Abstract
Hg accumulation in fish is influenced by several factors including seasonality. In the Amazon, ecosystems are marked by strong seasonal variation in precipitation, which leads to drastic changes in the water level of lakes and rivers. The aim of this study was to evaluate Hg levels in muscle of detritivorous, herbivorous and omnivorous fish from an Amazon lake (Madeira River Basin, Amazonas, Brazil) over four seasons (rising water, high water, falling water and low water). We hypothesized that total Hg concentration varies during the seasons. The results indicate that total Hg levels in detritivorous fish were higher in rising and low water seasons while in herbivorous and omnivorous fish the total Hg concentration was higher during the rising water season. The hypothesis was supported by the results. Additionally, the study provides evidence that Hg levels in fish with different feeding habits are influenced by the flood pulse of the Amazon region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is the third most dangerous contaminant according to the Agency for Toxic Substances and Disease Registry (ATSDR 2017), due to its high toxicity, widespread distribution and biomagnification potential. Several studies have demonstrated that, due to biomagnification, species that occupy higher trophic levels in food webs, like fish, have higher Hg levels in their organs in comparison with low trophic level organisms (e.g., plants and invertebrates) (Watras and Bloom 1992; Signa et al. 2017).
Hg accumulation in fish can vary geographically, as demonstrated by the meta-analysis of Lavoie et al. (2013). Those authors observed more intense Hg biomagnification in temperate aquatic environments in comparison with tropical ones. However, they considered only a small number of studies in the Amazon, a region known for high Hg concentrations in fish. In the Amazon, Hg has been extensively used in artisanal gold mining. Malm (1998) estimated that approximately 2000 tons of Hg from gold mining had been released in the Brazilian Amazon over the period from 1979 to 1994. Although anthropogenic emissions of Hg are significant, the studies of Roulet et al. (1988) and Roulet et al. (2001) showed that Amazon soils are naturally enriched with this metal. In agreement with this finding, Fadini and Jardim (2001) observed Hg concentration of 172 mg kg−1 in soils far from anthropogenic influence. Therefore, Amazonian soils act as reservoirs of Hg. However, during the rainy season, the transport of Hg accumulated in soils to adjacent aquatic environments is more intense due to the influence of the rain (Roulet et al. 2001), which could affect Hg accumulation in fish.
Amazonian environments are marked by strong seasonal variations in the rainfall which in turn results in alterations in water level of aquatic ecosystems (Sahoo et al. 2016). In the central Brazilian Amazon, during rising water and high water seasons, which can be considered as the rainy seasons, rainfall is more intense. In the high water season rivers overflow and interconnect with lakes, providing habitat and food for many fish species (Santos and Santos 2005; Melack et al. 2009; Correa et al. 2008; Queiroz et al. 2011). During the falling water and the low water seasons, which are considered as the dry seasons, rainfall reduces drastically and water level of aquatic ecosystems also reduces.
Seasonal variation in rainfall can also influence Hg bioavailability by changing Hg partition between particulate and dissolved phase. Maia et al. (2018) observed higher total Hg and methylmercury concentrations in the dissolved and particulate phase during the rainy seasons in white water lakes from the Amazon river basin. Dissolved Hg, especially methylmercury, is easily absorbed by fish due to its high bioavailability (Batley 1983). Maia et al. (2018) also observed that methylmercury is more prone to desorption of particles in black water lakes, due to low pH, which increase the concentration of methylmercury in the dissolved phase. Other studies in Amazon corroborate the pattern observed by Maia et al. (2018) like Kasper et al. (2017) that reported higher dissolved methylmercury concentration during the rainy season in Solimões/Amazon and Negro rivers. However, Pestana et al. (2018) observed different results with no relationship between seasonality and dissolved methylmercury levels in the Cuniã lake. Regarding biota, Roulet et al. (2000) observed influence of the seasonality on methylmercury accumulation in zooplankton in Tapajós river while Brito et al. (2017) found no influence of this parameter on Hg levels in plankton of Janaucá lake, Solimões river basin. The contrasting results reported by the aforementioned authors indicate the complexity of the Hg dynamics in Amazon aquatic ecosystems. It is important to keep searching for patterns for a better understanding of how the flood pulse can influence the Hg distribution in both abiotic and biotic compartments.
