Abstract
Anthropogenic habitat degradation and alien invasive species have led to the rapid decline of freshwater fish biodiversity globally. The knowledge of threatened species’ embryogenesis and larval development could be important for the design of appropriate conservation measures to reverse their decline. Here, we describe the embryonic and larval development of the globally threatened Peloponnese Valencia (Valencia robertae) to inform urgently needed ex situ and in situ conservation initiatives, such as safety stock creation, conservation translocation, and population monitoring. The development of V. robertae is described from the embryonic to the juvenile stage from in vivo imaging, for the first time in detail for this species and genus. Valencia robertae’s fertilised eggs are large (approximately 2 mm), spherical, macrolecithal, translucent, with negative buoyancy, filaments at the outer surface, and several oil globules. They have a long incubation period (approximately 18 days at 20 ± 1 °C) and, in laboratory conditions, a high hatching rate (84%, n = 89). Various types of chromatophores are visible in the embryo, incl. melanophores, xanthophores, and iridophores at the dorsal area of the eye and at the iris. Embryos at hatching measure approximately 5.4 mm SL (6.5 mm TL) and have well-developed caudal and pectoral fins, large eyes, and well-developed mouth; exogenous feeding starts at 24–48 h post hatching. Sequential formation of fins continues with the development of the anal and dorsal fins and is completed by the formation of the pelvic fins, at approximately 11 mm SL (25–30 days post hatching). The ecological and conservation implications of our findings are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inland waters within the Mediterranean basin are considered a freshwater biodiversity hotspot, with a large number of threatened, endemic species (Smith and Darwall 2006; Geiger et al. 2014). Among them, are three species of the genus Valencia, i.e., the Valencia toothcarp Valencia hispanica (Valenciennes 1846) endemic to eastern Spain, the Corfu Valencia Valencia letourneuxi (Sauvage 1880) endemic to southern Albania and north-western Greece, and the more recently described Peloponnese Valencia, Valencia robertae (Freyhof et al. 2014), endemic to south-western Greece (previously assigned to V. letourneuxi). These species were formerly included in the family Cyprinodontidae but now form the separate family Valenciidae (Kottelat and Freyhof 2007). Both V. hispanica and V. letourneuxi are critically endangered, according to the IUCN (Crivelli 2006a, b a, b), while Valencia robertae, the subject of this study, though not yet evaluated by IUCN, has been proposed to be assessed as critically endangered, with an extent of occurrence smaller than 100 km2 and an area of occupancy less than 10 km2 (Barbieri et al. 2015; Oikonomou et al. 2019; Freyhof et al. 2020).
The Peloponnese Valencia (and its congenerer V. letourneuxi) has a fragmented distribution, with extant populations found mainly in a few spring-fed systems, with rather stable thermal conditions and low salinity (Kalogianni et al. 2010 a, b). The most common sympatric native species of V. robertae are the Stymphalia minnow Pelasgus stymphalicus (Valenciennes 1844) and the Western Greece goby Economidichthys pygmaeus (Holly 1929) (Kalogianni et al. 2010b). Valencia robertae is threatened by habitat degradation, water pollution, and impact of the alien, invasive mosquitofish Gambusia holbrooki (Girard 1859) (Kalogianni et al. 2019; Kalogianni et al. 2022). A recent study has indicated that V. robertae has experienced a population decline of 91% within the period 2005–2018 (Kalogianni et al. 2022) and outlined the necessary measures to reverse this near collapse of the species, both in situ and ex situ, including conservation translocations and captive breeding. Knowledge of its embryogenesis and larval development can contribute to artificial propagation initiatives for conservation reintroduction in reclaimed habitats, and/or for conservation introduction in new habitats, satisfying the early life history requirements of the species (Vahed et al. 2018). Early development data will also permit comparisons with closely related genera (such as the species of the family Aphaniidae), while together with environmental and genetic data, can also help us to have a more accurate knowledge of the evolution and biogeography of the genus (Aral et al. 2011).
Here, we provide a detailed description of the eggs, free embryos, and larvae of V. robertae, with some emphasis on diagnostic characters allowing distinction from its co-occurring species in the field. The ecological and conservation implications of our study are discussed in relation to current and future challenges the species faces.
Materials and methods
Housing facilities
For housing the specimens, two 350 L aquariums (100 × 70 × 50 cm) were used. A layer of washed river gravel (7–10 mm) was placed as substrate. Artificial lighting in the aquarium was provided by two T5, 39w lamps emitting light suitable for aquariums, daylight spectrum (8500 °K), equipped with a timer. Water was filtered with JBL Cristall profie1501 filters (1400 l/h). Air supply was through Sera air 275 R plus oxygen pumps. In addition, cooling units (Chiller-Conditioning unit Teco TK500), set at 20 °C (± 1 °C) were used. In the aquariums, after the external filtration system, there was a UV Vecton 600 TMC water sterilisation system.
