Abstract
Aquatic ecosystems in the Amazon are exposed to mercury, mostly from natural sources. Hg accumulation in fish tissues poses a risk to the local population since fish is one of the main sources of protein in the region. The aim of this study was to evaluate Hg distribution in demersal and pelagic carnivorous fish between seasons in Puruzinho Lake in the Brazilian Amazon. Total Hg was quantified in 221 individuals of 8 species obtained during the high water and low water seasons. Two-way ANOVA indicated an interaction between foraging habitat and season. During high water, total Hg concentrations were similar between demersal and pelagic fish, while in low water, total Hg levels were higher in demersal fish. Pelagic and demersal fishes’ Hg levels were similar between the two seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a toxic metal released in the environment by anthropogenic sources (e.g., fossil fuel and coal combustion) and natural sources (e.g., atmospheric deposition, weathering). Although anthropogenic emission of Hg in the Brazilian Amazon has been as high as 2000 metric tons due to gold mining (Malm 1998), the study of Roulet et al. (1998) confirmed that natural sources of Hg are more important because Amazonian soils are enriched with this toxic metal on a geological scale.
Human exposure to Hg occurs mainly through food intake, especially of fish. Most of the Hg accumulated in the muscle of fish is in its most toxic form, methylmercury, which increases the risk to humans and biota in general (Hong et al. 2012). Due to its slow excretion rate and high affinity for thiol groups of proteins in fish muscle, methylmercury represents more than 85% of accumulated Hg species (Bloom 1992; Redmayne et al. 2000; Kehrig et al. 2008; Martorell et al. 2011; Burger et al. 2013; Paiva et al. 2017). Freshwater fish are one of the most important sources of animal protein in the Amazon (Freitas and Rivas 2006). For instance, fish consumption by the Puruzinho Lake riparian community (Madeira River Basin) is 406 g per capita day−1 (Oliveira et al. 2010), which is one order of magnitude higher than the national average (11 g per capita day−1) (Avegliano et al. 2015). The freshwater fish catch in the Amazon is the highest in the country, with an average of 280,000 tons per year (MPA 2009).
Hg accumulation in fish is influenced by several factors, like water pH, dissolved organic carbon and primary productivity (Sorensen et al. 2005; Brown et al. 2010; Poste et al. 2015). Vieira et al. (2018) reported that in Amazonian black water ecosystems, which have acid pH and high concentration of dissolved organic matter, methylmercury levels are higher in biotic and abiotic matrices in comparison with other aquatic ecosystems due to higher methylation rates. Several studies have indicated that higher levels of methylation of inorganic Hg are related to acidic environments (Ramlal et al. 1986; Bloom et al. 1991; Gilmour and Henry 1991; Spry and Wiener 1991; Ullrich et al. 2001). In contrast, high primary productivity has been related to low concentrations of Hg in aquatic biota due to algal biodilution, a process well described by other authors (Chen and Folt 2005; Pickhardt et al. 2005; Chen et al. 2008). The foraging habitat (demersal or pelagic) also plays a role in Hg accumulation (Eagles-Smith et al. 2008). Li and Xie (2016) and Jiang et al. (2018) reported that total Hg concentrations were higher in demersal than in pelagic fish. However, Rocha et al. (2015) observed similar Hg concentrations between the two foraging habitats while Lavoie et al. (2010) reported a stronger biomagnification in the pelagic food chain. From a human health safety standpoint, understanding the influence of foraging habitat on Hg levels is also important because commercial and subsistence fishing may focus on a particular food chain. For instance, commercial fishing in Western Amazonia is focused on the capture of demersal catfishes like Brachyplatystoma vailantii and Pseudoplatystoma filamentosum (Freitas and Rivas 2006).
Fish capture in the Amazon is highly influenced by seasonality. During the high water season (HW), the fish catch is higher in comparison with the low water season (LW) (Freitas et al. 2002). Additionally, consumption of piscivorous fish is higher during the high water season among the riparian population from the upper Madeira River Basin (Maurice-Bourgoin et al. 2000). Individuals from the Amazon region that have a lower intake of fish during the low water season showed lower Hg concentrations in hair (Maurice-Bourgoin et al. 2000). There is evidence that seasonal variation in Hg levels can influence Hg accumulation in humans.
