Abstract
The sheepshead minnow Cyprinodon variegatus has become a favoured model for laboratory studies because of their small size, rapid development, and tolerance of laboratory conditions. Here, we analyse sheepshead minnow post-embryonic development with the goal of providing a generally useful method for staging fish after embryogenesis. Groups of three females and two males were placed in breeding chambers designed for this experiment. More than 100 eggs were collected and maintained in seawater. Embryos were selected under a dissection microscope and placed in incubation dishes (50 per dish) at 26 °C. On day six, embryos hatched and larvae were transferred to 1 L beakers. To define a simplified normalization table for sheepshead minnow development, we measured each fish for its standard length and examined the fish for four externally evident traits: pigmentation pattern, caudal fin morphology, anal fin morphology, and dorsal fin morphology. The four traits were chosen, because they are easily visualized with standard laboratory equipment such as the stereomicroscope and camera. We have provided criteria for staging sheepshead minnows in studies of post-embryonic development. Our data suggest that dorsal and anal fin morphology may serve as a useful phenotype for defining metamorphic climax stages throughout post-embryonic development in C. variegatus. The staging systems we propose should facilitate detailed anatomical and developmental analyses in relation to ecotoxicological studies on potential disruption of the thyroid axis by xenobiotics and endocrine-disrupting compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sheepshead minnow Cyprinodon variegatus is a ray-finned fish belonging to the family Cyprinodontidae. It inhabits shallow, coastal waters along the Atlantic coast of North America, as well as the Gulf of Mexico (Mettee and Beckham 1978; Heitmuller et al. 1981). It has become a favoured model for laboratory studies because of their small size, rapid development, and tolerance to laboratory conditions (Cripe et al. 2009; Raimondo et al. 2009). Several studies demonstrate that C. variegatus is well suited for use in an in vivo screening assay for endocrine disruption and, possibly, as a sentinel species in estuarine field-monitoring programmes (Hemmer et al. 2001).
The metamorphosis is defined as the transition stage in development between an immature stage and the adult stage of the life cycle (Dye 2012). In teleosts, this transition commonly includes the formation of adult fins and ossification of fin rays, maturation of internal organs and sensory systems, formation of scales, modification of pigmentation, and allometric changes in body proportions (McMenamin and Parichy 2013). An essential tool for any model organism is a normal table of development for assigning individuals to particular stages of the life cycle (Hopwood 2007). Only with such a staging series can experiments be repeated and results compared across laboratories. A post-embryonic staging system is therefore needed because changes occur in a variety of traits during this period in sheepshead minnows. A normal table of sheepshead minnows development is available for embryonic stages (Kuntz 1917), but a staging series for post-embryonic development is still lacking.
Here, we analyse sheepshead minnow post-embryonic development with the objective of providing a generally useful system for assigning fish to stages after embryogenesis. By relating changes in several traits to size and age, we aim to provide an easy and rapid method to visually assign stages to living individual larvae. This staging system should facilitate detailed anatomical and developmental analyses of this important period of development.
Materials and methods
Adult Cyprinodon variegatus were purchased from Aquatic Research Organisms (ARO Inc. New Hampshire, USA) in April 2013. They were maintained according to the standardized test guidelines of EPA and OECD (EPA 2002; OECD 2013). Males and females were maintained in 150 L glass aquaria at 26 °C at a photoperiod of 14h:10h (L:D) and fed daily with frozen brine shrimp (Artemia nauplii) and flake food (Sera Vipan). The experiments were performed from March to May 2015. Groups of two males and three females were paired in five spawning boxes measuring 350 mm × 250 mm × 200 mm (length × width × height) for two hours (Cripe et al. 2009). Embryos were collected from spawning tanks. Embryos were selected under a dissection microscope and placed in Petri dishes (50 per dish) in an incubator (BCR-25, Jiangsu Best Electrics Co., Ltd) at 26 °C. On day 0, embryos hatched and larvae were transferred to a glass tank (1 litre working volume, 50 larvae per tank). From 0 day post-hatch (dph), larvae were fed on cultured brine shrimp.