Fish collection
In November 2018, a three-member team conducted sampling at the Mornos river basin (Western Greece) to collect V. robertae. The physicochemical parameters of the stream water at the time of sampling were as follows: conductivity 585 μS/cm, D.O. 9.27 mg/l, pH 7.5, salinity 0.27 ppt, and temperature 17.6 °C. A total of 107 individuals were collected and were transferred to HCMR laboratories in individual 500 ml plastic containers with oxygen supply; their condition was regularly checked during transport. After their transfer to the laboratory, with zero mortalities, fish were acclimatized to aquaria water conditions, which were similar to those of their natural environment. More specifically, the physicochemical parameters of the aquarium water where fish were placed were D.O. 9.36 mg/l, temperature 20 °C (± 1 °C), pH 8.1, and conductivity 653 μS/cm. Artificial lighting followed the natural photoperiod (12:12 March to 14:10 August). Eggs were obtained during the spring–summer breeding season of 2019 (F0) and 2020 (F0 and F1), through natural reproduction and under conditions similar to the natural host environment of the fish. Eggs were collected at regular intervals from the artificial breeding substrates (spawning mops prepared from woolen thread, attached to a stone) added in the aquaria. They were then placed (max three eggs, all collected simultaneously) in separate dark coloured, plastic “hatching” containers with water from the aquarium of the spawners. Water was half renewed every day with dechlorinated water to a total volume of 350 ml, with a methylene blue concentration of 0.3 ml/L (methylene blue alkaline Loeffler’s staining solution, UNI-CHEM Chemical Reagents) to prevent fungus contamination of the eggs.
Fish embryo rearing and imaging
Images were obtained using an OLYMPUS SZX7 stereoscope equipped with a Lumenera INFINITY2 camera. All images were examined, and measurements were obtained using the Media Cybernetics Image-Pro v10.0.5 system software. Embryo development inside the eggs was recorded every 24 h. For animal welfare reasons, an effort was made to minimise handling during the photo shoot. The egg was collected with the help of a small pipette, placed on a petri dish with a drop of water, and photographed under the stereoscope. The stereoscope lamp was turned on for a few seconds only, for focusing and photographing the egg, to prevent its overheating. No mortalities were recorded due to this procedure. Egg hatching time was determined from a subset of eggs not photographed; egg mean diameter (± SD) measurements were performed with double measurements per egg.
Free embryos and larvae rearing and imaging
After hatching, free embryos remained for three more days in the opaque “hatching” containers where from the second day they were offered Vipan Micron Nature and Vipan Baby Nature fish foods (Sera, Heinsberg, Germany), and live microworms, while from the fourth day, they were provided with live Artemia nauplii (Ocean Nutrition Brine Shrimp Eggs). At this stage, one-third of the water of the containers was replaced daily with water from the nursery aquarium. To limit stress, a small floating piece of native hornwort plants Ceratophyllum sp. (5–6 cm) was added in the containers, with the larvae often laying beneath it. On day 4, the larvae were transferred to the 60 L nursery aquarium with abundant floating plants. A low-flow filter operated in the aquarium with the help of air flow (Aquarium air filter, Aquanova NSF 0.60L).
Prior to photographing the larvae, each was placed in a plastic opaque container with a small plant and about 300 ml of aquarium water. A drop of clove oil solution (prepared with approx. 0.15 ml concentrate clove oil dissolved in 250 ml water) was added to the container. The larva was then carefully observed and immediately when it was anesthetised, it was collected with the help of a pipette, placed on a plexiglass slide at a laterial position and quickly photographed. After photographing, the larva was placed in a new container with aquarium water until it fully recovered and was then transferred to the aquarium. Length measurements, i.e. Stardard Length (SL) and Total length (TL), were made at the nearest 0.1 mm.