Understanding how Hg levels in fish fluctuate among seasons and foraging habitats in a region where the human population has high fish consumption is crucial. In this context, the aim of this study was to evaluate Hg distribution between pelagic and demersal carnivorous fish in two seasons (high water and low water) from an Amazonian lake.
Material and methods
The study area is Puruzinho Lake (63°6′0″W; 7°24′0″S), located 20 km from the urban region of the municipality of Humaitá, in the Madeira River Basin of Western Amazonia (Amazonas state, Brazil). Puruzinho Lake is a black water lake, which means that it is rich in dissolved organic matter and has acid pH, low primary productivity, and low concentrations of both dissolved elements and suspended particulate matter (Wissmar et al. 1981; Almeida et al. 2014). This lake is heavily influenced by the hydrological regime. During the high water (HW) season, the water column can reach 12.5 m, but in the low water (LW) season this value can drop to 0.30 m (Almeida et al. 2014). The following parameters also vary between seasons: pH (HW: 4.97 ± 0.23; LW: 5.48 ± 0.32); dissolved oxygen (HW: 3.40 ± 0.31 mg L−1; LW: 5.42 ± 0.49 mg L−1); water temperature (HW: 29.80 ± 1.30 °C; LW: 30.90 ± 1.60 °C); and electrical conductivity (HW: 8.36 ± 0.68 mS cm−1; LW: 14.27 ± 0.63 mS cm−1) (Almeida et al. 2014). Total Hg concentrations and organic matter content of bottom sediment in HW, reported by Almeida et al. (2014), were 84.20 ng g−1 dry wt and 7.88%, respectively, while during LW the reported results were 71.20 ng g−1 dry wt and 8.40%.
Sampling campaigns in the HW season occurred in December 2016, February 2017 and April 2017, while in the LW season they occurred in June 2017 and October 2017. Samples were obtained with help of local fisherman. Since the aim of the study was to evaluate the influence of foraging habitats, we considered only species with the same feeding habit. The eight sampled species are carnivorous. Demersal species obtained were Plasgioscion squamosissimus, Calophysus macropterus, Cichla pleiozona and Hoplias malabaricus, while pelagic species obtained were Ageneiosus inermis, Pellona flavipinnis, Pellona castelnaeana and Rhaphiodon vulpinus (Table 1). Information about foraging habitat was retrieved from Froese and Pauly (2019). A total of 221 (N = 221) specimens were caught. Total length (TL) and weight (TW) of specimens were measured after capture. Skinless white dorsal muscle was removed, frozen and transported to the laboratory. Although other tissues like gills, liver and kidney accumulate Hg (Mella et al. 2007; Azevedo et al. 2018), we did not consider these tissues. Keva et al. (2017) reported that, although liver has a higher turnover rate than muscle due to intense metabolic activity, THg concentration in both tissues varies significantly between seasons. Therefore, including liver in our analysis, or other tissues with fast turnover rates like kidney, would be redundant since muscle and liver vary similarly.
We do not measure Puruzinho Lake water level, however the Madeira River flow and water level, measured at the Humaitá station, were available from ANA (2019). As expected, both parameters showed higher values in the HW season (December 2016, February and April 2017) than in LW (June and October 2017). The summarized data about Madeira River flow and water level during December 2016 to October 2017 are presented in Azevedo et al. (2019).
Digestion of aliquots (0.2 g wet wt) of fish muscle samples for total Hg (THg) determination followed the method described by Bastos et al. (1998): (i) addition of 1 mL of 30% H2O2 and 4 mL of 65% HNO3: 98% H2SO4 (1:1); (ii) heating in a Tecnal digestion block (model: TE04/25) for 30 min at 70 °C; (iii) cooling at room temperature; (iv) addition of 5% KMnO4; heating for 15 min; (v) cooling at room temperature overnight; (vi) addition of 12% NH2OH.HCl; and (vii) addition of ultrapure water to a final volume of 12 mL. Measurements were performed by cold vapor atomic absorption spectrometry (CVAAS), using a flow injection mercury system (FIMS-400) from PerkinElmer (Germany). Blanks were used to control the quality of the reaction medium. Samples were analyzed in duplicate to evaluate the precision of the method. Certified material (DORM-2) was analyzed in triplicate at each 30 samples (recuperation: 99 ± 2.5%). Detection limit was 0.007 mg kg−1.
