05 and 0.01 levels by paired-sample t test or Mann–Whitney test, respectively
Subsurface DO differed significantly between the cover densities (F 3,32 = 115.11, P < 0.01, ANOVA) and between the cover types (F 3,32 = 7.80, P < 0.01, ANOVA) (Fig. 3b). Further analysis showed that the significant difference between the cover types only occurred in the high-cover treatment (P < 0.01, t test). The biomass of filamentous green algae declined significantly with increasing cover intensities in both the Lemna cover (χ2 = 10.55, P < 0.05, Kruskal–Wallis test) and the mesh cover (χ2 = 11.76, P < 0.01, Kruskal–Wallis test) (Fig. 3c). Paired comparison indicated that the biomass of filamentous algae was significantly higher in mesh cover than in Lemna cover under low- and mid-cover treatments (P < 0.05, t test). The filamentous algae was almost absent in the high-cover treatments of the two cover types.
TN concentrations differed significantly between the two cover types (F 1,32 = 6.43, P < 0.01, ANOVA) and among the four cover densities (F 3,32 = 4.26, P < 0.05, ANOVA) (Fig. 3d), while TP concentrations differed significantly between the two cover types (F 3,32 = 9.93, P < 0.01, ANOVA) but not among the four cover densities (F 1,32 = 0.27, P > 0.05, ANOVA) (Fig. 3e). The two variables were significantly lower in mesh cover than in Lemna cover under high-cover treatments (P < 0.05, t test); however, TN was significantly higher for mesh cover than Lemna cover under mid-cover treatments (P < 0.05, t test).
Growth of submerged macrophytes
The length of the longest leaf, the number of ramets and RGR of V. natans varied significantly over cover intensity and substrate type (Table 1; Fig. 4). The three variables declined significantly with decreasing clay content. On clay and mixed substrates, the length of the longest leaf had a mild increase under low- and mid-cover conditions compared to CK, and then decreased sharply under a high-intensity cover. Significant differences in the number of ramets and RGR were detected between the two cover types. There were significantly more ramets in Lemna cover than in mesh cover (P < 0.05, Kruskal–Wallis test) on clay substrate with low cover and on mixed substrate with mid cover. On clay and mixed substrates, RGR declined linearly with increasing cover intensity in mesh cover, but mildly increased under low- and mid-cover conditions of Lemna compared to CK. RGR was significantly higher in Lemna cover than in mesh cover, on clay substrate with low and mid cover, mixed substrate with mid cover and sand substrate with low and mid cover (P < 0.05, t test). Significant interactions were also detected between cover type and cover intensity and between substrate type and cover intensity.
Similar to V. natans, the length of the longest shoot, the number of lateral shoots and RGR of M. spicatum showed significant differences among the cover intensities and between the substrate types (Table 1; Fig. 4). The cover types significantly influenced RGR of M. spicatum, which was significantly higher in Lemna cover than in mesh cover, on clay substrate with mid cover and mixed substrate with low and mid cover (P < 0.05, t test). Unlike V. natans, however, the length of the longest shoot of M. spicatum was significantly different between the two cover types but the number of lateral shoots did not show significant differences between the cover types, suggesting a different response model for the two species. Shoots grew significantly quicker in Lemna cover than in mesh cover, on clay substrate with mid cover, and mixed and sand substrates with low cover (P < 0.05, t test). On the other hand, although the shoot growth and RGR of M. spicatum declined significantly on sand substrate than on the two other substrates, the extent of decline was markedly smaller compared to that of V. natans, suggesting M. spicatum has a relatively stronger ability to adapt to the sand substrate.
Chlorophyll fluorescence
The F v/F m values were significantly higher after 20 or 30 days of treatment than after 10 days for both species (Fig. 5; P < 0.01, ANOVA). The significant differences in F v/F m values occurred mainly among the cover intensities (Fig. 5; Table 1) and between the substrate types (Table 1). However, V. natans presented a significant difference in the cover types after 20 days of treatment, and significant interactions were also detected between cover type and cover intensity after 10 and 20 days of treatment. There were no significant correlations between F v/F m and cover types for M. spicatum, except for an interaction between cover type and cover intensity after 20 days of treatment. Moreover, it should be noted that F v/F m values showed a steady increasing trend with increasing cover intensities for most groups, especially after 10 days of treatment.