Results
Embryo development
A total of 22 embryos prior to hatching were photographed during the reproductive periods of 2019 and 2020 (May to August; Figs. 1–5). Fertilised eggs had a mean size of 1.93 mm (± 0.14, n = 21; Fig. 1), spherical in shape, transparent, with negative buoyancy, and a relatively narrow perivitelline space (Fig. 2). At 0–24 h (cleavage stage, Fig. 2a), a proliferation and gradual accumulation of cells at one pole of the fertilised eggs is observed, as well as hair filaments at the outer surface of the chorion. Inside the yolk, oil globules of varying numbers and sizes can be observed (Fig. 2a).
a Embryo 0–24 h (cleavage stage), cell division at the animal pole (upper left), oil globules (og); b embryo 24–48 h, developing neural tube (embryonic axis, ea), head detection; c embryo 48–72 h; d embryo 72–96 h, optic cups (oc) and brain vesicle development, melanophores (mc) at the embryo, and yolk. Scale bar 0.5 mm
At 24–48 h (day 2 after fertilisation; Fig. 2b), there is the appearance of the neural plate (embryonic axis). At 48–72 h, day 3 (Fig. 2c), the head starts being visible. At day 4 (Fig. 2d), the neural tube and head are more developed and there is formation of the optic cups. Small melanophores are present throughout the length of the embryo, and on the yolk. Brain vesicles that will form the forebrain, midbrain, and hindbrain are visible; also, somites are visible.
On day 5 (Fig. 3a), there is an increase in melanophores; also, myomeres are now discernible on either side of the neural tube. The heart beat is first detected and there is development of a transparent vascular network. The first xanthophores are also visible. On day 6 (120–144 h; Fig. 3b), there is an increase in xanthophores and the formation of otic vesicles. On day 7 (144–168 h; Fig. 3c), the vascular network becomes darker in colour. There is development of the brain, head, and eyes.
a Day 5, first xanthophores (xc), development of transparent vascular network, first heart beat; b day 6 formation of otic vesicles (ov); c day 7 darkening of vascular network (vn); d day 9, iridophores visible in the optic cups; e day 14, dorsal view of embryo, body and pectoral fin (pf) movement; f day 16, ventral view of the embryo. Scale bar 0.5 mm
On day 9 (168–216 h; Fig. 3d), iridophores in eyes are observed. Heart and blood vessels have a red colouring due to blood flow; the tail nearly reaches the head. From day 10 to day 13, the embryo increases in size.
On day 14 (Fig. 3e), the embryo contracts and rotates inside the egg, with also movements of the pectoral fins. On day 15, frequent sudden movements of the body, mouth, and eyes are also observed. On day 16 (Fig. 3f), embryonic development is complete. The mean hatching time of V. robertae eggs was 18 days (± 1 day, n = 16) at 20 °C (± 1 °C). Overall hatching rate was 84% (n = 89), eggs not hatched were infertile, and/or infected by fungi.
Free embryo and larval development
The stages of the free embryo and larval development of V. robertae are shown in Figs. 4–8. Mean free embryo length at hatching (0–24 h) was 5.29 SL ± 0.33 SD (n = 3).
At 5.41 mm SL (6.54 mm TL; Fig. 4), the first 24 h after hatching, the caudal and pectoral fins are developed, with 14 soft rays in the caudal fin. There is complete flexion of the urostyle. The abdominal area contains an oil globule (og), the yolk sac is laterally transparent, without chromatophores, and the visceral cavity filled with yolk. The primordial fin (prf) extends ventrally from the anus to the caudal fin and dorsally at one-third of the larval body length. There is no preanal primordial fin.
Already at 5.80 mm SL (Fig. 5; 24–48 h), there is swimbladder inflation, and the eyes are well developed with presence of many iridophores. There are scattered melanophores and xanthophores throughout the body with no clear pattern. There is high concentration of chromatophores at the ventral side, with remnant of yolk sac (ys) visible. Pectoral fins and caudal fin (with 16 rays) well developed, the anus is still closed.
Free embryo at 5.80 mm SL (24–48 h after hatching) with primordial fin (prf). Swim bladder (swb) inflation, with melanophores at the upper area. Anus still closed, yolk (y) still present. Mouth well developed. Caudal fin developed with 16 rays, pectoral fins (pf) developed. Eyes well developed covering almost half the area of the head with many chromatophores; a lateral view, b dorsal view, and c ventral view. Scale bar 1 mm
At 5.83 mm SL (Fig. 6a), 24–48 h, the anus is open. The peritoneum is opaque, with some presence of xanthophores. There is partial absorption of the yolk sac.
a Larva 5.83 mm SL, anus (an) open. b Larva 5.86 mm SL (24–48 h) food particles visible, remnants of the yolk sac present. c Larva 6.02 mm SL, presence of mesenchyme (ms) at the anal primordial fin, 16 soft rays on the caudal fin. d Larva 6.64 mm SL anal fin bud (afb), caudal fin with 18 distinct soft rays. Scale bar 1 mm
At 5.86 mm SL (24–48 h after hatching; Fig. 6b), exogenous feeding has started (food particles visible in the gut). Eyes are well developed, protruding slightly from the head, covering about one-third of the head length. The dorsal part of the eyes is covered by many melanophores and xanthophores (incl. iridophores), while on the iris, there is a large concentration of iridophores.