Statistical analyses were performed using the R environment (R Core Team 2018). To compare THg concentrations in pelagic and demersal fish between HW and LW, we conducted a two-way ANOVA. In the first step of this statistical analysis, the interaction term was evaluated. The interaction term, when significant (p < 0.05), indicates that seasonality influences Hg concentrations in fish of different feeding habits differently (e.g., during the transition from LW to HW period, Hg concentrations increase in demersal fish while decreasing in pelagic fish). In this situation, the Tukey post hoc test was performed to compare each combination of seasonality and feeding habits, since a comparison of each of the variables (seasonality and feeding habits) cannot be done individually. Data were transformed to square root to meet ANOVA requirements (linearity, normality and homoscedasticity of the residuals). We normalized concentrations by length, as suggested by Sccuder-Eikenberry et al. (2015), to remove the influence of size, following the formula: THg normalized = THg (concentration/total length). Total length is a better proxy for fish age than weight (Yi and Zhang 2012; Perugini et al. 2014). Therefore, by normalizing data by length the influence of age is also removed. The comparisons were carried out with the normalized data.
Results and discussion
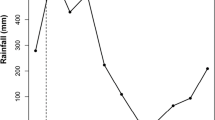
In general, demersal fish were bigger and heavier than pelagic fish (Table 2). The two-way ANOVA indicated interaction between seasons and the foraging habitat (interaction season × foraging habitat: F1,216 = 9.61; p= 0.002). This result suggests that seasonality influences THgNorm accumulation differently between demersal and pelagic fish. THgNorm concentration in demersal and pelagic fish was statistically similar between LW and HW (Tukey post hoc; Demersal: Effect size = 0.01, p= 0.22; Pelagic: Effect size = −0.02, p = 0.07) (Fig. 1). The comparison between demersal and pelagic fish showed that in HW season, THgNorm concentration was similar (Tukey post hoc; Effect size = −0.003, p= 0.97) while during LW season, THgNorm was significantly higher in demersal fish (Tukey post hoc; Effect size = −0.04, p< 0.0001) (Fig. 1).
Comparison of THgNorm concentrations among different demersal and pelagic fish during high water and low water seasons. Different letters indicate statistical differences (α = 0.05). Two-way ANOVA indicated an interaction between season and foraging habitat (interaction season × foraging habitat: F1,216 = 9.61; p= 0.002). There were no statistical differences in THgNorm in demersal and pelagic fish between seasons. THgnorm concentrations were higher in demersal fish compared to Pelagic fish only during low water (p < 0.0001)
Our results disagree with those of Azevedo et al. (2018), who observed higher concentration of THgNorm in fish from the lower Paraiba do Sul River Basin (southeastern Brazil) during a prolonged low water season. However, the hydrological regime in the Amazonian region is drastically different from the one found in southeastern Brazil. Additionally, the increased concentrations reported by Azevedo et al. (2018) were observed during an atypical low water season. In the Amazon region, Marshall et al. (2016) observed higher THg concentration in Paracheirodon axelrodi (Characidae) collected during LW season in the Negro River and Amaro et al. (2014) also observed higher concentration in Brachyplatystoma rousseauxii (Pimelodidae) from a fish market in Belem (Pará state) during LW. Although our results differed from these ones, other authors like Brito et al. (2017) reported that Hg concentration in zooplankton did not vary significantly along the seasons in Janaucá Lake, Solimões River, Amazon.