The increase of Hg bioavailability can impact the whole aquatic food chain. Roulet et al. (2000) attributed the increase in methylmercury concentrations in zooplankton from the dry season to the end of the rainy season to an increase in bioavailability during the rainy season. The primary producers like phytoplankton have great affinity for dissolved Hg, especially organic mercury, and the concentration in its cells can be 10,000 higher than in the water (Pickhardt and Fisher 2007; Carroll et al. 2011). Since Hg is slowly excreted (Bernhoft 2011), primary consumers (i.e., herbivores) that feed on phytoplankton would be exposed to high levels of Hg and secondary consumers (i.e., carnivores) would be exposed to even higher levels of Hg. On the other hand, during the rainy seasons (rising water and high water), the influx of Hg bound soil particles in the aquatic ecosystem is higher (Maia et al. 2018) and particulate Hg is less bioavailable to the biota (Batley 1983). Additionally, dissolved Hg can be diluted by intense rainfall (Maia et al. 2009), resulting in lower concentrations in this phase.
In this context, the aim of this study was to evaluate Hg concentration in fish with different feeding habits (detritivorous, herbivorous and omnivorous) in Puruzinho Lake (Madeira River Basin) over the four characteristics seasons of Amazon (rising, high, falling and low waters). Since fluctuation in rainfall among the seasons can influence Hg geochemistry, we expect differences in Hg concentrations in fish over the seasons.
Materials and methods
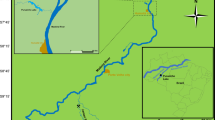
The study area was Puruzinho Lake, in the Madeira River Basin, western Amazonia, Brazil (63°6’0”W; 7°24’0”S). Puruzinho Lake is a black water ecosystem characterized by high concentration of dissolved organic matter, acid pH, low primary productivity and low suspended particulate material concentrations (Wissmar et al. 1981; Almeida et al. 2014). Black water ecosystems are suitable environments for Hg methylation due to their acid pH and high levels of dissolved organic matter (King et al. 1999). Puruzinho Lake is strongly influenced by seasonal hydrology. During the high water season, the Madeira River overflows and interconnects with Puruzinho Lake. Water levels change drastically between high water (12.5 m) and low water seasons (0.30 m) (Almeida et al. 2014) due to the Madeira River’s influence, which makes Puruzinho Lake a suitable environment to evaluate the influence of seasonality on Hg concentration in fish.
Sampling campaigns were carried out in the four seasons: rising water (RW) in December 2016 and February 2017; high water (HW) in April 2017; falling water (FW) in June 2017; and low water (LW) in October 2017. These seasons are mainly characterized by differences in rainfall intensity, and consequently water level of Puruzinho Lake. Figure 1 depicts the monthly rainfall measured at the Humaitá pluviometric station in Amazonas state (Puruzinho Lake is located 20 km from Humaitá) (INMET 2018). During the sampling campaigns, the rainfall followed the regular pattern in the region, with higher values in the RW and HW seasons (December 2016 to April 2017) and lower values in the FW and LW seasons (June 2017 to October 2017). Additionally, the Madeira river water level and flow (Table 1), measured at the Humaitá station (ANA 2019), reflected the rainfall pattern with higher values of both parameters during RW and FW seasons and lower values in FW and LW seasons (Table 1). Therefore, RW and HW can be considered as the rainy seasons and FW and LW as the dry seasons. We did not have the water level values of Puruzinho Lake, but based on the regular rainfall regime and the Madeira river water level and flow during the sampling (Fig. 1; Table 1), we assume that the lake’s water level is higher during the RW and HW and lower during FW and LW (Almeida et al. 2014). It is important to note that the sampling in HW (April 2017) was at the the peak of the rainy season because of the high rainfall values of the previous months, and the sampling in LW (October 2017) was at the peak of the dry season because of the low rainfall values in May, June, July, August and September (Fig. 1).
