Abstract
Selective serotonin re-uptake inhibitors are pharmaceuticals used to treat a range of psychological disorders. They are frequently found in surface waters in populated areas. In recent years, they have been shown to affect the behaviour of various aquatic organisms in a way that can have ecological effects. In this study, we exposed zebrafish of both sexes to nominally 0.00, 0.15 and 1.50 µg L−1 Escitalopram in flow-through tanks for three weeks. Subsequently, ten swimming behaviour parameters were quantified using high-resolution video tracking. There were noticeable gender differences in the behaviour responses to Escitalopram. Female fish exposed to 1.50 µg L−1 Escitalopram had a lower maximum swimming velocity, stopped less often and exhibited increased boldness (reduced thigmotaxis) compared to controls. Male fish exposed to 1.50 µg L−1 had a lower maximum swimming velocity compared to control fish. At the end of exposures, both length and weight of the females exposed to 1.50 µg L−1 Escitalopram were significantly less than the group of control fish. In addition, males exposed to 1.50 µg L−1 Escitalopram were significantly shorter than control fish. The behaviour, weight and body length of the fish exposed to nominally 0.15 µg L−1 was not significantly different from control fish in either sex. The results of this study demonstrate that Escitalopram can affect subtle but ecologically important aspects of fish behaviour and lends further credibility to the assumption that Escitalopram is an environmentally active pharmaceutical.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selective serotonin re-uptake inhibitors (SSRI) are a group of psychotropic, antidepressant pharmaceuticals. They exert their effect by blocking the re-uptake of serotonin (5-HT) into the pre-synaptic nerve ending, thereby prolonging the bioavailability of extracellular serotonin in the synaptic cleft. Because they are effective and have relatively mild side effects, they are the drug of choice in the treatment of depression and a range of other psychological disorders (Vaswani et al. 2003; Stahl et al. 1998). In recent years, the benefits of the SSRI group have been reflected in an increasing use across different European contries (Abbing-Karahahopian et al. 2014). For instance, a Danish study showed, that the per mille of children (5–17 year) who were prescribed a daily SSRI dose rose from 0,1 in 1995 to 3,3 in 2011 (Pottegård et al. 2014). The SSRI popularity are now resulting in environmental registrations and the activity of these anxiolytic drugs in natural environments have just recently started to be elucidated. Prevoius studies have reported, that the SSRIs will bioconcentrate in aquatic wildlife including the fish brain (Grabicova et al. 2014, 2015; Brooks et al. 2005; Schultz et al. 2010; Nakamura et al. 2008), where they can interfere with several 5-HT regulated fish behaviours. 5-HT is an important central neurotransmitter in the regulation of hormonal and neuronal signalling in mammals and other vertebrates. It´s involved in the regulation of appetite, reproduction and several behavioural processes (Santos et al. 2010). Similar functions have been found in fish (Pérez Maceira et al. 2014; Prasad et al. 2015; Winberg and Thörnqvist 2016). Furthermore, the activity of 5-HT plays a wide range of physiological, but different, regulatory roles in many invertebrates (Silva et al. 2015; Santos et al. 2010; Daughton and Ternes 1999). Drugs, like the SSRI´s, that increase the serotonergic tone could have a significant impact on the control of these biological processes.
SSRI drugs share many of the hallmarks of classical pollutants. They are highly lipophilic (Kwon and Armbrust 2008), exhibit poor degradation rates in sewage treatment plants (STPs) (Vasskog et al. 2008; Yuan et al. 2013) and are relatively persistent in the environment (Kwon and Armbrust 2005; Benotti and Brownawell 2009; Lam et al. 2004). Some of them, like Citalopram, bind readily to particles and sedimentation is likely the main route of elimination from the water body. In the sediment, they can remain for decades (Lahti and Oikari 2012), continuously exposing sediment feeding organisms. For those reasons, and because they were designed to alter human behaviour, the SSRIs have attracted attention as environmental pollutants in recent years. Escitalopram, the S-enantiomer of the 1:1 racemate Citalopram, is generally regarded as the most selective SSRI developed so far (Sánchez and Hyttel 1999).
The racemic mixture of S- and R-Citalopram has been detected in STP effluents dominated by household wastewater in concentrations ranging from 9.2 to 720 ng L−1 in Scandinavian countries (Vasskog et al. 2006; Wahlberg et al. 2008; Krog et al. 2015; Vasskog et al. 2008; Fick et al. 2011). Comparable SSRI concentrations in STP effluents have been measuered in other nations around the world (Silva et al. 2015).
Citalopram has been found in Danish and Swedish surface waters in the range between 3–92 and 6,6–210 ng L−1, respectively (Fick et al. 2011; Kragelund et al. 2015). A worst-case scenario is the detection of 76 µg L−1, which constitutes an extreme case from a site 150 m downstream a sewage treatment plant in India, which receives large amounts of wastewater from drug manufacturers (Fick et al. 2009). It has been predicted that the concentration needed in the aquatic environment to reach human therapeutic levels in fish is 141 ng L−1 for Citalopram (Fick et al. 2010). It should be kept in mind however, that although levels of individual SSRIs may be low, STP effluents often contain more than one SSRI resulting in a considerable total SSRI concentration (Schlüsener et al. 2015; Schultz et al. 2010; Vasskog et al. 2008). Some of the SSRI metabolites also retain some SSRI activity (Hiemke and Härtter 2000; Pawlowski et al. 1985), adding to the total SSRI impact.
SSRIs have a wide range of physiological effects on fish. Fluoxetine dissolved in water elevates the risk of embryonic abnormalities in Japanese medaka (Oryzias latipes) in the range between 0.1 and 5.0 µg L−1. At the lowest concentration, it increases the concentration of estradiol in plasma, indicating that the dose-response curve might not be linear (Foran et al. 2004). Fluoxetine at a concentration of 32 µg L−1 also reduces the levels of ovarian 17β-estradiol and the expression of the luteinising hormone receptor and follicle stimulating hormone receptor as well as clutch size in zebrafish (Lister et al. 2009). Effects on the hypothalamus-pituitary-interrenal (HPI) axis are also reported (Kreke and Dietrich 2008). The HPI axis is also known as the stress axis and is likely responsible for many of the reported behavioural effects of SSRI in fish.