Seasonal variation of fish Hg concentration can be considered a controversial issue, since many authors have reported the occurrence of this process (Farkas et al. 2003; Murphy et al. 2007; Zhang et al. 2012; Moreno et al. 2015; Azevedo et al. 2018) and others have observed no seasonal variation (Farkas et al. 2000; Foster et al. 2000; Burger et al. 2009). Mills et al. (2018) reported in a meta-analysis that seasonal variation in Hg concentration in fish is not related to waterbody type (e.g., lakes, rivers, reservoirs, etc.), fish size and trophic status. These authors observed that seasonal variation occurs when mean Hg concentration of the fish population increases. In 90% of the studies evaluated by Mills et al. (2018), significant seasonal variation was observed when the mean Hg concentration of the population exceeded 0.30 mg kg−1 (wet weight, non-normalized by fish size). The mean Hg concentrations of the demersal and pelagic fish populations in this study were 0.97 and 0.87 mg kg−1 (wet weight, non-normalized by fish size), respectively, which is almost 3 times higher than the mean 0.30 mg kg−1 suggested by Mills et al. (2018) as an indication of significant seasonal variation. Therefore, our results disagree with their meta-analysis.
It is important to mention that non-significant seasonal variation was observed at the guild level (i.e., pelagic carnivorous and demersal carnivorous). Species were pooled into guilds due to their similar feeding habitat and to increase representativeness. At the species level, only Cichla pleizona (demersal) and Rhaphiodon vulpinus (pelagic) had enough samples in both seasons to test for seasonal variation. The results showed, for both species, non-significant variation (Cichla pleiozona: Tukey post hoc; Effect size = −0.003, p= 0.69; Rhaphionodon vulpinus: Tukey post hoc; Effect size = 0.002, p= 0.81), which is consistent with the guild level results. However, more data are necessary to test if seasonal variation in THg concentration is species-specific.
The lower THg concentration in pelagic fish during the LW period may be derived from higher competition with demersal fish for food resources. Several studies have reported that competition for resources among fish is more intense during the low water season (Matthews 1998; Schlosser et al. 2000; Wantzen et al. 2002; Costa-Pereira et al. 2017). It is well established that feeding is the main pathway of fish exposure to THg. Therefore, an increase in competition for food could reduce the uptake of THg for the weakest competitor. During the LW, pelagic fish have to compete for resources with an environmentally more fit group (i.e., demersal fish), which may explain the lower THg concentration (Fig. 1).
During HW period, when the water column reaches approximately 8–12.5 m (Almeida et al. 2014), the inflow of resources to the Puruzinho Lake is higher, which may reduce the interspecific competition pressure (Costa-Pereira et al. 2017). Under these conditions, THg concentrations between pelagic and demersal fish were statistically similar (Tukey post hoc; Effect size = −0.003, p= 0.97) (Fig. 1). In contrast, during the LW, when the water column decreases to approximately 0.30–2.3 m (Almeida et al. 2014), interspecific pressure increased and THg concentrations were statistically higher (Tukey post hoc; Effect size = −0.04, p< 0.0001) (Fig. 1) in demersal than in pelagic fish, which suggests that the former group, in shallow water column conditions, is more exposed to the contaminant.
Besides ecological factors, geochemical features, like water chemistry, sediment resuspension and methylation rate, may also have contributed to the observed results. In black water ecosystems, dissolved organic carbon (DOC) plays an important role in Hg mobility and speciation (Silva et al. 2009). For instance, in LW season, when there is no input of fresh organic carbon from the drainage basin, black water ecosystems create a sunlight oxidative barrier that prevents the formation of dissolved gaseous mercury (DGM), thus reducing Hg export to the atmosphere, and degrades part of the dissolved methylmercury (i.e., photodemethylation) (Silva et al. 2009). Although methylmercury photodemethylation is higher during LW season, Bisinoti et al. (2007) observed higher levels of dissolved THg and methylmercury in the Negro River Basin during the LW season. Additionally, Bisinoti et al. (2007) also observed a negative relationship between THg and methylmercury and water level, indicating that the concentrations of these contaminants are lower in HW season due to dilution. Based on our results, the increase in THg and methylmercury availability during LW influences demersal fish more than pelagic fish.