The collected larvae were euthanized with MS-222 and then immersed in a solution of 1 % methylcellulose. The use of methylcellulose helped to place the larvae in perfect side view. Individuals were photographed using a Zeiss dissecting microscope Stemi 2000-C, a MOC-510 Müller® camera (5 MP), and an associated image acquisition software SI-Capture 3.6. A millimetre scale was placed in the background of every individual to obtain size information. Only individuals in perfect lateral view and having extended dorsal and anal fins were selected. Two larvae were photographed by post-hatch day from day 1 to 35 dph (limit corresponding approximately to the total absorption of the dorsal finfold). To define a simplified normalization table for sheepshead minnow maturation, we measured the standard length (SL, mm) for each fish, noticed the appearance of scales, and examined four externally evident traits: pigmentation pattern, caudal fin, anal fin, and dorsal fin morphology. The four traits were chosen, because they are easily visualized with standard laboratory equipment such as the stereomicroscope and camera. The larval and adult pigment patterns in sheepshead minnows are quite different, and the gradual changes during development were visually assessed (Kelsh and Parichy 2008; Kimmel et al. 1995; Quigley and Parichy 2002). We described the development of sheepshead minnow in 15 stages and related changes in several traits to size and age.
To identify which traits are more likely to reflect key developmentally regulated events, we related them to the ontogenetic pattern of thyroid hormones during development of the sheepshead minnow from embryo to adult, as determined in a previous study (Schnitzler et al. 2016). According to these hormone patterns, the development of these fish could be partitioned into periods of pre-metamorphosis (0–5 dph), pro-metamorphosis (6-9 dph), metamorphosis climax (9–18 dph), and post-metamorphosis (19–30 dph) (Raimondo et al. 2009; Schnitzler et al. 2016).
Results
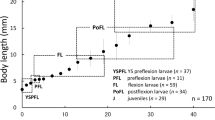
Each spawning female produced 15 to 30 embryos per spawn; fertilization could be assessed 24 hours after spawning by counting the number of opaque (fertile) and white (nonfertile) embryos. The fertilization rates were low and variable (40 % to 60 %), but the hatching rates were high (90 %) after an incubation time of 6 days. All tested larvae reached the juvenile stage in between 28 days post-hatching (Fig 1). With a daily observation of morphological characteristics of individuals, we could describe the development of sheepshead minnow in 15 stages [Table 1; Electronic Supplementary Material (ESM) S1]. Each includes fin ray morphology and the development of pigmentation.
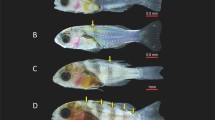
Pigment pattern. Pigmentation is an easily observable morphological trait whose patterns are linked to fish development. We defined 10 pigment patterns (Fig. 2a&b). In the pre-metamorphosis phase (Stage 1–3; 1-5 dph; 3–3.5 mm SL; Table 1; ESM S1), a first spotted line of six to seven clusters of melanophores is observed midlaterally along the trunk and tail. Isolated ventral spots of melanophores below the midlateral line were also observed, as well as on the proximal segments of the rays of the caudal fin. Further development results in the progression of the melanophores on the edges of the caudal rays, from proximal to distal. Melanophores scattered among the clusters on the midlateral line and extended to the ventral midline of the tail. During pro-metamorphosis (Stage 4–5; 6–9 dph; 3–4 mm SL; Table 1; ESM S1), cyanophores appeared below the midlateral line of the trunk and tail interspersed among the position of the melanophores. The melanophores start their migration on the dorsal fin. During metamorphosis climax (Stage 6–10; 9–18 dph; 3.5–6.5 mm SL; Table 1; ESM S1), we observed a thickening of the melanophores on the ventral line, especially along the edge of the anal fin. Additionally, melanophores appeared on the dorsal fin. Finally at 18 dph, a line of green chromatophores appeared slightly below the midlateral line of the trunk and tail, and we observed an intensification of pigmentation on the dorsal fin. In post-metamorphosis (Stage 11–15; 19–34 dph; 6–11 mm SL; Table 1; ESM S1), the dorsal melanophore clusters are in contact with those from the midlateral line in a way that melanophores largely cover the dorsal region of the trunk and tail. Iridophores appeared on the dorsal and anal fins. First scales were observed at 25 dph. On 28 dph, we observed the juvenile pigment pattern made of wide vertical bands of melanophores on the body.
Occurrence of pigmentation changes (a) in function of days post-hatching (dph) and (b) standard length (mm) of sheepshead minnows Cyprinodon variegatus. Number of caudal fin rays (c) in function of days post-hatching (dph) and (d) standard length (mm) of sheepshead minnows Cyprinodon variegatus. The shaded zone represents the metamorphic climax
Scales. Cycloid scales appeared at 26 dph during the post-metamorphosis period (Stage 11–15; 19–34 dph; 6–11 mm SL; Table 1; ESM S1). They first appeared on the ventral region and then their formation proceeded in a posterior-dorsal direction. On Stage 14, the scales showed three circuli, which were most visible in the midlateral margin of the trunk. At Stage 15, the scales were composed of four circuli, and the overlap among scales was larger than in Stage 14.