From an ecological point of view, behavioural effects are important because they have the potential to alter selective pressures, predation risk, growth and other factors that are important for both short term survival of the individual and long term stability of the population. Many of the documented effects of SSRI on fish behaviour are such that they may have an ecological effect by reducing growth or fitness, decreasing competitive ability or increasing predation risk. For example, Citalopram at a dose of 100 µg kg−1 b.w. reduces aggression in rainbow trout (Lepage et al. 2005) and 705 or 350 μg L−1 of fluoxetine had similar effects in Siamese fighting fish (Betta splendens) (Forsatka et al. 2014). Further, Citalopram at 0.15 and 1.50 μg L−1 significantly suppressed feeding in three-spine sticklebacks (Kellner et al. 2015) and 54 μg L−1 fluoxetine significantly decreased food intake and weight gain in goldfish (Carassius auratus) (Mennigen et al. 2010). Perhaps the most commonly reported consequences of SSRI exposure are anxiolytic effects when confronted with different stressors such as novel environments (Kellner et al. 2016; Egan et al. 2009; Olsén et al. 2014), conspecific alarm pheromone (Barbosa Junior et al. 2012) or predation risk (Weinberger and Klaper 2014).
In the current study, we used an automated tracking system with a high spatial and temporal resolution. We used Escitalopram at nominal concentrations of 0.00 µg L−1, 0.15 and 1.50 µg L−1. The middle concentration is clearly environmentally relevant and found in effluent dominated surface waters. A concentration of 1.50 µg L−1 is higher than normally found in nature, but was included in order to predict effects in worst-case scenarios. We used both sexes in order to increase the ecological relevance of the study and to investigate if any effects are sex dependent. The aim of the study was to gain a more detailed understanding of the behavioural effects of SSRI on fish and the possible consequences for both individual fish and fish populations. To our knowledge, this is the first study addressing the effects of this eutomer of Citalopram, Escitalopram, on fish behaviour.
Materials and methods
Specimens and experimental design
Totally three hundred adult wild type zebrafish (Danio rerio) of both genders were obtained from Credo Fish (Aalborg, Denmark), and transferred to a 100 × 45 × 45 cm (length × width × height) glass stock tank at Institute of Bioscience, Aarhus University, Denmark, filled with approximately 167 L of water. The water was supplied from a header tank containing aerated, demineralized water at 28 °C, mixed with tap water (16:1) and adjusted with NaCl (1.8 g L−1), resulting in a conductivity of 235 µS cm−1. Every second day, at least one third of the water was replaced. At delivery, the fish were about four month old and were acclimatized in the stock tank for three weeks. Fish were fed daily with TetraMin® (Tetra Werke, Melle, Germany) flake fodder in both the stock tank and later in the exposure tanks. The amount of food were daily regulated so that all food was eaten within five min (“the five-minute-rule”) (Lawrence 2007), but not eaten within one min.
Escitalopram oxalate ((S)-1-[3-(Dimethylamino) propyl]-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran-5-carbonitril, oxalate) (CAS 128196-01-0) (purity 99.8%, HPLC analysis with detection at 237 nm) was kindly donated by Lundbeck Pharma A/S, Denmark. A stock solution was prepared one day before exposure by dissolving 107.2 mg Escitalopram oxalate in 1000 mL milli-Q water and stored at 5 °C in darkness. From this stock solution, two 2-L working solutions at 56 and 574 µg L−1, respectively, were prepared. New working solutions were prepared weekly.
Six 47 × 29 × 19 cm glass exposure tanks each received 27 °C header tank water at a flow rate of 48.5 L day−1 in average via a peristaltic pump (Ole Dich Instrument Makers Aps, Hvidovre, Denmark). An outlet with a filter maintained the volume in the exposure tanks at approximately 26 L, resulting in a continuous flow-through system. Every exposure tank was equipped with a heating element set to 27 °C. The oxygen content (96.0 ± 0.42% of air saturation) was as recommended by OECD guideline no. 210 OECD (1992). The pH during exposure was 7.14 ± 0.02. The loads of ammonium, nitrite and nitrate were measured with Tetratest (TetraWerke, Melle, Germany). None exceeded the recommended values of 0.25 mg L−1 ammonium, 0.3 and 25 mg L−1 nitrate, respectively. Water quality, with respect to temperature, oxygen, pH, ammonium, nitrate and nitrite, was satisfying at all times during the experiment and there were no differences between tanks. The light regime during the experiment was 12:12 (light:dark) with a gradual increase in light intensity.
Six exposure tanks constituted the three exposure groups. A programmable peristaltic pump (Ismatec ICP-N, IDEX Health and Science GmbH, Wertheim–Mondfeld, Germany) continually dosed the control water and the Escitalopram working solutions at 128 mL day−1 in average to the inlet water, resulting in nominal tank concentrations at 0.00 (controls), 0.15 and 1.50 µg L−1, respectively. At the start of the three weeks exposure period, 150 preliminary sexed zebrafish were selected from the stock tank and sequentially distributed in the six exposure tanks. A typical male zebrafish has non-visible papilla, a slim body shape, a reddish coloration and a big anal fin with distinct markings. A typical female zebrafish has a large visible urogenital papilla, a round body shape, a bluish coloration and indistinct anal fin coloration (Eaton and Farley 1974; Brion et al. 2004; Nash et al. 2004). First later (see below), after dissection, the true gender could be established. A small surplus of fish allowed for some mortality during the exposure period. The behaviour measurements of each group (at least 20 male and 20 female fish) took two days. Consequently, in order to achieve the same exposure period for the three groups, we inserted a one-day delay between exposures of the groups. Faeces and fodder remains were daily removed from the tanks.
Swimming behaviour measurements
The spontaneous swimming behaviour was quantified for initially sexed 67 males and 64 females. The behaviour test tanks (19.1 × 14.4 × 11.0 cm) contained 6 cm header tank water (1.7 L) at ca. 27 °C. Four zebrafish, two females and two males, were measured simultaneously in individual tanks.