At 6.02 mm SL (Fig. 6c), mesenchyme (ms) appears in the primordial fin at the point where the anal fin will develop. Caudal fin has 16 soft rays. At this size, the yolk is completely absorbed.
At 6.64 mm SL (Fig. 6d), the caudal fin has 18 distinct soft rays. Many rays start to develop in the anal fin.
At 6.75 mm SL (Fig. 7a), mesenchyme is present in dorsal primordial fin, with no soft rays yet discernible, but it is rudimentarily separated from the rest of the dorsal primordial fin. There is further development of the anal fin. At 6.82 mm SL (Fig. 7b), the anal fin exceeds the width of the primordial ventral fin but it is still fully attached to it, with 10 soft rays visible.
a Larva at 6.75 mm SL, appearance of mesenchyme (ms) at dorsal primordial fin. b Larva at 6.82 mm SL, 10 soft rays at anal fin. c Larva at 7.38 mm SL, anal fin more developed with 12 soft rays, dorsal fin with 8 rays visible, heavy concentration of melanophores on the dorsal area. d Larva at 8.6 mm SL, pelvic fin buds (pfb) visible. Full absorption of dorsal primordial fin. Scale bar 1 mm
At 7.38 mm SL (Fig. 7c), the anal fin is more developed with 12 soft rays visible, while there are eight rays visible in the dorsal fin (Fig. 23.1). The primordial fin is still present between the anus and anal fin and between the anal and the caudal fin.
At 8.6 mm SL (Fig. 7d), the pelvic fin buds (pfb) are visible. There is full absorption of the dorsal primordial fin, while there is a small last rudiment of the primordial ventral fin between the anus and anal fin and between the anal and the caudal fin.
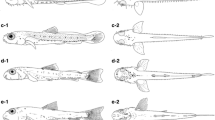
At 10.66 mm SL (Fig. 8a, b), juvenile fish are fully developed. Adult fish exhibit pronounced sexual dimorphism (Fig. 9a, b). Figure 9 c shows the characteristic reflection of V. robertae larva eyes.
Discussion
In this study, we present for the first time in detail the embryonic and larval development of the Peloponnese Valencia V. robertae, in laboratory conditions, a threatened freshwater fish, endemic to Greece, in need of targeted conservation measures (Kalogianni et al. 2022). The species embryogenesis and larval development starts with external fertilisation of macrolecithal eggs with average diameter approx. 2 mm, similar to that reported for a fertilized V. letourneuxi egg attached to a plant collected from the field (Barbieri et al. 2000).
In comparison to the phylogenetically related genus, Aphanius (Piller et al. 2022) and V. robertae (as well as V. letourneuxi) have larger eggs in diameter, as the size of the eggs in the studied Aphanius species (Cyprinodontidae) does not exceed 1.71 mm, in the perimediteranean A. fasciatus (Valenciennes 1821) (see Mordenti et al 2012), in A. mento (Heckel 1843) 1.58 mm (see Sezen and Olmez 2012), and in A. sophiae (Heckel 1847) 1.45 mm (see Masoudi et al. 2018; but see Motamedi et al. 2019 for A. hormuzensis eggs with mean size 1.60 ± 0.20 mm). Also, hatching time of V. robertae was found to be 18 (± 1) days at 20 °C (± 1 °C), while maximum hatching time in Aphanius species does not exceed 14 days, i.e., in A. sophiae, see Masoudi et al. (2018); for other Aphanius species, see Motamedi et al. (2019), Vahed et al. (2018), and Sezen and Olmez (2012).
Hatching time in freshwater fishes depends on many environmental factors, the most important of which is water temperature (Korwin-Kossakowski 2012, 2008). Brown et al. (2011) reported that eggs of the killifish Fundulus grandis Baird and Girard, 1853 (Fundulidae) incubated at lower temperatures, had a longer incubation time and the larvae were more developed (larger) and had less yolk than eggs incubated and hatched at higher temperatures. The relatively long developmental time of V. robertae compared to the Aphanius species may be due to the relatively low temperature of its spring habitats, compared to the Aphanius genera which live in generally warmer environments, such as the eurythermal A. fasciatus (Chaibi et al. 2015) and subtropical A. dispar group. This long developmental time possibly creates disadvantageous prospects for V. robertae under current and future environmental variations due to global warming scenarios. Conversely, rapid incubation and rapid larval growth have been reported as advantageous for the persistence of freshwater fish species in harsh and unstable environments (Karageorgou et al. 2024).