Caudal fin. For the caudal fin, we identified 10 morphological stages (Fig 2c&d). When fin rays emerge, the shape of the fin is blunt. The presence of 10 condensed fin rays, with a maximum of 5 segments, marks the beginning of caudal fin development. In the following days, the caudal fin progressed to 12 and 14 rays with up to 8 segments. Then these rays begin to thicken during the pre- and pro-metamorphosis phase (Stage 1–5; 1-9 dph; 3-4 mm SL; Table 1; ESM S1). During metamorphosis climax (Stage 6–10; 9–18 dph; 3.5-6.5 mm SL; Table 1; ESM S1), we observed a multiplication of rays from 16 up to 20. At the post-metamorphosis stage (Stage 11–15; 19–34 dph; 6–11 mm SL; Table 1; ESM S1), pairs of rays were continuously added at 18, 22, 25, and 30 dph to reach the final number of 28 rays.
Anal fin. For the anal fin, seven phenotypic stages could be defined (Fig. 3a&b). During the pre-metamorphosis phase (Stage 1–3; 1–5 dph; 3–3.5 mm SL; Table 1; ESM S1), the finfold was present and the anal fin was not visible. We observed the development of anlage of the anal fin around 6–7 dph, during pro-metamorphosis (Stage 4–5; Table 1; ESM S1). The first three rays of the anal fin appeared at 7 dph, incipient inside the finfold. During metamorphosis climax (Stage 6–10; 9–18 dph; 3.5–6.5 mm SL; Table 1; ESM S1), the three rays emerged and deformed the finfold at 9 dph. During the metamorphic period, we observed additions of fin rays between 10 and 18 dph to reach 10 rays finally. The resorption of the ventral finfold was achieved at 17 dph (Stage 9).
Number of anal fin rays (a) in function of days post-hatching (dph) and (b) standard length (mm) of sheepshead minnows Cyprinodon variegatus. Number of dorsal fin rays (c) in function of days post-hatching (dph) and (d) standard length (mm) of sheepshead minnows Cyprinodon variegatus. The shaded zone represents the metamorphic climax
Dorsal fin. We defined eight stages (Fig. 3c, d). During pre-metamorphosis (Stage 1–3; 1–5 dph; 3.0–3.5 mm SL; Table 1; ESM S1), the finfold included the dorsal and anal fins. During pro-metamorphosis (Stage 4–5; 6-9 dph; 3–4 mm SL; Table 1; ESM S1), the first two dorsal fin rays appeared around 7–8 dph, still within the finfold. During metamorphosis climax (Stage 6–10; 9–18 dph; 3.5–6.5 mm SL; Table 1; ESM S1), they emerged out of the finfold around 9–10 dph. Then, during the metamorphic period, we observed additions of rays from 4 up to 11 rays on 18 dph. During the post-metamorphosis (Stage 11–15; 19–34 dph; 6–11 mm SL; Table 1; ESM S1), the anterior part of the dorsal finfold began to resorb. A notch became visible between the posterior part of the dorsal fin and the finfold at 18 dph. There was a continued resorption of the posterior part of the dorsal finfold from behind the dorsal fin, until the total resorption was completed at 29 dph.
Discussion
Generally, sheepshead minnows hatch after 5 to 7 days and remain as larvae for approximately 28 days, during which they metamorphose to the juvenile stage lasting approximately 35 days prior to sexual maturation (Raimondo et al. 2009), consistent with the observation we made in this study.
The growth rate and development of fish are influenced by many factors including: water quality, temperature, population density, genetics, food quality, and food availability. These external environmental factors were constant under our standardized laboratory conditions. The sheepshead minnow is widely used in ecotoxicological studies that only recently began to focus on the potential disruption of the thyroid axis by xenobiotics and endocrine-disrupting compounds. Therefore, we may focus on hormonal contributions to teleost metamorphosis. The thyroid hormones play an important role in the growth and development of fish, as reviewed previously (Power et al. 2001; Yamano 2005). Thyroid hormones are key regulators of teleost metamorphosis. Whole body concentrations of thyroid hormones show typical changes during these periods of teleost fish development, indicating that the ontogeny of thyroid hormones is related to specific morphological characteristics that represent early development (Chang et al. 2012; Crane et al. 2004; de Jesus and Hirano 1992; Johns et al. 2009; Kawakami et al. 2003; Klaren et al. 2008; Shiao et al., 2008; Szisch et al. 2005; Yamano 2005). In sheepshead minnow, an association between an increase in thyroid hormone levels with larval metamorphosis was recently demonstrated around 9 and 18 days post-hatching (Schnitzler et al. 2016).