The four test tanks were placed on a sheet of glass 50 cm above diffusely lit white paper (79 lux). The entire setup was enclosed in a metal frame covered with a blackout curtain in order to exclude visual disturbances. When viewed from above, this arrangement resulted in clear silhouettes of the four fish. The scenario was recorded for 30 min. A GigE Vision colour progressive scan (non-interlaced) CCD camera (Leutron Vision, Switzerland) was mounted approximately 50 cm above the test tank. The digital video signal from the camera consisted of a 1024 × 768 pixel image, giving a 0.39 mm spatial resolution of the visual field. The camera was interfaced with a three Gigahertz personal computer. The behavioural measurements were controlled by the MOTIO vision system (Department of Bioscience, University of Aarhus, Denmark). Prior to recording, the interior of each of the four test tanks was framed by software windows (Area Of Interest) plus a 60 × 60 mm software Region Of Interest (ROI) in the centre of the tank. Appropriate ranges of grey-levels, areas, perimeters and form, respectively, corresponding to the fish silhouettes were likewise set in the software. The centroids of the pixel assemblages fulfilling both grey-level and shape criteria were calculated and recorded as the x,y positions of the four fish. The resultant time series of x, y coordinates were stored in a primary data file together with information on the experiment and calibration factors for the conversion of pixel values into millimetres. During the 30-min recording, the images were captured at 16 frames per second (16 Hz). Subsequently, these time-series of fish positions were transformed into series of connected vectors and analysed by the MOTIO software. If the pixel assemblages did not fulfil both grey-level and shape criteria (fish not identified), the latest know position was written to the data file. When visible again, the new position and the last known position were connected with a vector. Of course, this result in a small underestimate of the true path. Our quality criterion was that the fish should be visible for at least 70% of the recording time in order to qualify for further analyses.
The male and female swimming behaviour were evaluated on the basis of the following ten parameters; (1) total swimming distances (m), (2) Mean maximum swimming velocity maintained for 1 sec (mm s−1), (3) average swimming velocity (mm s−1), (4) number of stops, (5) time swimming (s), (6) turning rate per second swum (degrees s−1), (7) turning bias between right (negative degrees s−1) and left turns (positive degrees s−1), (8) time spent in tank centre, ROI (s), (9) swimming distance in ROI (m), (10) number of visits to ROI. Additionally, for each track, frequency distributions of swimming velocities (vector lengths) were extracted from the data file. All velocities between > 1 and 300 mm s−1 were clasified into 20 catagories where each 15 mm s−1 width interval represents the time spent here during the recording period.
Determination of gender
After the 30-min behaviour measurement, each test fish was killed in 0 °C water, lightly dried on filter paper, and placed on the right side under an Olympus SZ 40 dissection microscope (Olympus, Hatagaya, Tokyo, Japan). Hereafter, the length was measured to the nearest mm and the fish was weighed. The Fulton condition factor was calculated as 100 × weight/length3. Finally, under the dissection microscope, the gender was definitively established by examining the appearance of the gonads.
Actual concentrations of Escitalopram in tank water
Water was sampled from the six exposure tanks in the middle of the exposure period. The samples were quick-frozen and stored at −18 °C before analysed by HPLC-MS without any further sample preparation.
Twenty-five µL was injected onto the HPLC column. The HPLC-MS system used consisted of a Hewlett Packard 1100 series chromatograph (Palo Alto, California, USA) equipped with a Quatpump, a degasser, column oven (colcomp), an auto sampler (ALS), and a MSD detector. Data were collected using the HP ChemStaion software, version 6.03 (Palo Alto, California, USA).
The analytical column was a reversed phase Kinetex C18 (Phenomenex, Værløse, Denmark) column (4.6 mm I.D. x 50 mm, 2.6 µm particles). A linear gradient system was applied. Eluent A consisted of 0.1% formic acid in MilliQ water and eluent B 0.1% formic acid in methanol. The gradient was applied from 30% B to 90 % B from 0–4 min, maintained at 90% B for 1 min and then returned to 30% B over 0.5 min. The flow-rate was 0.5 mL/min. The MSD detector was equipped with an electrospray interface and used in positive mode for SIM detection of the mass:m/z = 325.1.
The fragmentor voltage was set to 100 V. The voltage over the capillary was kept at 5000 V. The temperature of the nitrogen drying gas was set to 350 °C with a flow-rate of 9 L/min. The nebulizer gas pressure was 60 psig. The limit of quantification is 0.05 ng/mL.
Statistical analyses
Differences in body weight, length and behaviour were detected using ANOVA. If the ANOVA where data did not comply with normality, logarithmic transformations resulted in normality and ANOVA was carried out. Female-male differences were examined with the t-test. The χ2-test was used for comparison of frequency distributions, presuming that the period between data points precluded correlation. All statistical tests were performed in SPSS 24.0 for Windows (IBM Corporation, New York, USA) except the χ2-tests, which were performed in Excel (Microsoft Office 2016). Data are presented as mean value ± S.E.M. and using a significance level of 0.05.
Results
The actual concentrations of Escitalopram in the 0.15 and 1.50 µg L−1 exposure tanks were 67 and 100% of the nominal concentrations, revealing that the 0.15 µg L−1 concentration was lower than intended. In the text, tables and figures below, the actual concentrations at 0.00, 0.10 and 1.50 µg L−1 are presented. The concentration of Escitalopram in the control tanks were below the limit of detection and was therefore assumed to be zero. There was no mortality in any of the six tanks during the three weeks of exposure.
Body length and weight
In the female zebrafish, body length and body weight were significantly lower after three weeks of exposure to the highest Escitalopram concentration (Fig. 1 and Table 1). In males, the body lengths were also significantly shorter after exposure to Escitalopram at 1.50 µg L−1, and there was a significant weight difference between the two Escitalopram exposure groups (Fig. 1 and Table 1). Mean Fulton condition factor for the females was 2.16 ± 0.07 (n = 19) for the control group, 1.99 ± 0.05 (n = 18) for the 0.10 µg L−1 group and 2.03 ± 0.05 (n = 21) for the 1.50 µg L−1 group. In the male fish, the mean Fulton condition factor was 1.60 ± 0.06 (n = 18) for the control group, 1.70 ± 0.04 (n = 18) for the 0.10 µg L−1 group and 1.72 ± 0.03 (n = 23) for the 1.50 µg L−1 group. There were no statistically effects of treatment on the Fulton condition factor in either females (F = 2.24, p = 0.116) or males (F = 2.30, p = 0.109).
Body lengths (a) and weights (b) in female ( ) and male (
) and male ( ) zebrafish after three weeks exposure to 0, 0.10 and 1.50 µg L−1 Escitalopram. Different letters denote statistically significant differences (ANOVA; Tukey post hoc test) with the significance level at 0.05. Error bars denote SEM. N for the females are, 19, 18, 21 and for the males 18, 18, 23 for the 0, 0.10 and 1.50 µg L−1groups, respectively
) zebrafish after three weeks exposure to 0, 0.10 and 1.50 µg L−1 Escitalopram. Different letters denote statistically significant differences (ANOVA; Tukey post hoc test) with the significance level at 0.05. Error bars denote SEM. N for the females are, 19, 18, 21 and for the males 18, 18, 23 for the 0, 0.10 and 1.50 µg L−1groups, respectively
Swimming behaviour
Of the totally 64 females and 67 males recorded in the test tanks, only 58 females (19, 18 and 21 for the 0.00, 0.10 and 1.50 µg L−1 groups, respectively) and 59 males (18, 18 and 23 for the three concentrations) qualified for further behaviour analyses by visual inspection of displayed tracks and the criterion of at least 70% visibility.