A total of 391 specimens (N = 391) were caught with the help of local fisherman. Specimens were grouped based on their feeding habits: detritivorous, herbivorous and omnivorous. Detritivorous fish consisted of Potamorhina altamazonica, Potamorhina latior, Prochilodus nigricans, Psectrogaster amazonica, Semaprochilodus insignis, Hemiodus unimaculatus and Psectrogaster rutiloides; herbivorous fish consisted of Mylossoma duriventre and Schizodon fasciatus; and omnivorous fish consisted of Pimelodus blochiiTriportheus albus and Leporinus friderici. Although food diversity and availability to fish can change seasonally, trophic ecology studies can provide a pattern of fish diets. We defined the food habits of the studied species based on Cella-Ribeiro et al. (2017) that compiled detailed data about stomach content and biology of several fish species from the Madeira River Basin. The only species whose feeding habit was not determined based on Cella-Ribeiro et al. (2017) was Leporinus friderici, due to the low number of stomachs analyzed by those authors (N = 9). Leporinus friderici feeding habit was classified as omnivorous based on Santos (1982), Durães et al. (2001) and Marçal-Simabuku and Peret (2002). Specimens were measured to obtain total length (cm) and total weight (g) (Table 2).
Total Hg (THg) determination in muscle (0.2 g wet wt) followed the protocol described by Bastos et al. (1998): addition of 1 mL of 30%H2O2 and 4 mL of 70% HNO3:98% H2SO4 (1:1); heating in a digestion block (model TE04/25) for 30 min in 70 °C; cooling to room temperature; addition of 5% KMnO4; heating for 15 min; cooling to room temperature overnight; addition of 12% NH2OH.HCl; and addition of ultrapure water to a final volume of 12 mL (MilliQ Plus, Millipore, Bedford, MA, USA). Determination was conducted by CV-AAS with a FIMS 400 Flow Injection Mercury System (PerkinElmer, Germany). Detection limit of the method was 0.007 mg kg−1. Certified material (DORM-2) was analyzed in triplicate at each 30 samples (recuperation: 99 ± 2.5%). Additionally, each sample was analyzed two times to test precision. Blanks were used for quality control.
To compare THg levels among seasons, we normalized concentrations by length, as suggested by Sccuder-Eikenberry et al. (2015), to remove the influence of size, following the formula: THg normalized = THg concentration / total length. Sccuder-Eikenberry et al. (2015) recommended multiplying THg normalized by the mean total length of the species. However, the statistical analysis conducted with the data multiplied by the mean total length and not multiplied showed the same patterns. Therefore, we present in this paper the normalization without the multiplication by the mean total length. Comparisons were conducted with ANOVA and the Tukey post-hoc test (α = 0.05). When necessary, data were transformed with Box-Cox (Venables and Ripley 2002) to fit ANOVA premises. Two-way ANOVA (α = 0.05) was used to test for interactions between seasons and feeding habits. Linear regressions (α = 0.05) were used to test for relationship between THg concentration and size (total length and total weight). Statistical analyses were performed using the R software (R Core Team 2018).
It is well known that Hg, especially methylmercury, forms strong bonds with thiol compounds in muscle proteins, which in turn leads to a long half-life in the organism. Also, due to this strong bond, methylmercury is slowly excreted and is the major Hg chemical species accumulated in fish muscle (Ikingura and Akagi 2003). This high half-life of Hg could influence the interpretation of our results, since we compared individuals in different seasons. However, the environmental conditions of the study area and the size of the fish can mitigate this issue because methylmercury excretion rate is positively correlated with water temperature and negatively with body size (Trudel and Rasmussen 1997). In the Amazon region, water temperature is high the whole year (INMET 2018), which can reduce Hg half-life. Additionally, the weight of the sampled individuals was similar to the ones that presented the highest Hg excretion rates in the long-term experiment of Trudel and Rasmussen (1997). Those authors also observed that fish chronically exposed to methylmercury excrete this contaminant 2.1 times faster than acutely exposed fish. Since there is no point source of Hg in the study area, it is more likely that fish in Puruzinho Lake are chronically exposed to the contaminant, thus showing a high excretion rate. Therefore, we assume that the Hg concentration observed in a fish sample in a given season represents that season and not past periods.