Although Hg methylation and accumulation are distinct processes, increases in the methylation rate can influence THg accumulation in fish (Gabriel et al. 2014). For instance, Marusczak et al. (2011) attributed the low concentrations of Hg in arctic charr to low methylation rates. Higher methylation rates increase the concentration of bioavailable Hg in aquatic environments (Marvin-DiPasquale et al. 2003; Hammerschmidt and Fitzgerald 2004; Hollweg et al. 2010), which in turn influence all the links of the food chain (Lacerda and Malm 2008). It is possible that increases in mercury methylation rates during LW season cannot explain higher levels of THg in demersal fish. Almeida et al. (2014) observed that during this season in Puruzinho Lake, pH became less acid (HW: 4.97 ± 0.23; LW: 5.48 ± 0.32) and dissolved oxygen levels increased (HW: 3.40 ± 0.31 mg L−1; LW: 5.42 ± 0.49 mg L−1). Therefore, the better conditions for higher methylation rate are present in HW instead of LW season.
By definition, demersal fish have close contact with bottom sediment, and Almeida et al. (2014) observed higher remobilization of THg associated with the bottom sediment during the LW season. Additionally, Mason et al. (2003) and Kalnejais et al. (2007) reported that sediment resuspension is an important source of metals in the water column. Therefore, the higher THg concentrations in demersal than in pelagic fish during LW may be related to this process.
Conclusions
Although the Amazon is considered a hotspot for Hg and several studies have evaluated the element’s concentrations in fish, this is to our knowledge the first study to investigate the variation in Hg accumulation between foraging habitats. Our results indicate that the interaction between seasonality and foraging habitat can influence THg concentration in demersal and pelagic fish. During LW, demersal species showed higher levels of THg than pelagic species. In the HW season, THg concentrations were similar between the two foraging habitats. Significant seasonal variation in THg was not observed. This study provides evidence that foraging habitat is a factor able to influence Hg accumulation in carnivorous fish during the low water season.
References
Agência Nacional de Águas (ANA) (2019) Rede Hidrometeorológica Nacional. Sistema HIDRO—Telemetria, Estação Humaitá, Código. http://www.snirh.gov.br/gestorpcd/gerarGrafico.aspx. 15630000.
Almeida R, Bernardi JVE, Oliveira RC, Carvalho DP, Manzatto AG, Lacerda LD, Bastos WR (2014) Flood pulse and spatial dynamics of Mercury in sediments in Puruzinho lake, Brazilian Amazon. Acta Amaz 44:99–106
Amaro CSO, Junior DR, Silva MCF, Lima AAS, Santos GFS, Pinheiro MCN (2014) Total mercury (Hg-T) concentration in marketable fish in different seasonal periods at Ver-o-Peso Market, Belém, Pará State, Brazil. Rev Pan Amaz Saúde 5:53–60
Avegliano RP, Maihara VA, Silva FF (2015) Development of the food list for Brazilian total diet study. Food Sci Technol 35:207–212
Azevedo LS, Pestana IA, Rocha ARM, Meneguelli-Souza AC, Lima CAI, Almeida MG, Bastos WR, Souza CMM (2018) Drought promotes increases in total Mercury and methylmercury concentrations in fish from the lower Paraíba do Sul river, southeastern Brazil. Chemosphere 202:483–490
Azevedo LS, Pestana IA, Nery AFC, Bastos WR, Souza CMM (2019) Influence of the flood pulse on mercury accumulation in detritivorous, herbivorous and omnivorous fish in Brazilian Amazonia. Ecotoxicology 28:478–485
Bastos WR, Malm O, Pfeifer WC, Cleary D (1998) Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon, Brazil. Rev Ciên Cult 50:255–260
Bisinoti MC, Júnior ES, Jardim WF (2007) Seasonal behavior of mercury species in waters and sediments from the Negro River Basin, Amazon, Brazil. J Braz Chem Soc 18:544–553
Bloom NS, Watras CJ, Hurley JP (1991) Impact of acidification on the methylmercury cycle of remote seepage lakes. Water Air Soil Pollut 56:477–491