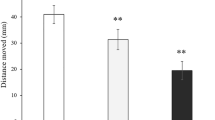
The swimming behaviour in females was more affected by the Escitalopram treatment than in males, at least at the highest Escitalopram concentration. There was a non-significant increase in the females’ swimming distance with increasing Escitalopram concentrations, which was partly due to a slight increase in the average swimming velocity and a significantly lower number of swimming quiescence periods for the fish exposed to 1.50 µg L−1 (Fig. 2a,c; Table 2). In contrast, the maximum velocity, maintained for 1 sec, increased in the group of females exposed to 0.10 µg L−1 Escitalopram, but significantly dropped in the group receiving 1.50 µg L−1 (Fig. 2b; Table 2). Escitalopram did not significantly alter any of the remaining swimming behaviour components measured in this study (Table 2).
Swimming behaviour components in female ( ) and male (
) and male ( ) zebrafish after three weeks exposure to 0, 0.10 and 1.50 µg L−1 Escitalopram. (a) swum distance; (b) maximum velocity maintained for one second; (c) average velocity; (d) time in central area of aquarium; (e) swum distance in central area of aquarium; (f) number of visits to central area of aquarium. Different letters denote statistically significant differences (ANOVA; Tukey post hoc test) with the significance level at 0.05. Error bars denote SEM. N for the females are, 19, 18, 21 and for the males 18, 18, 23 for the 0, 0.10 and 1.50 µg L−1groups, respectively
) zebrafish after three weeks exposure to 0, 0.10 and 1.50 µg L−1 Escitalopram. (a) swum distance; (b) maximum velocity maintained for one second; (c) average velocity; (d) time in central area of aquarium; (e) swum distance in central area of aquarium; (f) number of visits to central area of aquarium. Different letters denote statistically significant differences (ANOVA; Tukey post hoc test) with the significance level at 0.05. Error bars denote SEM. N for the females are, 19, 18, 21 and for the males 18, 18, 23 for the 0, 0.10 and 1.50 µg L−1groups, respectively
When introduced to a new environment, zebrafish typicaly display thigmotaxis, swimming in close proximity to the tank walls (Baiamonte et al. 2016; Nema et al. 2016). This phenomenon is a sign of anxiety (Baiamonte et al. 2016; Nema et al. 2016) and can be interpreted as a defensive behaviour against potential predators in fish (Sharma et al. 2009). Thus, a higher activity level of the zebrafish in the central area of the test tank can be considered as an increased boldness (Winberg and Thörnqvist 2016). This boldness was stimulated in the females by Escitalopram exposure. Female zebrafish, exposed to the highest Escitalopram concentration (1.50 µg L−1), visited the central area (ROI) significantly more frequent, spent significantly more time there and were swimming significantly longer distances in the ROI compared to the unexposed control group (Fig. 2d–f; Table 2).
In male zebrafish, the highest exposure concentration at 1.50 µg L−1 significantly suppressed the mean maximum velocity (Fig. 2; Table 3) but otherwise, the remaining swimming parameters only demonstrated statistically non-significant variations from the unexposed fish (Fig. 2a–c; Table 3) with one exception. The total time of active swimming (Table 3) was significantly higher in both exposure groups. In males, there was no statistically significant increase in the time spent in the central area (ROI) of the tank (increased boldness) although it is evident from Fig. 2d that there is a strong tendency of spending more time in the central area with increasing Escitalopram concentrations. There were only minor variations in the swum distance within the ROI and number of visits to this area (Fig. 2e, f; Table 3).
Escitalopram response patterns differed between female and male zebrafish. Unexposed males swum significantly faster (t-test; p = 0.009) than the corresponding control females with a significantly lower (t-test; p = 0.013) turning rate. At 0.1 µg L−1, females increased their maximum swimming velocity, whereas it decreased in males (Fig. 2b). Also, it is worth noticing that at 0.1 µg L−1, the males visited the central area significantly more often (t-test; p = 0.048) than females. At 1.5 µg L−1, the reverse was true.
The frequency distributions of velocities revealed that both sexes spent significantly more time at lower velocities after the highest (1.50 µg L−1) Escitalopram exposure (females, χ2 = 36, df 20, p < 0.020; males, χ2 = 33, df 20, p < 0.025). From the graphic representation, it is not quite as evident that also the velocity frequency distribution of the lower (0.10 µg L−1) group was significantly different from the control group (females, χ2 = 34, df 20, p < 0.025; males, χ2 = 33, df 20, p = 0.025). Even between the two Escitalopram exposed groups there were significant differences in the administration of swimming velocities (females, χ2 = 74, df 20, p < 0.005; males, χ2 = 69, df 20, p < 0.001).
Discussion
The current study demonstrates that exposure to relatively low levels of Escitalopram may have both direct effects on fitness in the form of reduced size and gives rise to changes in zebrafish behaviour. The lower, environmentally relevant, concentration at 0.10 µg L−1 had no significant effects on zebrafish behaviour, which is in agreement with Porseryd et al. (2017), who found no effect of Citalopram at 0.10 µg L−1 on the zebrafish locomotor activity. On the other hand, the 15 times higher concentration at 1.50 µg L−1 affected several of the quantified behaviour components in at least one sex. Escitalopram also affected the mean maximum velocity in both sexes, increased the time spent and distance swum in the central part of the test tank (ROI) as well as the number of entries into the ROI by the females. In addition, the female and male response to treatment were significantly different regarding body weight, mean maximum velocity, number of visits to the ROI and distance swum inside the ROI.
Escitalopram affected body length and body weight in both sexes during the three weeks of exposure. Please note that this conclusion is based upon a random distribution of the fish to the test tanks and thus assuming an equal size distribution before exposure. This cannot be verified since we, in order to minimize stress, did not measure length and weight before exposure. Most likely, the size difference can be attributed to reduced food intake and thus inhibition of growth rate. Several authors (Kellner et al. 2015; Mennigen et al. 2009; Silva et al. 2015; Weinberger and Klaper 2014) have previously demonstrated suppressed feeding rates in fish in response to SSRI exposure. For instance, another SSRI pharmaceutical, Fluoxetine, decreased feeding rates and weight gain in female goldfish (Mennigen et al. 2009, 2010) and hybrid striped bass (Gaworecki and Klaine 2008).