Results and discussion
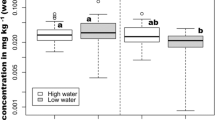
THg concentrations in the detritivorous guild were statistically higher during the RW and LW in comparison with HW and FW (p= 0.01), while herbivorous guild THg levels were highest in RW (p= 0.001) and THg levels in the omnivorous guild were statistically higher in the RW (p= 0.0001) (Fig. 2; Table 3). The results indicate that THg levels in all guilds vary along the seasons.
Most of the detritivorous fish sampled in this study belong to the order Characiforme (families Prochilodontidae and Curimatidae). These species have phytoplankton as their primary source of carbon (Araujo-Lima et al. 1986; Forsberg et al. 1993). Nascimento et al. (2006) reported that THg concentration in plankton from Puruzinho Lake was higher during the RW (337.0 ng g−1), which explains the higher levels observed in detritivorous fish during this seasons (Fig. 1). The two species herbivorous species, Mylossoma duriventre and Schizodon fasciatus, feeds mainly on C3 and C4 plants during RW (Oliveira et al. 2010) while phytoplankton constitutes an insignificant part of its diet. Despite this, THg levels in herbivorous was also higher during RW. The same pattern was observed in omnivorous fish that have Triportheus albus as its most sampled specie in RW. Triportheus albus feeding habit is a mix of insects and plants (Cella-Ribeiro et al. 2017) and phytoplankton is not often consumed by this specie. Therefore, higher THg levels in phytoplankton during RW can explain the higher levels of THg in detritivorous fish but not in herbivorous and omnivorous fish. Other factors like water physico-chemical parameters may have influenced THg accumulation in fish. Black water ecosystems, such as Puruzinho Lake, provide optimum conditions to increase Hg methylation and bioavailability, like low pH and high concentration of dissolved organic matter (Watras et al. 1995; Vieira et al. 2018).
During the HW season, the Madeira River’s main course overflows and invades Puruzinho Lake (Queiroz et al. 2011), resulting in an inflow of white waters. This is one of the three water groups in the Amazon and is characterized by high levels of suspended particulate matter (SPM) and neutral pH (Vieira et al. 2018). Therefore, the inflow of white waters in Puruzinho Lake may reduce Hg bioavailability to biota due to the high affinity of Hg for SPM (Maia et al. 2018). THg bound to SPM is less available to biota (Batley 1983) while dissolved Hg has strong affinity to phytoplankton biomass (Carroll et al. 2011). Since phytoplankton are the primary source of carbon for detritivorous fish (Araujo-Lima et al. 1986; Forsberg et al. 1993), the reduction of Hg bioavailability to phytoplankton during HW certainly influenced THg concentration in fish (Driscoll et al. 2007; Chen et al. 2012). Additionally, the high inflow of SPM during the HW may reduce phytoplankton growth due to increase in water turbidity (Northcote et al. 2009).
The influence the Madeira River’s main course on Puruzinho Lake was weaker during the FW. In this season, THg concentration in detritivorous and herbivorous fish were similar to that in the HW, possibly due to the presence of white waters from the Madeira River. During the LW, when there was no influence of the river, the THg concentration in detritivorous and omnivorous fish showed different patterns. In detritivorous fish, THg levels increased significantly and were statistically similar to those in the RW season, while in omnivorous fish, THg concentrations decreased significantly. The observed pattern in detritivorous fish was expected since there was no more influence of white waters in the lake and the optimum condition for increased Hg methylation was reestablished (Vieira et al. 2018). However, omnivorous fish’s THg levels did not increase in LW. This result may be related to the omnivorous fish diet rather than Hg bioavailability. The most sampled omnivorous species in LW was Triportheus albus, which may have switched to a more plant based diet in LW due to a lower input of terrestrial insects in the lake. In this season, rainfall is low (Fig. 1) which negatively impacts the influx of allochthonous food items (Rezende and Mazzoni 2005; Lamberti and Gregory 2007). This certainly influences the diet of Triportheus albus, since Cella-Ribeiro et al. (2017) reported that terrestrial arthropods correspond to 56.39% of the animal protein for this species. Therefore, a plant-based diet can result in lower THg levels in omnivorous fish.