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017
Brito BC, Forsberg BR, Kasper D, Amaral JHF, Vasconcelos MRR, Sousa OP, Cunha FAG, Bastos WR (2017) The influence of inundation and lake morphometry on the dynamics of mercury in the water and plankton in an Amazon floodplain lake. Hydrobiologia 790:35–48
Brown D, Goncharov A, Paul E, Simonin H, Carpenter DO (2010) The relationship between Adirondack Lake pH and levels of mercury in yellow perch. J Aquat Anim Health 22:280–290
Burger J, Jeitner C, Donio M, Shukla S, Gochfeld M (2009) Factors affecting mercury and selenium levels in New Jersey flatfish: low risk to human consumers. J Toxicol Environ Health 72:853–860
Burger J, Gochfeld M, Jeitner C, Donio M, Pittfield T (2013) Sushi consumption rates and mercury levels in sushi: ethnic and demographic differences in exposure. J Risk Res 16:1057–1075
Chen CY, Pickhardt PC, Xu MQ, Folt CL (2008) Mercury and arsenic bioaccumulation and eutrophication in Baiyangdian Lake, China. Water Air Soil Pollut 190:115–127
Chen CY, Folt CL (2005) High plankton densities reduce mercury biomagnification. Environ Sci Technol 39:115–121
Costa-Pereira R, Tavares LER, Camargo PB, Araújo MS (2017) Seasonal population and individual niche dynamics in a tetra fish in the Pantanal wetlands. Biotropica 49:531–538
Eagles-Smith CA, Suchanek TH, Colwell AE, Anderson NL (2008) Mercury trophic transfer in a eutrophic lake: the importance of habitat-specific foraging. Ecol Appl 18:196–212
Farkas A, Salánki J, Varanka I (2000) Heavy metal concentrations in fish of Lake Balaton. Lakes Reserv 5:271–279
Farkas A, Salánki J, Specziár A (2003) Age- and size-specific patterns oh heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res 37:959–964
Foster EP, Drake DL, DiDomenico G (2000) Seasonal changes and tissue distribution of mercury in largemouth bass (Micropterus salmoides) from Dorena Reservoir, Oregon. Arch Environ Contam Toxicol 38:78–82
Freitas CEC, Batista VS, Inhamuns AJ (2002) Strategies of small-scale fisheries on the Central Amazon floodplain. Acta Amaz 32:1–7
Freitas CEC, Rivas AAF (2006) A pesca e recursos pesqueiros na Amazônia ocidental. Ciência e Cult 58:30–32
Froese R, Pauly D (eds) (2019). World Wide Web electronic publication. FishBase. www.fishbase.org, version (04/2019)
Gabriel MC, Howard N, Osborne TZ (2014) Fish mercury and surface water sulfate relationships in the Everglades Protection Area. Environ Manag 53:583–593
Gilmour CC, Henry EA (1991) Mercury methylation in aquatic systems affected by acid deposition. Environ Pollut 71:131–169
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol 38:1487–1495
Hollweg TA, Gilmour CC, Mason RP (2010) Mercury and methylmercury cylcing in sediments of the mid-Atlantic continent and slope. Limnol Oceanogr 55:2708–2722
Hong YS, Kim YM, Lee KE (2012) Methylmercury exposure and health effects. J Prev Med Public Health 63:257–264
Jiang Z, Xu N, Liu B, Zhou L, Wang J, Wang C, Dai B, Xiong W (2018) Metal concentrations and risk assessment in water, sediment and economic fish species with various habitat preferences and trophic guilds from Lake Caizi, Southeast China. Ecotoxicol Environ Saf 157:1–8
Kalnejais LH, Martin WR, Signell RP, Bothner MH (2007) Role of sediment resuspension in the remobilization of particulate-phase metals from coastal sediments. Environ Sci Technol 41:2282–2288
Kehrig HA, Howard BM, Malm O (2008) Methylmercury in a predatory fish (Cichla spp.) inhabiting the Brazilian Amazon. Environ Pollut 154:68–76
Keva O, Hayden B, Harrod C, Kahilainen KK (2017) Total mercury concentrations in liver and muscle of European whitefish (Coregonus lavaretus (L.)) in a subarctic lake—assessing the factors driving year-round variation. Environ Pollut 231:1518–1528
Lacerda LD, Malm O (2008) Mercury contamination in aquatic ecosystems: an analysis of the critical areas. Estud Av 22:173–190