Another reason why treatment affected the body weight in the females may be that they carry eggs and consequently have a more variable weight than the males. Reduced food intake may reduce egg production and therefore weight in the females. A more disturbing possibility is that Escitalopram could have intrinsic properties that affect egg production. Serotonin participates in the modulation of the reproductive neuroendocrine (HPG) axis (Toufexis et al. 2014), which consists of a communication between the hypothalamus, pituitary and the gonads. Among other important functions in fish, the sex steroid estradiol and associated receptors play crucial roles for the growing oocyte (Chakraborty et al. 2011; Menuet et al. 2004; Rani et al. 2010) and the SSRI fluoxetine reduces the levels of circulating estradiol as well as expression of oestrogen receptor mRNA in some brain regions in goldfish (Mennigen et al. 2008). However, except for determination of gender, gonads were not examined in the current study, implying that this possibility remains to be investigated.
The Fulton condition factor decreased slightly with treatment in the females and increased in the males, but there were no significant effects in either of the two genders, indicating that the relationship between weight and length did not change.
General swimming behaviour was moderately affected by Escitalopram in both sexes, with a few exceptions. The distances swum during the 30 min measuring period, average swimming velocity and turning behaviour were statistically similar for the three groups. The mean maximum velocity, which is the highest velocity maintained by the fish for 1 sec was significantly reduced by the high Escitalopram treatment in both sexes, demonstrating a reduced usage of higher swimming velocities. Further, both females and males demonstrated a more continuous swimming activity under the influence of Escitalopram. The number of swimming quiescence periods decreased significantly in females exposed to the highest Escitalopram concentration and the time in locomotor activity increased significantly in males exposed to both concentrations. It has previously been reported that anxiety increases locomotor activity in zebrafish and that the SSRI fluoxetine inhibits this stress-induced increase (Giacomini et al. 2016). In the Siamese fighting fish (Betta splendens) (Kohlert et al. 2012) and in Chinook salmon (Oncorhynchus tshawytscha) (Clements and Schreck 2007), fluoxetine reduces baseline locomotor activity measured as vertical and horizontal movement in grid covered test arenas, respectively. The current study supports these findings.
The frequency distribution of employed velocities signifies how the fish administer its swimming velocity. Typically, both terrestrial and aquatic animals spend most time at lower velocities with exponentially decreasing time allocated to increasing velocities (Baatrup and Bayley 1993; Henriksen et al. 2016). In the present study, the distributions were significantly different between the three treatments in both genders, clearly demonstrating that Escitalopram alters this component of the general swimming behaviour. Thus, Escitalopram decreases the time allocated to higher velocities, supporting a suppressed locomotor activity. It is worth noticing that the biological importance of this quantitative result still needs to be elucidated.
The wall-seeking (thigmotactic) swimming behaviour diminished in female zebrafish under the influence of Escitalopram. The number of entries into the central area of the tank (ROI), the time spent in the ROI and the distance swum within the ROI significantly increased in females treated with 1.50 µg L−1 Escitalopram, indicating a severe reduction of thigmotaxis. Other fish studies have also repported increased central movement in the test aquariums after chronic SSRI exposure (Clements and Schreck 2007; Ansai et al. 2016). No effect of treatment on those variables was evident in the male fish, which demonstrates sex-dependent effects on anxiety/antipredator behaviours. In their novel tank experiment, Porseryd et al. (2017) found that Citalopram at 0.1 µg L−1 significantly increased the number of transitions to the upper half of the tank but no effect on latency to first transition in male zebrafish. The phenomenon of thigmotaxis as an anxiety response is well established in zebrafish (Baiamonte et al. 2016; Jantzen et al. 2016; Nema et al. 2016). Despite this, studies of sex differences in the effects of SSRI on zebrafish thigmotaxis appear to be almost absent. In one study on zebrafish behavioural responses to the SSRI fluoxetine, Singer et al. (2016) found that there were no sex-dependent effects on zebrafish thigmotaxis. However, this study also found a positive correlation between fluoxetine exposure and zebrafish thigmotaxis, which is inconsistent with thigmotaxis as a stress related behaviour. Secondly, this could indicate that explorative abilities are more sensitive to long-term SSRI exposure than the spontaneous swimming behaviour, at least in female zebrafish. Boldness is a behavioural trait that has been described to vary on a shy-bold continumm in fish (Wilson et al. 1994) and a determining factor of an animal’s behavioural profile (Sih et al. 2004). Thus, this trait might be used to predict the behavioural outcome of various daily situations and challenges in fish and thereby become ecolologically important. In addition to locomotor activity (Bell 2005; Moretz et al. 2007), boldness in fish has been positivity linked to aggressiveness (Huntingford 1976) and has proven suitable in predicting social status (Dahlbom et al. (2011). Despite possible advantages, an increased boldness may have severe ecological consequences leading to a higher predation risk. For example, bolder individuals of guppy (Poecilia reticulata) has been shown to suffer higher mortality rates than their shoalmates caused by a relativily higher degree of predator inspection (Dugatkin 1992). In agreement with this assumed increased predation risk, juvenile Piauçu fish (Leporinus microcephalus) did not respond to alarm signals from conspecifics after Fluoxetine administration (10 µg g−1 b.w) (Barbosa Junior et al. 2012) and four weeks of Sertraline exposure (3–30 µg L−1) reduced shelter-seeking behaviour in adult fathead minnows (Pimephales promelas) (Valenti et al. 2012). Boldness in male zebrafish was not significantly altered by Escitalopram treatment. As evident from Fig. 2 and Table 3, the time spent in the central area of the test tank increased from on average 93 s in the control group to 168 s in the 1.50 µg L−1 exposure group, but with a substantial variation among the male fish. This suggests a strong trend towards an increased boldness, supporting that boldness does not appear to differ between the sexes in zebrafish (Way et al. 2015; Moretz et al. 2007). Still, if there are sex-dependent differences in the action of Escitalopram, this certainly calls upon attention considering the effects on other vertebrates, including humans.
In conclusion, Escitalopram affects body weight and length as well as a range of behavioural variables in zebrafish, including boldness, a presumed ecological relevant trait. The effects were more pronounced in females than in males, indicating a possible effect on the reproductive axis. The effects were only evident at the higher concentration, which exceeds that commonly found in nature even in close proximity of sewage treatment plants. While the results of this study indicate that the concentrations found in nature are safe for zebrafish it should be keept in mind that wildlife organism seldom are exposed to one single SSRI and previous studies on three-spine sticklebacks have found effects on feeding behaviour at 0.15 µg L−1 Citalopram (Kellner et al. 2015), corresponding to 0.075 µg L−1 Escitalopram. This species difference highlights the complexity of environmental SSRI effects.