In the V. robertae spring habitat, where fish were collected, but also to the other spring–fed habitats of the species, water temperatures do not exceed 20 °C in summer (Kalogianni et al. 2010a). Feiner et al. (2016) showed a correlation between egg size and low water temperature in walleyes Sander vitreus (Mitchill 1818), so similarly it is possible that the larger V. robertae egg size compared to Aphanius sp., eggs may also be related to the relatively low temperature of its specialized aquatic habitats. Valencia robertae (and V. letourneuxi) spring habitats are also characterized by thermal stability (with low temperature variation, see Kalogianni et al. 2010a, b) that may alternatively explain the species larger egg size. Kamal et al. (2009) compared egg diameter of two populations of A. sophiae and reported that the egg size of a population in a stable environment (spring-fed habitat) was significantly larger than the egg size of the population in a changing environment (river), postulating that A. sophiae reduced egg size in the river as a compensation to the unstable environment (Kamal et al. 2009). In summary, there appears to be a correlation between larger egg size with lower temperatures, longer hatching time and environmental stability.
In the V. robertae embryo, melanophores are first observed at 96 h, and the first appearance of xanthophores was observed 24 h later. In comparison, available information on Aphanius species indicates the first detection of melanophores between 21 and 75 h after fertilisation, while there is no reference on xanthophores. The intense presence of melanophores and dark pigmentation in the V. robertae embryos (at 72–96 h, 4 days) and in the larva may be an adaptation for protection from UV radiation and for camouflage, helping to avoid predators (Mueller and Neuhauss 2014; Macaya et al. 2019). On the fourth day, small otic vesicles were also visible in the V. robertae embryo; otic vesicles are visible much earlier in A. sophiae (30 h; Masoudi et al. 2018) and in A. vladykovi Coad, 1988 (26 h; Vahed et al. 2018b). Soft rays of caudal and pectoral fins are visible in the embryo prior to hatching.
A high concentration of reflective iridescent cells was also observed in the eye region of V. robertae embryos and larvae. Iridophores are visible already from embryonic day 9 in the egg, around the eye in the area of the iris but also in the dorsal part of the eyes. The concentration of iridophores in the eye area remains high throughout life; however, the degree of their reflection seems to vary, which may be an indication that this can be regulated by the fish themselves in different environmental conditions. Various explanations for their usefulness have been proposed; it has been postulated that reflective irises help to camouflage the eyes by creating a silvery reflection (Gur et al. 2018). Furthermore, they increase visual acuity, enabling the already well-developed eye to become functional at a very early stage, crucially for the developing larvae (Gur et al. 2018) and, additionally, for photolocation of potential predators (Santon et al. 2020).
The V. robertae free embryos, at hatching, have a well-developed mouth, large head, large eyes, developed pectoral fins, and a well-developed rounded caudal fin, while they retain melanophores and xanthophores, throughout the body and yolk with an oil droplet. At 24–48 h after hatching, swim bladder inflation is observed, also food particles, marking the start of exogenous feeding and the start of the larval stage. The first fin to develop from the primordial fin in V. robertae larva is the anal fin, followed by the dorsal fin and lastly the pelvic fins. A similar sequential formation of fins has been reported for V. letourneuxi at similar lengths, using wild caught larva (Barbieri et al. 2000). However, pelvic fin buds in V. robertae were first observed at 8.6 mm SL, while in V. letourneuxi at 9.5 mm SL (Barbieri et al. 2000). Full absorption of the primordial fin and full development of the pelvic fin was observed in V. robertae at 10.66 mm SL, while this occurred in V. letourneuxi larvae between 10 and 12.9 mm SL (Barbieri et al. 2000).
Ecological and conservation implications
A recent study demonstrated the rapid decline of both V. robertae and V. letourneuxi in Greece over the last 14 years (2005–2018; see Kalogianni et al. 2022). The most important factors for this decline are the presence of the alien Eastern mosquitofish and the degradation of their natural habitats, mainly through pollution, as well as water abstraction, in-filling and draining. Comparatively, its sympatric minnows and gobies indicated a lower negative trend and an increasing trend respectively for the same time period (Kalogianni et al. 2022). The different population trends of the two killifishes compared to their sympatrics in the same habitats may be related to their different reproduction strategies. Compared to the killifishes, Pelasgus minnows and Economidichthys pygmaeus gobies lay a larger number of smaller or equal sized eggs respectively, with a prolonged reproductive period, shorter hatching time, and smaller-size embryos at hatching compared to V. robertae, though they reach similar adult size; for example, in P. stymphalicus, mean egg size is approximately 1.3 mm, hatching in 5–7 days at 19 °C, embryos at hatching have a NL (notochord length) of 4.7 mm (Daoulas et al 1995; for E. pygmaeus see Daoulas et al. 1993, also Barbieri et al. 2015); significantly, the Economidichthys gobies also exhibit parental care. We suggest that the negative impacts of environmental degradation as well as the impacts of alien invasive species, such as the mosquitofish, are possibly more acute on species, such as V. robertae, that lay fewer eggs, do not exhibit parental care, and have a longer hatching time and ontogenetic development compared to their sympatric natives (and co-occurring aliens). In addition, these biological traits possibly increase the vulnerability of the species’ embryos and larvae to stochastic water stress events, such as wetted habitat desiccation, as hydrological impairment was identified as an important pressure in the species extant habitats (Kalogianni et al. 2022).