The pigment pattern and caudal fin development demonstrate a slow transition throughout development from embryonic stages to juveniles without a clear link to the metamorphic thyroid hormone peaks demonstrated in a previous paper (Schnitzler et al. 2016). On the other hand, we identified an abrupt variation of dorsal and anal fin morphology that occurred during the metamorphic thyroid hormone peaks, likely reflecting key developmentally regulated events. This can be visualized in Figs. 2 and 3. Indeed, the plots for dorsal and anal fin rays reveal a characteristic S-shaped curve, indicating that the addition of these fin rays is faster during the period around the thyroid hormone peaks, whereas pigment pattern events and the count of caudal fin rays reveal a linear transition throughout development.
The formation of adult pigmentation is a common feature of sheepshead minnow metamorphosis in which adult pigment patterns result from the spatial arrangements of black melanophores, yellow xanthophores, and iridescent iridophores (Kelsh and Parichy 2008; Kimmel et al. 1995; Quigley and Parichy 2002). Here, the pigment pattern demonstrates a slow transition throughout development from embryonic stages to adulthood without a clear link to the metamorphic thyroid hormone peaks. However, studies showed that inhibited thyroid hormone synthesis by goitrogens delayed the development of metamorphic pigmentation in zebrafish Danio rerio (see Brown 1997). Others showed that a treatment with high levels of T4 inhibits adult melanophore development in flatfish (Yoo et al. 2000), eels (Jegstrup and Rosenkilde 2003), and zebrafish (McMenamin and Parichy 2013), but the biological significance of these observations remains obscure. Currently, it is unknown if thyroid hormone is directly required by pigment cells or if thyroid hormones exert an indirect influence through other cellular intermediaries.
Similarly, caudal fin development demonstrates a slow transition throughout development in Cyprinodon variegatus. This pattern differs from the one observed in the Japanese conger eel Conger myriaster. In C. myriaster, the number of rays in the dorsal, anal, and caudal fins increases at the beginning of the thyroid hormone peaks and the number of rays in the dorsal, anal, and caudal fins was complete at the end of the thyroid hormone fluctuations (Kawakami et al. 2008).
Dorsal and anal fin morphology underwent abrupt variations during the metamorphic thyroid hormone peaks of C. variegatus. The implications of thyroid hormones during fin development are still poorly explored. In fish, mesenchymal cells generate fin endoskeleton, with rays growing from proximal to distal ends (McMenamin and Parichy 2013). The positioning and outgrowth of these rays are regulated by numerous pathways (Marí‐Beffa and Murciano 2010; Sims et al. 2009). Deiodinase enzymes are important in thyroid hormone homeostasis by regulating the serum and cytoplasmic levels of T3. Type 3 deiodinase can only deiodinate the inner ring of T4 and T3 and is the major inactivating enzyme (Jarque and Piña 2014). In zebrafish, fin regeneration is accompanied by enhanced expression of type 3 deiodinase, and the regenerative progress is retarded when type 3 deiodinase activity is pharmacologically blocked (Bouzaffour et al. 2010), which suggests that local T3 degradation promotes regeneration. The roles of these thyroid hormone factors during normal fin development remain largely unknown.
Our results agree with those from Olson and Watabe (1980), who noticed that scale formation in C. variegatus is initiated between 26 and 30 dph (Olson and Watabe 1980). In the present study, the first scales appeared at 26 dph, largely after the metamorphic thyroid hormone peaks. The integumentary structure of larval teleosts is primarily composed of epidermis. The skin becomes progressively stratified and complex at metamorphosis, when fibroblasts initiate scale formation (Chang and Hwang 2011; Rakers et al. 2010). The thyroid hormone potentially regulates these fibroblasts, as keratin expression is regulated directly by the thyroid hormone (Infante et al. 2007). Nevertheless, further research is needed to determine the precise roles of the thyroid hormone in promoting the integumentary metamorphosis of teleosts.
Although thyroid hormone signalling plays an important role in promoting metamorphosis in teleosts, the precise mechanisms by which thyroid hormones affect morphological and physiological events remain largely unknown. We have provided criteria for staging sheepshead minnows in studies of post-embryonic development. Our data suggest that dorsal and anal fin morphology may serve as a useful phenotype for defining the metamorphic climax stages throughout post-embryonic development in C. variegatus. The proposed staging systems should facilitate detailed anatomical and developmental analyses in relation to ecotoxicological studies on potential disruption of the thyroid axis by xenobiotics and endocrine-disrupting compounds.