References
Abbing-Karahagopian V, Huerta C, Souverein PC, de Abajo F, Leufkens HGM, Slattery J et al. (2014) Antidepressant prescribing in five European countries: application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur J Clin Pharmacol 70(7):849–857
Ansai S, Hosokawa H, Maegawa S, Kinoshita M (2016) Chronic fluoxetine treatment induces anxiolytic responses and altered social behaviors in medaka, Oryzias latipes. Behav Brain Res 303:126–136. https://doi.org/10.1016/j.bbr.2016.01.050
Baatrup E, Bayley M (1993) Quantitative analysis of spider locomotion employing computer-aided video tracking. Physiol Behav 54(1):83–90
Baiamonte M, Parker MO, Vinson G, Brennan CH (2016) Sustained effects of developmental exposure to ethanol on Zebrafish anxiety-like behaviour. PLoS ONE 11(2):e0148425. https://doi.org/10.1371/journal.pone.0148425
Barbosa Junior A, Alves FL, ASP Pereira, Ide LM, Hoffmann A (2012) Behavioral characterization of the alarm reaction and anxiolytic-like effect of acute treatment with fluoxetine in Piauçu fish Physiol Behav 105(3):784–790. https://doi.org/10.1016/j.physbeh.2011.10.007
Bell AM (2005) Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473. https://doi.org/10.1111/j.1420-9101.2004.00817.x
Benotti MJ, Brownawell BJ (2009) Microbial degradation of pharmaceuticals in estuarine and coastal seawater. Environ Pollut 157(3):994–1002. https://doi.org/10.1016/j.envpol.2008.10.009
Brion F, Tyler CR, Palazzi X, Laillet B, Porcher JM, Garric J, Flammarion P (2004) Impacts of 17β-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio). Aquat Toxicol 68(3):193–217
Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD et al. (2005) Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem 24(2):464
Chakraborty T, Shibata Y, Zhou LY, Katsu Y, Iguchi T, Nagahama Y (2011) Differential expression of three estrogen receptor subtype mRNAs in gonads and liver from embryos to adults of the medaka, Oryzias latipes. Mol Cell Endocrinol 333:47–54. https://doi.org/10.1016/j.mce.2010.12.002
Clements S, Schreck CB (2007) Chronic administration of fluoxetine alters locomotor behavior, but does not potentiate the locomotor stimulating effects of CRH in Juvenile Chinook Salmon (Oncorhynchus Tshawytscha). Comp Biochem Physiol Part A 147(1):43–49. https://doi.org/10.1016/j.cbpa.2006.11.011
Dahlbom SJ, Lagman D, Lundstedt-Enkel K, Sundström LF, Winberg S (2011) Boldness predicts social status in Zebrafish (Danio rerio). PLoS ONE 6(8):e23565. https://doi.org/10.1371/journal.pone.0023565
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107(Suppl 6):907–938
Dugatkin LA (1992) Tendency to inspect predators predicts mortality risk in the guppy (Poecilia reticulata). Behav Ecol 3(2):124–127. https://doi.org/10.1093/beheco/3.2.124
Eaton RC, Farley RD (1974) Spawning cycle and egg-production of Zebrafish, Brachydanio rerio, in laboratory. Copeia 1:195–204. https://doi.org/10.2307/1443023
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI et al. (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in Zebrafish. Behav Brain Res 205(1):38–44. https://doi.org/10.1016/j.bbr.2009.06.022
Fick J, Lindberg RH, Tysklind M, Larsson DGJ (2010) Predicted critical environmental concentrations for 500 pharmaceuticals. Reg Toxicol Pharmacol 58(3):516–523. https://doi.org/10.1016/j.yrtph.2010.08.025
Fick J, Lindberg RH, Kaj L, Brorström-Lundén E (2011) Results from the Swedish National Screening Pro-gramme 2010 (No. Subreport 3. Pharmaceuticals). Swedish Environmental Research Institute
Fick J, Soederstrom H, Lindberg RH, Phan C, Tysklind M, Larsson DGJ (2009) Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem 28(12):2522–2527. https://doi.org/10.1897/09-073.1
Foran CM, Weston J, Slattery M, Brooks BW, Duane B, Huggett DB (2004) Reproductive assessment of Japanese Medaka (Oryzias Latipes) Following a Four-Week Fluoxetine (SSRI) exposure. Arch Environ Cont Toxicol 46(4):511–517. https://doi.org/10.1007/s00244-003-3042-5
Forsatka MN, Nematollahi MA, Amiri BM, Huang W-B (2014) Fluoxetine inhibits aggressive behaviour during parental care I male fighting fish (Betta splendens, Regan). Ecotoxicology 23:1794–1802. https://doi.org/10.1007/s10646-014-1345-0
Gaworecki KM, Klaine SJ (2008) Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat Toxicol 88:207–213. https://doi.org/10.1016/j.aquatox.2008.04.011
Giacomini ACVV, Abreu MS, Giacomini LV, Siebe AMl, Zimerman FF, Rambo CL, Mocelin R, Bonan CD, Piato AL, Barcellos LJG (2016) Fluoxetine and Diazepam acutely modulate stress induced-behavior. Behav Brain Res 296:301–310. https://doi.org/10.1016/j.bbr.2015.09.027
Giebułtowicz J, Nałęcz-Jawecki G (2014) Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol Environ Saf 104:103–9. https://doi.org/10.1016/j.ecoenv.2014.02.020. June
Grabicova K, Lindberg RH, Östman M, Grabic R, Randak T, Larsson DGJ et al. (2014) Tissue-specific bioconcentration of antidepressants in fish exposed to effluent from a municipal sewage treatment plant. Sci Total Environ 488–489:46–50
Grabicova K, Grabic R, Blaha M, Kumar V, Cerveny D, Fedorova G et al. (2015) Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res 72:145–153