Knowledge of the reproductive strategy and early life history requirements of threatened fish species is a prerequisite for their successful management and conservation through breeding for safety stock creation and in situ translocation to un-occupied habitats (George et al. 2009). For the extant populations in the wild, patterns of abundance and distribution of larvae also provide insights on anthropogenic pressures on the target species and its habitats, such as river regulation or river flow fluctuation (flash flood events) and the subsequent larval dispersal (Humphries and Lake, 2000; Lechner et al. 2016). Knowledge of the species ontogeny is also crucial for population assessments of threatened species as, identifying larvae in the field, might provide more reliable information about the status of their populations, as well as obviously a measure of the success of any previous in situ translocation effort, as it would indicate a self-reproducing population. Finally, information on larval ontogeny can be crucial in locating areas with larval accumulation and growth, thus informing plans for the conservation of these important habitats.
Larval taxonomy has proven to be a reliable tool in fish species identification, when early life stages present many similarities (Oliveira et al. 2021). In our case, it is fortunate that the reflective eyes of V. robertae larvae due to the heavy presence of iridophores permits the reliable detection of V. robertae larvae macroscopically in a habitat, without even handling the fish. Characteristics such as the absence of the pre-anal primordial fin and the well-developed caudal fin are also those that readily distinguish V. robertae larvae from the larvae of its co-occurring mosquitofish, cyprinids and gobiids, when larvae of these species are collected in the wild to be examined in the laboratory (Barbieri 2020).
Data availability
Data will be made available on reasonable request.
References
Aral F, Şahınöz E, Doğu Z (2011) Embryonic and larval development of freshwater fish. In: Aral F, Dogu Z (eds) Recent advances in fish farms. Intechopen, pp 83–94
Barbieri R, Daoulas C, Psarras T, Stoumboudi MT, Economou AN (2000) The biology and ecology of Valencia letourneuxi Sauvage 1880 (Valenciidae) — prospects for conservation. Mediterr Mar Sci 1(2):75–90
Barbieri R, Zogaris S, Kalogianni E, Stoumboudi MTh, Chatzinikolaou Y, Giakoumi S, Kapakos Y, Kommatas D, Koutsikos N, Tachos V, Vardakas L, Economou AN (2015) Freshwater fishes and lampreys of Greece: an annotated checklist. In: Monographs on Marine Sciences, no 8. Hellenic Centre for Marine Research, Athens, Greece, p 13
Barbieri R (2020) Description of the ontogenic development of the cyprinids of Louros river. phD thesis, University of Ioannina, Greece (in Greek), 132 https://doi.org/10.12681/eadd/48565
Brown CA, Gothreaux CT, Green CC (2011) Effects of temperature and salinity during incubation on hatching and yolk utilization of Gulf killifish Fundulus grandis embryos. Aquaculture 315(3–4):335–339. https://doi.org/10.1016/j.aquaculture.2011.02.041
Chaibi R, Si Bachir A, Chenchouni H (2015) New inland sites for the Mediterranean killifish (Aphanius fasciatus Valenciennes, 1821) in the Sahara Desert of Algeria. J Appl Ichthyol 31(6):1072–1076. https://doi.org/10.1111/jai.12892
Crivelli AJ (2006a) Valencia hispanica. In: IUCN Red List of Threatened Species. Version 2012.2. https://doi.org/10.2305/IUCN.UK.2006.RLTS.T22829A9392487.en
Crivelli AJ (2006b) Valencia letourneuxi. In: IUCN Red List of Threatened Species. Version 2012.2. https://doi.org/10.2305/IUCN.UK.2006.RLTS.T22830A9393054.en
Daoulas C, Economou AN, Psarras T, Barbieri-Tseliki R (1993) Reproductive strategies and early development of three freshwater gobies. J Fish Biol 42(5):749–776. https://doi.org/10.1111/j.1095-8649.1993.tb00382.x
Daoulas C, Psarras T, Barbieri-Tseliki R, Economou AN (1995) Early development of Pseudophoxinus stymphalicus (Cyprinidae) from lake Trichonis. Greece. Cybium 19(1):89–93. https://doi.org/10.26028/cybium/1995-191-005