Discussion
The experimental cover and substrate treatments engendered various significant results. One conclusion is that low- and mid-cover densities generally enhanced the leaf/shoot growth of both submerged macrophytes under most cover types and substrate types. RGR did not decrease significantly, and was even mildly enhanced in several cases, in the treatment of Lemna cover with low- and mid-intensity covers on clay and mixture substrates. These results corroborate our hypothesis that on nutrient-rich substrates, submerged macrophytes can successfully develop under a low-intensity cover of floating vegetation. The biomass of both submerged macrophytes generally increased more under the Lemna cover than the mesh cover, which aligns with our initial hypothesis that other mechanisms, besides shade, may also be responsible for the submerged macrophyte growth. It was confirmed in this experiment that macrophytes have faster growth in length of longest leaf/shoot, RGR and ramet/shoot number on nutrient-rich clay and mixed substrates than on nutrient-deficient sand—a finding that has been well documented in the literatures (Xie et al., 2007; Jiang et al., 2008). The increased F v/F m values with both the duration of treatment and the cover intensity could have been caused by a higher proportion of open reaction centres (higher values of F m), which could be attributed to an increase in Chl a content and PSII efficiency (Parr et al., 2002; Redondo-Gómez et al., 2007). These suggest that both studied macrophytes might be adapted fast to low solar irradiance from shading. Moreover, our results demonstrate that these two macrophytes with different growth forms have clearly different growth strategies in response to cover densities.
Our result indicated that the growth of the longest leaf/shoot of both submerged macrophytes was stimulated mildly by low- and mid-cover, but not high-cover conditions. A similar increase in shoot length at low light has been found in laboratory studies with Potamogeton crispus (Tobiessen & Snow, 1984) and Vallisneria americana (Morris et al., 2004). In a cover experiment with free-floating Azolla filiculoides and Lemna minuta, Janes et al. (1996) found that Elodea nuttallii significantly elongated, but P. crispus showed no such response under same cover conditions. Therefore, the growth stimulation in response to low light is apparently intensity-dependent and species-dependent.
Although direct light depletion seems to have an important effect on biomass production in our experiments, it is probably not the only factor explaining RGR variations of both submerged species under different cover densities. The fact that RGR of both species were much higher under Lemna cover than mesh cover at low- and mid-cover densities, suggests that, additional mechanisms may play a role. In the current experimental system, despite the small differences in light intensity and DO concentration that occurred between both cover types, both variables were generally higher under mesh cover than Lemna cover and thus should be more conducive to RGR. Thus, we assume that the different responses of RGR between both cover types could indeed be attributed to the emergence of unexpected filamentous green algae. Experimental evidence has reported that filamentous algae have negative effects on the growth of submerged plants (Phillips et al., 1978; Simpson & Eaton, 1986; Ozimek et al., 1991; Asaeda et al., 2004; Sultana et al., 2010). In the current experiment, the biomass of filamentous algae was significantly higher in mesh cover than in Lemna cover at low- and mid-cover densities. The filamentous algae that appeared may further shade submerged plants. However, the mechanism that causes the difference in biomass of filamentous algae between the two cover types is unclear. We suppose that filamentous algae might suffer more from nutrient limitation under Lemna cover than mesh cover, since both free-floating macrophyte and filamentous algae can only acquire nutrients from the water column. This hypothesis is supported by the fact that both TN and TP concentrations were significantly lower in mesh cover than in Lemna cover in high-cover treatments, but inconsistent with the fact that a higher TN concentration was present in the medium Lemna cover condition. It is unclear whether such an increase was due to the decomposition of L. minor and/or filamentous algae, which could result from nutrient-limited death. Moreover, L. minor that was present may also have an allelopathic effect on the alga (Parr et al., 2002). Our reported results appear to be the first describing the inductive effect of free-floating macrophytes on the growth of submerged macrophytes. However, the interaction mechanism among free-floating macrophyte, filamentous algae and submerged plants still needs further clarification.
Our results confirm that these two submerged species with different growth forms have clearly different tolerance levels and growth strategies in response to cover shade. The rosette-forming V. natans increased its biomass by producing more ramets in relatively favourable low- and mid-cover conditions and nutrient-rich substrates, while the canopy-forming M. spicatum adopted a strategy of rapid elongation. In line with these results, Vallisneria is an acknowledged low-light-tolerant species. It has been reported that Vallisneria can persist under a dense canopy of M. spicatum (Boylen et al., 1999), and can even survive over 3 months beneath 100% shading (Blanch et al., 1998, Morris et al., 2004). It should be noted that despite the fact that these two species with different growth forms adequately tolerated severe light attenuation, the current conclusion is obtained from a small spatial scale and short-term experiment. However, the special capability of canopy-forming species with rapid elongation should allow them to rapidly colonise the subsurface layer and hence more effectively avoid damage imposed by a reduction of light intensity and dissolved oxygen. If allowed a larger temporal or spatial scale, we presume that the canopy-form species should adapt even better to free-floating cover.