Henriksen PG, Beedholm K, Baatrup E (2016) Differences in reproductive behavior between spawning and non-spawning zebrafish pairs and the effects of 17α-Ethinylestradiol (EE2). Toxics 4:22–33. https://doi.org/10.3390/toxics4030022
Hiemke C, Härtter S (2000) Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 85(1):11–28. https://doi.org/10.1016/S0163-7258(99)00048-0
Huntingford FA (1976) The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus Aculeatus. Anim Behav 24(2):245–260. https://doi.org/10.1016/S0003-3472(76)80034-6
Jantzen CE, Annunziato KM, Cooper KR (2016) Behavioral, morphometric, and gene expression effects in adult zebrafish (danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquat Toxicol 180:123–30. https://doi.org/10.1016/j.aquatox.2016.09.011
Krog JS, Hansen MS, Holm E, Hjulsager CK, Chriél M, Pedersen K et al. (2015) Influenza A(H10N7) Virus in Dead Harbor Seals, Denmark. Emerg Infect Dis 21(4):684–687
Kragelund C, Litty K, Lindholst S, Langerhuus AT, Møller T, Rasmussen HU et al. (2015) Miljø-og energieffektiv rensning af miljøfremmede stoffer i særligt be-lastet spildevand. Miljøministeriet, Naturstyrelsen
Kellner M, Porseryd T, Hallgren S, Porsch-Hällström I, Hansen SH, Olsén KH (2016) Waterborne citalopram has anxiolytic effects and increases locomotor activity in the three-spine stickleback (Gasterosteus Aculeatus). Aquat Toxicol 173:19–28. https://doi.org/10.1016/j.aquatox.2015.12.026
Kellner M, Porseryd T, Porsch-Hällström I, Hansen SH, Olsén KH (2015) Environmentally relevant concentrations of Citalopram partially inhibit feeding in the three-spine stickleback (Gasterosteus Aculeatus). Aquat Toxicol 158:165–70. https://doi.org/10.1016/j.aquatox.2014.11.003
Kohlert JG, Mangan BP, Kodra C, Drako L, Long E, Simpson H (2012) Decreased aggressive and locomotor behaviors in Betta splendens after exposure to fluoxetine. Psychol Rep 110(1):51–62. https://doi.org/10.2466/02.13.PR0.110.1.51-62
Kreke N, Dietrich DR (2008) Physiological endpoints for potential SSRI interactions in fish. Crit Rev Toxicol 38(3):215–47. https://doi.org/10.1080/10408440801891057
Kwon J-W, Armbrust KL (2005) Degradation of citalopram by simulated sunlight. Environ Toxicol Chem 24(7):1618. https://doi.org/10.1897/04-522R.1
Kwon J-W, Armbrust KL (2008) Aqueous solubility, N-octanol–water partition coefficient, and sorption of five selective serotonin reuptake inhibitors to sediments and soils. Bull Environ Contam Toxicol 81(2):128–35. https://doi.org/10.1007/s00128-008-9401-1
Lahti M, Oikari A (2012) Vertical distribution of pharmaceuticals in lake sediments-citalopram as potential chemomarker. Environ Toxicol Chem 31(8):1738–44. https://doi.org/10.1002/etc.1901
Lam MW, Young CJ, Brain RA, Johnson DJ, Hanson MA, Wilson CJ, Richards SM, Solomon KR, Mabury SA (2004) Aquatic persistence of eight pharmaceuticals in a microcosm study. Environ Toxicol Chem 23(6):1431. https://doi.org/10.1897/03-421
Lawrence C (2007) The husbandry of Zebrafish (Danio rerio): a review. Aquaculture 269(1):1–20. https://doi.org/10.1016/j.aquaculture.2007.04.077
Lepage O, Larson ET, Mayer I, Winberg S (2005) Serotonin, but not melatonin, plays a role in shaping dominant–subordinate relationships and aggression in Rainbow Trout. Horm Behav 48(2):233–42. https://doi.org/10.1016/j.yhbeh.2005.02.012
Lister A, Regan C, Van Zwol J, Van Der Kraak G (2009) Inhibition of egg production in Zebrafish by Fluoxetine and municipal effluents: a mechanistic evaluation. Aquat Toxicol 95(4):320–29. https://doi.org/10.1016/j.aquatox.2009.04.011
Mennigen JA, Martyniuk CJ, Crump K, Xiong H, Zhao E, Popesku J, Anisman H, Cossin AR, Xia X, Trudeau VL (2008) Effects of fluoxetine on the reproductive axis of Female Goldfish (Carassius Auratus). Physiol Genom 35(3):273–82. https://doi.org/10.1152/physiolgenomics.90263.2008
Mennigen JA, Harris EA, Chang JP, Moon TW, Trudeau VL (2009) Fluoxetine affects weight gain and expression of feeding peptides in the female Goldfish Brain. Regul Pept 155(1–3):99–104. https://doi.org/10.1016/j.regpep.2009.01.001
Mennigen JA, Sassine J, Trudeau VL, Moon TW (2010) Waterborne fluoxetine disrupts feeding and energy metabolism in the Goldfish Carassius Auratus. Aquat Toxicol 100(1):128–37. https://doi.org/10.1016/j.aquatox.2010.07.022
Menuet A, Le Page Y, Torres O, Kern L, Kah O, Pakdel F (2004) Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of ERalpha, ERbeta1 and ERbeta2. J Mol Endocrinol 32:975–86
Moretz JA, Martins EP, Robison BD (2007) Behavioral syndromes and the evolution of correlated behavior in Zebrafish. Behav Ecol 18(3):556–62. https://doi.org/10.1093/beheco/arm011
Nakamura Y, Yamamoto H, Sekizawa J, Kondo T, Hirai N, Tatarazako N (2008) The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): Acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 70(5):865–873
Nash JP, Kime DE, Van der Ven LTM, Wester PW, Brion F, Maack G et al. (2004) Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Health Perspect 112(17):1725–1733. http://www.jstor.org/stable/3435909
Nema S, Hasan W, Bhargava A, Bhargava Y (2016) A novel method for automated tracking and quantification of adult Zebrafish behaviour during anxiety. J Neurosci Met 271:65–75. https://doi.org/10.1016/j.jneumeth.2016.07.004.