Feiner ZS, Wang HY, Einhouse DW, Jackson JR, Rutherford ES, Schelb C et al (2016) Thermal environment and maternal effects shape egg size in a freshwater fish. Ecosphere 7(5):e01304. https://doi.org/10.1002/ecs2.1304
George AL, Kuhajda BR, Williams JD, Cantrell MA, Rakes PL, Shute JR (2009) Guidelines for propagation and translocation for freshwater fish conservation. Fisheries 34(11):529–545. https://doi.org/10.1577/1548-8446-34.11.529
Gur D, Nicolas JD, Brumfeld V, Bar-Elli O, Oron D, Levkowitz G (2018) The dual functional reflecting Iris of the Zebrafish. Adv Sci 5(8):1800338. https://doi.org/10.1002/advs.201800338
Jarić I, Lennox RJ, Kalinkat G, Cvijanović G, Radinger J (2019) Susceptibility of European freshwater fish to climate change: species profiling based on life-history and environmental characteristics. Glob Chang Biol 25(2):448–458. https://doi.org/10.1111/gcb.14518
Kalogianni E, Giakoumi S, Andriopoulou A, Chatzinikolaou Y (2010) Feeding ecology of the critically endangered Valencia letourneuxi (Valenciidae). Aquat Ecol 44:289–299. https://doi.org/10.1007/s10452-009-9253-8
Kalogianni E, Kapakos Y, Oikonomou A, Giakoumi S, Zimmerman B (2022) Dramatic decline of two freshwater killifishes, main anthropogenic drivers and appropriate conservation actions. J Nat Conserv 67:126191. https://doi.org/10.1016/j.jnc.2022.126191
Kamal S, Bakhtiyari M, Abdoli A, Eagderi S, Karami M (2009) Life-history variations of killifish (Aphanius sophiae) populations in two environmentally different habitats in central Iran. J Appl Ichthyol 25(4):474–478. https://doi.org/10.1111/j.1439-0426.2009.01242.x
Karageorgou E, Kapakos Y, Barbieri R, Vardakas L, Karakatsouli N, Kalogianni E (2024) Ontogeny of a threatened freshwater minnow: Implications for conservation. Acta Zool. https://doi.org/10.1111/azo.12492
Keivany Y, Soofiani N (2004) Contribution to the biology of Zagros tooth-carp, Aphanius vladykovi (Cyprinodontidae) in central Iran. Environ Biol Fishes 71:165–169. https://doi.org/10.1007/s10641-004-0106-y
Korwin-Kossakowski M (2012) Fish hatching strategies: a review. Reviews in Fish Biol Fish 22(1):225–240. https://doi.org/10.1007/s11160-011-9233-7
Korwin-Kossakowski M (2008) The influence of temperature during the embryonic period on larval growth and development in Carp, L, and Grass Carp,(Val.): Theoretical and Practical Aspects. Fish Aquat Life 16(3):231–314. https://doi.org/10.2478/s10086-008-0020-6
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Kottelat, Cornol, Switzerland and Freyhof, Berlin, Germany, p 646
Lechner A, Keckeis H, Humphries P (2016) Patterns and processes in the drift of early developmental stages of fish in rivers: a review. Rev Fish Biol Fish, 26, 471–489. https://springerlink.bibliotecabuap.elogim.com/article/https://doi.org/10.1007/s11160-016-9437-y
Macaya C, Lam N, Vila I (2019) Embryological development of the high-altitude killifish Orestias ascotanensis Parenti 1984 (Teleostei: Cyprinodontidae). Environ Biol Fishes, 102, 675–684. https://springerlink.bibliotecabuap.elogim.com/article/https://doi.org/10.1007/s10641-019-00859-6
Masoudi M, Esmaeili HR (1847) Ebrahimi M (2018) Embryology and early ontogeny of an endemic tooth-carp fish, Aphanius sophiae (Heckel. J Appl Ichthyol 34(3):622–632. https://doi.org/10.1111/jai.13630
Monti F, Marcelli M, Fastelli P, Fattorini N (2021) Pushed to the edge: Environmental factors drive ecological responses of Aphanius fasciatus when in sympatry with invasive Gambusia holbrooki. Aquat Conserv.: Mar Freshw Ecosyst 31(9):2547–2559. https://doi.org/10.1002/aqc.3600
Mordenti O, Di Biase A, Zaccaroni A, Bastone G, Scaravelli D (2012) Induced reproduction of Aphanius fasciatus by ecophysiological conditioning and hormonal treatment in fresh and marine water Isr J Aquac. - Bamidgeh , 64. https://doi.org/10.46989/001c.20658