To test if the relationship between Hg and size varies over the seasons, we performed linear regressions with the data from the four seasons (Table 4). Weak positive relationships between size and THg (Table 4) were observed in the detritivorous guild (RW and LW) and a stronger positive relationship was observed in the herbivorous guild during RW and FW. Omnivorous and herbivorous guilds showed negative relationships in RW and HW, respectively (Table 4). The results showed no clear trend for relationship between size and THg levels among seasons. However, only during RW was the relationship significant for all guilds. Although it is well established that THg concentration increases with fish size (Kraepiel et al. 2003; Campbell et al. 2010; Bosch et al. 2016), Roulet and Maury-Brachet (2001) observed that significant relationships between size and THg concentration in Amazonian fish is rare. In agreement with this author Bastos et al. (2008) reported a non-significant relationship between size and THg in 86 species from the Madeira River Basin during the dry and rainy seasons.
In general, the capture of migratory species in the Amazon region is higher during the RW and HW (Freitas et al. 2002). Therefore, increases in THg level during these seasons may result in a higher risk of exposure to fish consumers. In the Brazilian Amazon, preference for fish among the population is 70.7%, against 14.7% and 12.2% for beef and chicken, respectively (Lopes et al. 2016). The study of Oliveira et al. (2010) showed that the fish eating among the people living near Puruzinho Lake was 406 g day−1, which is very high. It is important to advise consumers, especially those who consume fish often, to have a more mixed diet during the rainy seasons due to higher levels of THg.
We recognize that Hg exposure to consumers can be influenced by which species is consumed. However, THg concentration among most species (Table 3) was in same order of magnitude. The only exception was the herbivore Mylossoma duriventre, which showed lower mean THg levels (Table 3). This suggests that risk of exposure to THg by intake of most of the detritivorous, omnivorous and herbivorous species of Puruzinho Lake is similar.
Conclusions
THg concentration in detritivorous, herbivorous and omnivorous fish was influenced by the flood pulse. In detritivorous fish, THg levels were higher during Rising Water, decreased in high water and falling water and increased again in low water. Herbivorous fish showed a similar pattern, with higher concentration of THg in Rising Water and lower concentration in high water and falling water. We suggest that this pattern is explained by the strong influence of the “white waters” from the Madeira River that invade Puruzinho Lake during high water and falling water. Also, phytoplankton THg concentration is higher during the Rising Water season in comparison with other seasons. When the seasons were grouped in rainy (Rising Water and high water) and dry (falling water and low water) seasons, THg concentration were higher during the rainy season in detritivorous, herbivorous and omnivorous fish.