Olsén KH, Ask K, Olsén H, Porsch-Hällström I, Hallgren S (2014) Effects of the SSRI citalopram on behaviours connected to stress and reproduction in Endler guppy. Poecilia wingei Aquat Toxicol 151:97–104. https://doi.org/10.1016/j.aquatox.2014.02.011
Organisation for Economic Cooperation and Development (OECD) (1992) Fish, Early Life Stage Toxicity Test. Guideline No. 210. OECD, Paris, France
Pawlowski L, Nowak G, Górka Z, Mazela H (1985) Ro 11-2465 (Cyan-Imipramine), Citalopram and Their N-Desmethyl metabolites: effects on the uptake of 5-Hydroxytryptamine and Noradrenaline in vivo and related pharmacological activities. Psychopharmacology 86(1–2):156–63. https://doi.org/10.1007/BF00431702
Pérez Maceira JJ, Mancebo MJ, Aldegunde M (2014) The involvement of 5-HT-like receptors in the regulation of food intake in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 161:1–6
Porseryd T, Kellner M, Caspillo NR, Volkova K, Elabbas L, Ullah S, Olsén H, Dinnétz P, Hällström IP (2017) Combinatory effects of low concentrations of 17α-etinylestradiol and citalopram on non-reproductive behaviour in adult zebrafish (Danio rerio). Aquat Toxicol 193:9–17. https://doi.org/10.1016/j.aquatox.2017.10.001
Pottegård A, Zoëga H, Hallas J, Damkier P (2014) Use of SSRIs among Danish children: a nationwide study. Eur Child Adolesc Psychiatry 23(12):1211–1218
Prasad P, Ogawa S, Parhar IS (2015) Role of serotonin in fish reproduction. Frontiers in Neuroscience 9
Rani KV, Sehgal N, Goswami SV, Prakash O (2010) Relative potencies of natural estrogens on vitellogenin and choriogenin levels in the Indian freshwater spotted snakehead, Channa punctata: in vivo and in vitro studies. Fish Physiol Biochem 36:587–595. https://doi.org/10.1007/s10695-009-9332-8
Sánchez C, Hyttel J (1999) Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 19(4):467–89. https://doi.org/10.1023/A:1006986824213
Santos LHMLM, Araújo AN, Fachini A, Pena A, Delerue-Matos C, Montenegro MCBSM (2010) Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater 175(1–3):45–95
Schlüsener MP, Hardenbicker P, Nilson E, Schulz M, Viergutz C, Ternes TA (2015) Occurrence of Venlafaxine, other antidepressants and selected metabolites in the Rhine Catchment in the face of climate change. Environ Pollut 196:247–56. https://doi.org/10.1016/j.envpol.2014.09.019
Schultz MM, Furlong ET, Kolpin DW, Werner SL, Schoenfuss HL, Barber LB, Blazer VS, Norris DO, Vajda AM (2010) Antidepressant pharmaceuticals in two U.S. Effluent-Impacted Streams: occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ Sci Technol 44(6):1918–25. https://doi.org/10.1021/es9022706
Sharma S, Coombs S, Patton P, Perera TBde (2009) The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax). J Comp Physiol A 195(3):225–240. https://doi.org/10.1007/s00359-008-0400-9
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19(7):372–378. https://doi.org/10.1016/j.tree.2004.04.009
Silva LJG, Pereira AMPT, Meisel LM, Lino CM, Pena A (2015) Reviewing the serotonin reuptake inhibitors (SSRIs) footprint in the aquatic biota: uptake, bioaccumulation and ecotoxicology. Environ Pollut 197:127–143. https://doi.org/10.1016/j.envpol.2014.12.002
Singer ML, Oreschak K, Rhinehart Z, Robison BD (2016) Anxiolytic effects of fluoxetine and nicotine exposure on exploratory behavior in Zebrafish. PeerJ 4:e2352. https://doi.org/10.7717/peerj.2352
Stahl SM (1998) Basic psychopharmacology of antidepressants: Part 1. Antidepressants have seven dis-tinct mechanisms of action. J Clin Psychiatry 59:5–14
Toufexis D, Rivarola MA, Lara H, Viau V (2014) Stress and the reproductive axis. J Neuroendocrinol 26(9):573–86. https://doi.org/10.1111/jne.12179
Valenti TW, Gould GG, Berninger JP, Connors KA, Keele NB, Prosser KN, Brooks BW (2012) Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (ssri) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ Sci Technol 46(4):2427–2435. https://doi.org/10.1021/es204164b
Vasskog T, Anderssen T, Pedersen-Bjergaard S, Kallenborn R, Jensen E (2008) Occurrence of selective serotonin reuptake inhibitors in sewage and receiving waters at Spitsbergen and in Norway. J Chromatogr A 1185(2):194–205. https://doi.org/10.1016/j.chroma.2008.01.063
Vasskog T, Berger U, Samuelsen P-J, Kallenborn R, Jensen E (2006) Selective serotonin reuptake inhibitors in sewage influents and effluents from Tromsø, Norway. J Chromatogr A 1115(1–2):187–95. https://doi.org/10.1016/j.chroma.2006.02.091
Vaswani M, Linda FK, Ramesh S (2003) Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 27(1):85–102
Wahlberg C, Sverige Naturvårdsverket, Stockholm vatten and IVL Svenska miljöinstitutet (2008) Avloppsreningsverkens förmåga att ta hand om läkemedelsrester och andra farliga ämnen: redovisning av regeringsuppdrag: 512-386-06 Rm. Stockholm: Naturvårdsverket
Way GP, Kiesel AL, Ruhl N, Snekser JL, McRobert SP (2015) Sex differences in a shoaling-boldness behavioral syndrome, but no link with aggression. Behav Proc 113:7–12. https://doi.org/10.1016/j.beproc.2014.12.014
Weinberger J, Klaper R (2014) Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the Fish Pimephales Promelas (Fathead Minnow). Aquat Toxicol 151:77–83. https://doi.org/10.1016/j.aquatox.2013.10.012
Wilson DS, Clark AB, Coleman K, Dearstyne T (1994) Shyness and boldness in humans and other animals. Trends Ecol Evol 9(11):442–446. https://doi.org/10.1016/0169-5347(94)90134-1
Winberg S, Thörnqvist P-O (2016) Role of brain serotonin in modulating fish behavior. Curr Zool 62(3):317–323. https://doi.org/10.1093/cz/zow037
Woldegiorgis A (2011) SSLs Mätningar Av Läkemedel I Vatten Och Korresponderande Regional Försäljning Av Läkemedel I Storstockholm
Yuan S, Jiang X, Xia X, Zhang H, Zheng S (2013) Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere 90(10):2520–25. https://doi.org/10.1016/j.chemosphere.2012.10.089
Acknowledgements
This work was supported by the Faculty of Science and Technology, Aarhus University, Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experiments were conducted in accordance with the guidelines by The Danish Animal Experiments Inspectorate (permission 2012-15-2934-00246).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nielsen, S.V., Kellner, M., Henriksen, P.G. et al. The psychoactive drug Escitalopram affects swimming behaviour and increases boldness in zebrafish (Danio rerio). Ecotoxicology 27, 485–497 (2018). https://doi.org/10.1007/s10646-018-1920-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-018-1920-x