Increased nutrient loading and asymmetric competition between floating and submerged species may initiate a switch to floating plant domination (Scheffer et al., 2003). However, such a switch could be misjudged if the competitive capability or tolerance of submerged plant to free-floating macrophyte is underestimated. Our results, in conjunction with current research in this field, demonstrate that substantial submerged growth is still possible under a low- and mid-intensity cover of free-floating macrophytes. Mechanisms of rapid stem elongation or high tolerance to low-light intensity might mainly contribute to the response and adaption of submerged plants to cover of free-floating macrophytes.
References
Abdel-Tawwab, M., 2006. Effect of free-floating macrophyte, Azolla pinnata, on water physico-chemistry, primary productivity and the production of Nile Tilapia, Oreochromis niloticus, L., and common carp, Cyprinus carpio L., in fertilized earthen ponds. Journal of Applied Aquaculture 18: 21–41.
Asaeda, T., M. Sultana, J. Manatunge & T. Fujino, 2004. The effect of epiphytic algae on the growth and production of Potamogeton perfoliatus L. in two light conditions. Environmental and Experimental Botany 52: 225–238.
Barko, J. W. & R. M. Smart, 1986. Sediment-related mechanisms of growth limitation in submersed macrophytes. Ecology 67: 1328–1340.
Bicudo, D. D. E. C., B. M. Fonseca, L. M. Bini, L. O. Crossetti, C. E. Bicudo & T. Araújo-Jesus, 2007. Undesirable side-effects of water hyacinth control in a shallow tropical reservoir. Freshwater Biology 52: 1120–1133.
Blanch, S. J., G. G. Ganf & K. F. Walker, 1998. Growth and recruitment in Vallisneria americana as related to average irradiance in the water column. Aquatic Botany 61: 181–205.
Boylen, C. W., L. W. Eichler & J. D. Madsen, 1999. Loss of native aquatic plant species in a community dominated by Eurasian watermilfoil. Hydrobiologia 415: 207–211.
Caraco, N., J. C. Cole, S. Findlay & C. Wigand, 2006. Vascular plants as engineers of oxygen in aquatic systems. Bioscience 56: 219–225.
Carignan, R. & J. Kallf, 1980. Phosphorus sources for aquatic weeds: water or sediment? Science 207: 987–989.
Chambers, P. G., E. E. Prepas, M. L. Bothwell & H. R. Hamilton, 1989. Roots versus shoots in nutrient uptake by aquatic macrophytes. Canadian Journal of Fisheries and Aquatic Sciences 45: 435–439.
Eriksson, P. G. & S. E. B. Weisner, 1997. Nitrogen removal in a wastewater reservoir: the importance of denitrification by epiphytic biofilms on submersed vegetation. Journal of Environmental Quality 26: 905–910.
Feuchtmayr, H., R. Moran, K. Hatton, L. Connor, T. Heyes, B. Moss, I. Harvey & D. Atkinston, 2009. Global warming and eutrophication: effects on water chemistry and autotrophic communities in experimental hypertrophic shallow lake mesocosms. Journal of Applied Ecology 46: 713–723.
Huang, X. F., W. M. Chen & Q. M. Cai, 1999. Survey, Observation and Analysis of Lake Ecology. Standards Press of China, Beijing.
Janes, R. A., J. W. Eaton & K. Hardwick, 1996. The effects of floating mats of Azolla filiculoides Lam. and Lemna minuta Kunth on the growth of submerged macrophytes. Hydrobiologia 340: 23–26.
Jiang, J. H., C. F. Zhou, S. Q. An, H. B. Yang, B. H. Guan & Y. Cai, 2008. Sediment type, population density and their combined effect greatly charge the short-time growth of two common submerged macrophytes. Ecological Engineering 34: 79–90.
Madsen, T. V. & N. Cedergreen, 2002. Sources of nutrients to rooted submerged macrophytes growing in a nutrient-rich stream. Freshwater Biology 47: 283–291.
Meerhoff, M., C. Fosalba, C. Bruzzone, N. Mazzeo, W. Noordoven & E. Jeppesen, 2006. An experimental study of habitat choice by Daphnia: plants signal danger more than refuge in subtropical lakes. Freshwater Biology 51: 1320–1330.
Meerhoff, M., C. Iglesias, F. T. E. Mello, J. M. Clemente, E. Jensen, T. L. Lauridsen & E. Jeppesen, 2007. Effects of habitat complexity on community structure and predator avoidance behaviour of littoral zooplankton in temperate versus subtropical shallow lakes. Freshwater Biology 52: 1009–1021.
Morris, K., P. C. Bailey, P. I. Boon & L. Hughes, 2003. Alternative stable states in the aquatic vegetation of shallow urban lakes II. Catastrophic loss of aquatic plants consequent to nutrient enrichment. Marine and Freshwater Research 54: 201–215.