Lavoie RA, Hebert CE, Rail JF, Braune BM, Yumvihoze E, Hill LG, Lean DRS (2010) Trophic structure and mercury distribution in a gulf of St. Lawrence (Canada) food web using stable isotope analysis. Sci Total Environ 408:5529–5539
Li J, Xie X (2016) Heavy metal concentrations in fish species from three Gorges Reservoir, China, after impoundment. Bull Environ Contam Toxicol 96:616–621
Malm O (1998) Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ Res 77:73–78
Marshall BG, Forsberg BR, Thomé-Souza M, Peleja R, Moreira MZ, Freitas CEC (2016) Evidence of mercury biomagnification in the food chain of the cardinal tetra Paracheirodon axelrodi (Osteichthyes: Characidae) in the Rio Negro, central Amazon, Brazil. J Fish Biol 89:220–240
Martorell I, Perello G, Martí-Cid R, Llobet JM, Castell V, Domingo JL (2011) Human exposure to arsenic, cadmium, mercury, and lead from foods in Catalonia, Spain: temporal trend. Biol Trace Elem Res 142:309–322
Marusczak N, Larose C, Dommergue A, Paquet S, Beaulne JS, Maury-Brachet R, Lucotte M, Nedjai R, Ferrari CP (2011) Mercury and methylmercury concentrations in high altitude lakes and fish (Arctic charr) from the French Alps related to watershed characteristics. Sci Total Environ 409:1909–1915
Marvin-DiPasquale M, Agee JG, Bouse R, Jaffe B (2003) Microbial cycling of mercury in contaminated pelagic and wetland sediments of San Pablo Bay, California. Environ Geol 43:260–267
Mason R, Kim EH, Porter E, Soulen H (2003) The impact of sediment resuspension on mercury and methylmercury fate, transport and bioaccumulation is shallow estuaries. Geochim Cosmochim Acta 67:274–274
Matthews WJ (1998) Patterns in freshwater fish ecology. Chapman & Hall, New York, NY
Maurice-Bourgoin L, Quiroga I, Chincheros J, Courau P (2000) Mercury distribution in waters and fishes of the upper Madeira rivers and mercury exposure in riparian Amazonian populations. Sci Total Environ 260:73–86
Mella M, Randi MAF, Ventura DF, Carvalho CEV, Pelletier E, Oliveira Ribeiro CA (2007) Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicol Environ Saf 68:426–435
Mills N, Cashatt D, Weber MJ, Pierce CL (2018) A case study and meta-analysis of seasonal variation in fish mercury concentrations. Ecotoxicology 27:641–649
Moreno CE, Fjeld E, Deshar MK, Lydersen E (2015) Seasonal variation of mercury and δ15N in fish from lake Heddalsvatn, southern Norway. J Limnol 74:21–30
MPA—Ministério da Pesca e Agricultura (Ministry of Fishery and Agriculture) (2009) Amazônia, Aquicultura e Pesca. Plano de Desenvolvimento Sustentável. Ministry of Fishery and Agriculture. Extracted from www.agricultura.gov.br/assuntos/aquicultura-e-pesca
Murphy GW, Newcomb TJ, Orth DJ (2007) Sexual and seasonal variations of mercury in smallmouth bass. J Freshw Ecol 22:35–143
Oliveira RC, Dórea JG, Bernardi JVE, Bastos WR, Almeida R, Manzatto AG (2010) Fish consumption by traditional subsistence villagers of the Rio Madeira (Amazon): impact on hair mercury. Ann Hum Biol 37:629–642
Paiva EL, Milani RF, Boer BS, Quintae KD, Morgano MA (2017) Methylmercury in fish species used in preparing sashimi: a case study in Brazil. Food Control 80:104–112
Perugini M, Visciano P, Manera M, Zaccaroni A, Olivieri V, Amorena M (2014) Heavy metal (As, Cd, Hg, Pb, Cu, Zn, Se) concentrations in muscle and bone of four commercial fish caught in the central Adriatic Sea, Italy. Environ Monit Assess 186:2205–2213
Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD (2005) Impacts of zooplankton composition and algal enrichment on theaccumulation of mercury in an experimental freshwater food web. Sci Total Environ 339:89–101
Poste AE, Muir DCG, Guildford SJ, Hecky RE (2015) Bioaccumulation and biomagnification of mercury in African lakes: the importance of trophic status. Sci Total Environ 506-507:126–136
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Austria, Vienna. http://www.R-project.org/