Motamedi M, Teimori A, Masoumi AH, Mohammadzadeh Shaghooei P, Mousavi SE (2019) Early embryonic development of brackish water Killifish Aphanius hormuzensis (Teleostei, Aphaniidae) inhabiting coastal environment in Southern Iran. J Appl Ichthyol 35(6):1260–1268. https://doi.org/10.1111/jai.13955
Mueller KP, Neuhauss SC (2014) Sunscreen for fish: co-option of UV light protection for camouflage. PLoS ONE 9(1):e87372. https://doi.org/10.1371/journal.pone.0087372
Oliveira LS, Cajado RA, Silva FK, Bialetzki A, Zacardi DM (2021) Larval development of the freshwater croaker Pachypops fourcroi (La Cépède 1802) (Perciformes: Sciaenidae). J Fish Biol 99(6):2056–2059. https://doi.org/10.1111/jfb.14894
Piller KR, Parker E, Lemmon AR, Lemmon EM (2022) Investigating the utility of Anchored Hybrid Enrichment data to investigate the relationships among the Killifishes (Actinopterygii: Cyprinodontiformes), a globally distributed group of fishes. Mol Phylogenet Evol 173:107482. https://doi.org/10.1016/j.ympev.2022.107482
Sanjarani Vahed N, Esmaeili HR, Masoudi M, Ebrahimi M (2018) Embryonic and early development of the Zagros tooth-carp, Aphanius vladykovi (Actinopterygii: Cyprinodontidae). J Morphol 279(6):747–756. https://doi.org/10.1002/jmor.20807
Santon M, Bitton PP, Dehm J, Fritsch R, Harant UK, Anthes N (1919) Michiels N K (2020) Redirection of ambient light improves predator detection in a diurnal fish. Proc Royal Soc B 287:20192292. https://doi.org/10.1098/rspb.2019.2292
Teimori A, Esmaeili HR, Hamidan N, Reichenbacher B (2018) Systematics and historical biogeography of the Aphanius dispar species group (Teleostei: Aphaniidae) and description of a new species from Southern Iran. J Zoolog Syst Evol Res 56(4):579–598. https://doi.org/10.1111/jzs.12228
Teletchea F, Fostier A, Kamler E, Gardeur JN, Le Bail PY, Jalabert B, Fontaine P (2009) Comparative analysis of reproductive traits in 65 freshwater fish species: application to the domestication of new fish species. Rev Fish Biol Fish 19:403–430. https://doi.org/10.1007/s11160-008-9102-1
Vahed NS, Esmaeili HR, Masoudi M, Ebrahimi M (2018) Towards the conservation of a critically endangered species, Aphanius farsicus: embryogenesis and development. Environ Biol Fishes 101:193–202. https://doi.org/10.1007/s10641-017-0691-1
Acknowledgements
The authors wish to thank P. Kouraklis for assistance in fish collection and L. Vardakas for a friendly review of the paper. This work forms part of the PhD thesis of Y. Kapakos at the Department of Applied Hydrobiology, Agricultural University of Athens (AUA), Greece.
Funding
This work was conducted within the frame of project DECAGON funded by the A.G.Leventis Foundation and the Zoological Society of London (ZSL).
Author information
Authors and Affiliations
Contributions
Y. Kapakos, B. Zimmerman, and E. Kalogianni contributed to the study conception and design. Material preparation and data collection were performed by Y. Kapakos and analysis performed by Y. Kapakos and E. Kalogianni. The first draft of the manuscript was written by Y. Kapakos, R, Barbieri, and E. Kalogianni. All authors contributed and approved the final manuscript. Funding acquisition by B. Zimmerman and E. Kalogianni.
Corresponding author
Ethics declarations
Ethics approval
Τhe Hellenic Centre for Marine Research (HCMR) had secured all necessary permits for fish collection from the Greek Ministry of Environment, Energy and Climate Change (permit 9ZE24653Π-ΖΟ6, 20/7/2016; the HCMR Research Ethics Committee was still under development when this research was conducted). Fish handling in the field and the laboratory at HCMR complied with Greek guidelines on the protection of animals used for scientific purposes (Official Journal of the Greek Government No. 106/30 April 2013), where applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kapakos, Y., Barbieri, R., Zimmerman, B. et al. Embryonic and larval development of a highly threatened killifish: ecological and conservation implications. Environ Biol Fish 107, 293–305 (2024). https://doi.org/10.1007/s10641-024-01529-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-024-01529-y