Morris, K., K. A. Harrison, P. C. E. Bailey & P. I. Boon, 2004. Domain shifts in the aquatic vegetation of shallow urban lakes: the relative roles of low light and anoxia in the catastrophic loss of the submerged angiosperm Vallisneria americana. Marine and Freshwater Research 55: 749–758.
Netten, J. J. C., G. H. P. Arts, R. Gylstra, E. H. Van Nes, M. Scheffer & R. M. M. Roijackers, 2010. Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii in mesocosms. Fundamental and Applied Limnology 177: 125–132.
Netten, J. J. C., J. van Zuidam, S. Kosten & E. T. H. M. Peeters, 2011. Differential response to climatic variation of free-floating and submerged macrophytes in ditches. Freshwater Biology 56: 1761–1768.
O’Farrell, P., P. de Tezanos Pinto, P. L. Rodríguez, G. Chaparro & H. N. Pizarro, 2009. Experimental evidence of the dynamic effect of free-floating plants on phytoplankton ecology. Freshwater Biology 54: 363–375.
Ozimek, T., E. Pieczyska & A. Hankiewicz, 1991. Effects of filamentous algae on submerged macrophyte growth: a laboratory experiment. Aquatic Botany 41: 309–315.
Parr, L. B., R. G. Perkins & C. F. Mason, 2002. Reduction in photosynthetic efficiency of Cladophora glomerata, induced by overlying canopies of Lemna spp. Water Research 36: 1735–1742.
Phillips, G., D. Eminson & B. Moss, 1978. A mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquatic Botany 4: 103–126.
Redondo-Gómez, S., E. Mateos-Naranjo, A. J. Davy, F. Fernández-Muñoz, E. M. Castellanos, T. Luque & F. M. Enrique, 2007. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Annals of Botany 100: 555–563.
Sand-Jensen, K., N. L. Pedersen, I. Thorsgaard, B. Moeslund, J. Borum & K. P. Brodersen, 2008. 100 years of vegetation decline and recovery in Lake Fure, Denmark. Journal of Ecology 96: 260–271.
Scheffer, M., & E. H. Van Nes, 2007. Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584: 455–466.
Scheffer, M., S. Hosper, M. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279.
Scheffer, M., S. Szabó, A. Gragnani, E. H. Van Nes, S. Rinaldi, N. Kautsky, J. Norberg, R. M. M. Roijackers & R. J. M. Franken, 2003. Floating plant dominance as a stable state. The Proceedings of the National Academy of Sciences of United States of America 100: 4040–4045.
Simpson, P. S., J. W. Eaton & K. Hardwick, 1980. The influence of environmental factors on apparent photosynthesis and respiration of the submersed macrophyte Elodea canadensis. Plant, Cell and Environment 3: 415–423.
Simpson, P. S. & J. W. Eaton, 1986. Comparative studies of the photosynthesis of the submerged macrophyte Elodea canadensis and the filamentous algae Cladophora glomerata and Spirogyra sp. Aquatic Botany 24: 1–12.
Su, W., G. Zhang, Y. Zhang, H. Xiao & F. Xia, 2004. The photosynthetic characteristics of five submerged aquatic plants. Acta Hydrobiologia Sinica 28: 391–395.
Sultana, M., T. Asaeda, M. E. Azim & T. Fujino, 2010. Morphological responses of a submerged macrophyte to epiphyton. Aquatic Ecology 44: 73–81.
Szabo, S., M. Scheffer, R. Roijackers, B. Waluto, M. Braun, P. T. Nagy, G. Borics & L. Zambrano, 2010. Strong growth limitation of a floating plant (Lemna gibba) by the submerged macrophyte (Elodea nuttallii) under laboratory conditions. Freshwater Biology 55: 681–690.
Titus, J. E. & M. S. Adams, 1979. Coexistence and the comparative light relations of the submersed macrophytes Myriophyllum spicatum L., and Vallisneria Americana Michx. Oecologia 40: 273–286.
Tobiessen, P. & P. D. Snow, 1984. Temperature and light effects on the growth of Potamogeton crispus in Collins Lake NYS. Canadian Journal of Botany 62: 2822–2826.
Xie, Y. H., W. B. Luo, B. Ren & F. Li, 2007. Morphological and physiological responses to sediment type and light availability in roots of the submerged plant Myriophyllum spicatum. Annals of Botany 100: 1517–1523.
Acknowledgments
This study was supported by the National Science Foundation of China (30970469) and the National S&T Major Project (2008ZX07102-005). We thank Diana Chen for language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sidinei Magela Thomaz
Rights and permissions
About this article
Cite this article
Lu, J., Wang, Z., Xing, W. et al. Effects of substrate and shading on the growth of two submerged macrophytes. Hydrobiologia 700, 157–167 (2013). https://doi.org/10.1007/s10750-012-1227-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1227-5