References
Agência Nacional de Águas (ANA) (2019): Rede Hidrometeorológica Nacional, Sistema HIDRO - Telemetria, Estação Humaitá, Código 15630000. http://www.snirh.gov.br/gestorpcd/gerarGrafico.aspx
Agency for Toxic Substances and Disease Registry (ATSDR) (2017) U.S. Department of Health and Human Services. Priority list of hazardous substances. Digital report. https://www.atsdr.cdc.gov/spl/. Accessed 4 Dec 2017
Almeida R, Bernardi JVE, Oliveira RC, Carvalho DP, Manzatto AG, Lacerda LD, Bastos WR (2014) Flood pulse and spatial dynamics of Mercury in sediments in Puruzinho Lake, Brazilian Amazon. Acta Amazonica 44:99–106
Araujo-Lima CARM, Forsberg BR, Victoria R, Martinelli LA (1986) Energy sources for detritivorous fishes in the Amazon. Science 234:1256–1258
Bastos WR, Malm O, Pfeifer WC, Cleary D (1998) Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon, Brazil. Rev. Ciên. Cult. 50:255–260
Bastos WR, Rebelo MF, Fonseca MF, Almeida R, Malm O (2008) A description of mercury in fishes from the Madeira River Basin, Amazon, Brazil. Acta Amazonica 38:431–438
Batley GE (1983) The current status of trace element speciation studies in natural waters. In: Leppard GG (Ed.) Trace element speciation in surface waters and its ecological implications. Plenum, New York, NY, pp. 17–36
Bernhoft RA (2011) Mercury toxicity and treatment: a review of literature. J Environ Public Health 2012:460508
Bosch AC, O’Neill B, Sigge GO, Kerwath SE, Hoffman LC (2016) Mercury accumulation in Yellowfin tuna (Thunnus albacares) with regards to muscle type, muscle position and fish size. Food Chem 190:351–356
Brito BC, Forsberg BR, Kasper D, Amaral JHF, Vasconcelos MRR, Sousa OP, Cunha FAG, Bastos WR (2017) The influence of inundation and lake morphometry on the dynamics of mercury in the water and plankton in an Amazon floodplain lake. Hydrobiologia 790:35–48
Campbell LM, Balirwa J, Dixon D, Hecky R (2010) Biomagnification of mercury in fish from Thruston Bay, Napoleon Gulf, Lake Victoria (East Africa). African J Aquatic Sci 29:91–96
Carroll RWH, Memmott J, Warwick JJ, Fritsen CH, Bonzongo JC, Acharya K (2011) Temporal variation of mercury associated with different phytoplankton size fractions in Lahontan Reservoir, Nevada. Water Air Soil Pollut 217:221–232
Cella-Ribeiro A, Torrente-Vilara G, Lima-Filho JA, Doria CRC (2017) Ecologia e Biologia de peixes do Rio Madeira. EDUFRO, Porto Velho
Chen CY, Kamman N, Williams J, Bugge D, Taylor V, Jackson B, Miller E (2012) Spatial and temporal variation in mercury bioaccumulation by zooplankton in Lake Champlain (North America). Environ Pollut 161:343–349
Correa SB, Crampton WGR, Chapman LJ, Albert JS (2008) A comparison of flooded forest and floating meadow fish assemblages in an upper Amazon floodplain. J Fish Biol 72:1–16
Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen T, Kamman NC, Munson R (2007) Mercury contamination in remote forest and aquatic ecosystems in the Northeastern U.S.: sources, transformations and management options. Bioscience 57:17–28
Durães R, Pompeu PS, Godinho AAL (2001) Alimentação de quatro espécies de Leporinus (Characiformes, Anostomidae) durante a formação de um reservatório no sudeste do Brasil. Iheringia, Sér.Zool., Porto Alegre 90:183–191
Fadini PS, Jardim WF (2001) Is the Negro River Basin (Amazon) impacted naturally occurring mercury? Sci Total Environ 275:71–82
Forsberg BR, Araujo-Lima CARM, Martinelli LA, Victoria RL, Bonassi JA (1993) Autotrophic carbon sources for fish of the central amazona. Ecology 74:643–652
Freitas CEC, Batista VS, Inhamuns AJ (2002) Strategies of small-scale fisheries on the Central Amazon floodplain. Acta Amazonica 32:1–7
Ikingura JR, Akagi H (2003) Total mercury and methylmercury levels in fish from hydroelectric reservoirs in Tanzania. Sci Total Environ 304:355–368