Ramlal PS, Rudd JWM, Hecky RE (1986) Methods for measuring specific rates of mercury methylation and degradation and their use in determining factors controlling net rates of mercury methylation. Appl Environ Microbiol 51:110–114
Redmayne AC, Kim JP, Closs GP, Hunter KA (2000) Methyl mercury bioaccumulation in long-finned eels, Anguilla dieffenbachii, from three rivers in Otago, New Zealand. Sci Total Environ 262:37–47
Rocha ARM, Di Beneditto APM, Pestana IA, Souza CMM (2015) Isotopic profile and Mercury concentration in fish of the lower portion of the rio Paraíba do Sul watershed, southeastern Brazil. Neotrop Ichthyol 13:723–732
Roulet M, Lucotte M, Saint-Aubin A, Tran S, Rheault I, Farella N, Silva EJ, Dezencourt J, Passos CJS, Soares GS, Guimarães JRD, Mergler D, Amorim M (1998) The geochemistry of Hg in Central Amazonian soils developed on the Alter-do-Chao formation of the lower Tapajos river valley, Para State, Brazil. Sci Total Environ 223:1–24
Schlosser IJ, Johnson JD, Knotek WL, Lapinska M (2000) Climate variability and size-structured interactions among juvenile fish along a lake-stream gradient. Ecol 81:1046–1057
Sccuder-Eikenberry BC, Riva-Murray K, Knightes CD, Journey CA, Chasar LC, Brigham ME, Bradley PM (2015) Optimizing fish sampling for fish–mercury bioaccumulation factors. Chemosphere 135:467–473
Silva GS, Bisinoti MC, Fadini PS, Margarelli G, Jardim WF, Fostier AH (2009) Major aspects of the mercury cycle in the Negro River Basin, Amazon. J Braz Chem Soc 20:1127–1134
Sorensen JA, Kallemeyn LW, Sydor M (2005) Relationship between mercury accumulation in young-of-the-year yellow perch and water-level fluctuations. Environ Sci Technol 39:9237–9243
Spry DJ, Wiener JG (1991) Metal bioavailability and toxicity to fish in low-alkalinity lakes: a critical review. Environ Pollut 71:243–304
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ SciTechnol 31:241–293
Vieira M, Bernardi JVE, Dórea JG, Rocha BCP, Ribeiro R, Zara LF (2018) Distribution and availability of mercury and methylmercury in different waters from the Rio Madeira Basin, Amazon. Environ Pollut 235:771–779
Wantzen KM, Machado FD, Voss M, Boriss H, Junk WJ (2002) Seasonal isotopic shifts in fish of the Pantanal wetland, Brazil. Aquat Sci 64:239–251
Wissmar RC, Richey JE, Stallard RF, Edmond JM (1981) Plankton metabolism and carbon processes in the Amazon River, its tributaries, and floodplain waters, Peru- Brazil, May-June 1977. Ecology 62:1622–1633
Yi YJ, Zhang SH (2012) The relationships between fish heavy metal concentrations and fish size in the upper and middle reach of Yangtze River. Procedia. Environ Sci 13:1699–1707
Zhang L, Campbell LM, Johnson TB (2012) Seasonal variation in mercury and food web biomagnification in Lake Ontario, Canada. Environ Poll 161:178–184
Acknowledgements
This work was supported by the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through CNPq/CT-Universal project (Grant no. 458977/2014-4). We are grateful to IBAMA (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis) for the fish collection license (DIFAP/IBAMA no. 091). Special thanks go to the fisherman Raimundo Nonato dos Santos (“Leleco”). This study was also financed in part by Coordenação de Aperfeiçoamento de Pessoa de Nível Superior—Brazil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable national guidelines for the care and use of animals were followed. Fish sampling was approved by Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) (license: DIFAP/IBAMA no. 091).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azevedo, L.S., Pestana, I.A., da Costa Nery, A.F. et al. Variation in Hg accumulation between demersal and pelagic fish from Puruzinho Lake, Brazilian Amazon. Ecotoxicology 28, 1143–1149 (2019). https://doi.org/10.1007/s10646-019-02118-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02118-x