INMET, Instituto Nacional de Meteorologia, 2018. http://www.inmet.gov.br. Accessed Dec 2018
Kasper D, Forsberg BR, Amaral JHF, Py-Daniel SS, Bastos WR, Malm O (2017) Methylmercury modulation in Amazon rivers linked to basin characteristics and seasonal flood-pulse. Environ Sci Technol 51:14182–14191
King JK, Saunders FM, Lee RF, Jahnke RA (1999) Coupling mercury methylation rates to sulfate reduction rates in marine sediments. Environmental Toxicology. Chemistry 18:1362–1369
Kraepiel AML, Keller K, Chin HB, Malcolm EG, Morel FMM (2003) Sources and variations of mercury in tuna. Environ Sci Technol 37:5551–5558
Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM (2013) Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ Sci Technol 47:13385–13394
Lamberti GA, Gregory SV (2007) CPOM transport, retention, and measurement. In:Hauer FR, Lamberti GA (eds.) Methods in stream ecology. 2nd edn. Academic Press, San Diego, p. 273–289
Lopes IG, Oliveira RG, Ramos FM (2016) Perfil do consumo de peixe da população brasileira. Biota Amazônia 6:62–65
Maia PD, Maurice L, Tessier E, Amouroux D, Cossa D, Pérez M, Moreira-Turcq P, Rhéault I (2009) Mercury distribution and exchanges between the Amazon river and connected floodplain lakes. Sci Total Environ 407:6073–6084
Maia PD, Maurice L, Tessier E, Amouroux D, Cossa D, Moreira-Turcq P, Etcheber H (2018) Role of the floodplain lakes in the methylmercury distribution and exchanges with the Amazon River, Brazil. J Environ Sci 68:24–40
Malm O (1998) Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ Res 77:73–78
Marçal-Simabuku MAe, Peret AC (2002) Alimentação de peixes (Osteichthyes, Characiformes) em duas Lagoas de uma Planíce de Inundação brasileira da Bacia do rio Paraná. Interciência 27:299–306
Melack J, Novo EMLM, Forsberg BR, Piedade MTF, Maurice L (2009) Floodplain ecosystem processes. In: Keller M, Bustamante M, Gash J, Silva PD (eds.). Amazonia and global change. American Geophysical Union, Washington, D.C., USA, pp. 525–541
Nascimento EL, Gomes JPO, Almeida R, Bastos WR, Bernardi JVE, Miyai RK (2006) Mercurio no plâncton de um lago natural amazônico, Lago Puruzinho (Brasil). J Braz Soc Ecotoxicol 1:1–6
Northcote TG, Pick FR, Fillion DB, Salter SP (2009) Interaction of nutrients and turbidity in the control of phytoplankton in a large Western Canadian Lake prior to major watershed impoundments. Lake Reserv Manage 21:261–276
Oliveira RC, Dórea JG, Bernardi JVE, Bastos WR, Almeida R, Manzatto AG (2010) Annals Human Biol 37:629–642
Pestana IA, Almeida MG, Bastos WR, Souza CMM (2018) Total Hg and methylmercury dynamics in a river-floodplain system in the Western Amazon: influence of the seasonality, organic matter and physical and chemical parameters. Sci Total Environ 656:388–399
Pickhardt PC, Fisher NS (2007) Accumulation of inorganic and methylmercury by freshwater phyto plankton in two contrasting water bodies. Environ Sci Technol 41:125–131
Queiroz MMA, Horbe AMC, Moura CAV (2011) Mineralogia e química dos sedimentos de fundo do médio e baixo Madeira e de seus principais tributários e Amazonas e Brasil. Acta Amazonica 41:453–464
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for statistical computing, Austria, Vienna, http://www.R-project.org/
Rezende CF, Mazzoni R (2005) Seasonal variation in the input of allochthonous matter in an Atlantic Rain Forest stream, Ilha Grande- RJ. Acta Limnologica Brasiliensia 17:167–175
Roulet M, Lucotte M, Saint-Aubin A, Tran S, Rhéault I, Farella N, Silva EJ, Dezencourt J, Sousa Passos CJ, Guimarães JRD, Mergler D, Amorim M (1988) The geochemistry of mercury in 43 central Amazonian soils developed on the Alter-do-Chão formation of the lower Tapajos River Valley, Para State, Brazil. Sci Total Environ 223:1–24
Roulet M, Lucotte M, Guimarães JRD, Rheault I (2000) Methylmercury in water, seston, and epiphyton of an Amazonian river and its floodplain, Tapajós River, Brazil. Sci Total Environ 261:43–59
Roulet M, Lucotte M, Canuel R, Farella N, Goch YGF, Peleja JRP, Guimarães JRD, Mergler D, Amorim M (2001) Spatio-temporal geochemistry of mercury in waters of the Tapajós and Amazon rivers, Brazil. Limnol Oceanogr 46:1141–1157
Roulet M, Maury-Brachet R (2001) Le mercure dans les organisms aquatiques amazoniens. In: Carmouse JP, Lucotte M, Boudou A (eds.) Le mercure en Amazonie. Rôle de L' homme et de L'environment, risques sanitaries. IRD editions, Paris, p. 494
Sahoo PK, Guimarães JTF, Souza-Filho PWM, Silva MS, Silva Junior RO, Pessim G, Moraes BC, Pessoa PFP, Rodrigues TM, Costa MF, Dall’Agnol R (2016) Influence of seasonal variation on the hydro-biogeochemical characteristics of two upland lakes of the southeastern Amazon, Brazil. Anais da Academia Brasileira de Ciências 88:2211–2227
Santos GM (1982) Caracterização, hábitos alimentares e reprodutivos de quatro espécies de “aracus” e considerações ecológicas sobre o grupo no lago Janaucá-AM (Osteychthyes, Characoidei, Anostomidae). Acta Amazonica 12:713–739
Santos GM, Santos ACM (2005) Sustentabilidade da pesca na Amazônia. Estudos Avançados 54:165–182
Sccuder-Eikenberry BC, Riva-Murray K, Knightes CD, Journey CA, Chasar LC, Brigham ME, Bradley PM (2015) Optimizing fish sampling for fish–mercury bioaccumulation factors. Chemosphere 135:467–473
Signa G, Mazzola A, Tramati CD, Vizzini S (2017) Diet and habitat use influence Hg and Cd transfer to fish and consequent biomagnification in a highly contaminated area: Augusta Bay (Mediterranean Sea). Environ Pollut 230:394–404
Trudel M, Rasmussen JB (1997) Modeling the elimination of mercury. Environ Sci Technol 31:1716–1722
Venables WN, Ripley BD (2002) Modern Applied Statistics with S., 4th edn. Springer, New York, NY, https://doi.org/10.1007/978-0-387-21706-2
Vieira M, Bernardi JVE, Dórea JG, Rocha BCP, Ribeiro R, Zara LF (2018) Distribution and availability of mercury and methylmercury in different waters from the Rio Madeira Basin, Amazon. Environ Pollut 235:771–779
Watras CJ, Bloom NS (1992) Mercury and methylmercury in individual zooplankton: implications for bioaccumulation. Limnol. Oceanogr. 37:1313–1318
Watras CJ, Morrison KA, Host J, Bloom NS (1995) Concentration of mercury species in relation to other site-specific factors in the surface waters of northern Wisconsin lakes. Limnol. Oceanogr. 40:556–565
Wissmar RC, Richey JE, Stallard RF, Edmond JM (1981) Plankton metabolism and carbon processes in the Amazon River, its tributaries, and floodplain waters, Peru- Brazil, May-June 1977. Ecology 62:1622–1633
Acknowledgements
This work was supported by the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through CNPq/CT-Universal project (Grant no. 458977/2014-4). We are grateful to IBAMA (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis) for the fish collection license (DIFAP/IBAMA no. 091). We extend special thanks to the fisherman Raimundo Nonato dos Santos. This study was also financed in part by Coordenação de Aperfeiçoamento de Pessoa de Nível Superior – Brazil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable national guidelines for the care and use of animals were followed. Fish sampling was approved by Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) (license: DIFAP/IBAMA no. 091).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azevedo, L.S., Pestana, I.A., Nery, A.F.d.C. et al. Influence of the flood pulse on mercury accumulation in detritivorous, herbivorous and omnivorous fish in Brazilian Amazonia. Ecotoxicology 28, 478–485 (2019). https://doi.org/10.1007/s10646-019-02044-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02044-y