References
Bouzaffour M, Rampon C, Ramaugé M, Courtin F, Vriz S (2010) Implication of type 3 deiodinase induction in zebrafish fin regeneration. Gen Com Endocrinol 168:88–94
Brown DD (1997) The role of thyroid hormone in zebrafish and axolotl development. Pro Natl Acad of Sci U S A 94:13011–13016
Chang WJ, Hwang PP (2011) Development of zebrafish epidermis. Birth Defects Res C Embryo Today 93:205–214
Chang J, Wang M, Gui W, Zhao Y, Yu L, Zhu G (2012) Changes in thyroid hormone levels during zebrafish development. Zool Sci 29: 181–184
Crane HM, Pickford DB, Hutchinson TH, Brown JA (2004) Developmental changes of thyroid hormones in the fathead minnow, Pimephales promelas. General and Comparative Endocrinology 139:55–60
Cripe GM, Hemmer BL, Goodman LR, Vennari JC (2009) Development of a methodology for successful multigeneration life-cycle testing of the estuarine sheepshead minnow, Cyprinodon variegatus. Arch Environ Contam Toxicol 56:500–508
de Jesus EGT, Hirano T (1992) Changes in whole body concentrations of cortisol, thyroid hormones, and sex steroids during early development of the chum salmon, Oncorhynchus keta. Gen Comp Endocrinol 85:55–61
Dye FJ (2012) Dictionary of developmental biology and embryology. John Wiley & Sons Inc, Hoboken
EPA (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. US Environmental Protection Agency, Washington
Heitmuller PT, Hollister TA, Parrish PR (1981) Acute toxicity of 54 industrial chemicals to sheepshead minnows (Cyprinodon variegatus). Bull Environ Contam Toxicol 27:596–604
Hemmer MJ, Hemmer BL, Bowman CJ, Kroll KJ, Folmar LC, Marcovich D, Hoglund MD, Denslow ND (2001) Effects of p-nonylphenol, methoxychlor, and endosulfan on vitellogenin induction and expression in sheepshead minnow (Cyprinodon variegatus). Environ Toxicol Chem 20:336–343
Hopwood N (2007) A history of normal plates, tables and stages in vertebrate embryology. Int J of Dev Biol 51:1–26
Infante C, Manchado M, Asensio E, Canavate JP (2007) Molecular characterization, gene expression and dependence on thyroid hormones of two type I keratin genes (sseKer1 and sseKer2) in the flatfish Senegalese sole (Solea senegalensis Kaup). BMC Dev Biol 7:118
Jarque S, Piña B (2014) Deiodinases and thyroid metabolism disruption in teleost fish. Environ Res 135:361–375
Jegstrup I, Rosenkilde P (2003) Regulation of post‐larval development in the European eel: thyroid hormone level, progress of pigmentation and changes in behaviour. J Fish Biol 63:168–175
Johns SM, Kane MD, Denslow ND, Watanabe KH, Orlando EF, Villeneuve DL, Ankley GT, Sepulveda MS (2009) Characterization of ontogenetic changes in gene expression in the fathead minnow (Pimephales promelas). Environ Toxicol Chem 28:873–880
Kawakami Y, Tanda M, Adachi S, Yamauchi K (2003) Characterization of thyroid hormone receptor α and β in the metamorphosing Japanese conger eel, Conger myriaster. Gen and Comp Endocrinol 132:321–332
Kawakami Y, Nozaki J, Seoka M, Kumai H, Ohta H (2008) Characterization of thyroid hormones and thyroid hormone receptors during the early development of Pacific bluefin tuna (Thunnus orientalis). Gen Comp Endocrinol 155:597–606
Kelsh RN, Parichy DM (2008) Pigmentation. In: Finn RN, Kapoor BG (eds) Fish Larval Physiology. Science Publishers, Enfield, New Hampshire, pp. 27–49
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Developmental dynamics 203:253–310
Klaren PHM, Wunderink YS, Yufera M, Mancera JM, Flik G (2008) The thyroid gland and thyroid hormones in Senegalese sole (Solea senegalensis) during early development and metamorphosis. Gen Compa Endocrinol 155:686–694
Kuntz A (1917) Notes on the embryology and larval development of five species of teleostean Fishes. Fish Bull Fish and Wildl Serv 34:409–429
Marí‐Beffa M, Murciano C (2010) Dermoskeleton morphogenesis in zebrafish fins. Dev Dyn 239:2779–2794
McMenamin SK, Parichy DM (2013) Metamorphosis in teleosts. Curr Top Dev Biol 103:127–165
Mettee MF, Beckham MC (1978) Notes on the breeding behavior, embryology and larval development of Cyprinodon variegatus Lacepède in aquaria. Tulane Stud Zool Botany 20:137–148
OECD (2013) OECD Guidelines for testing of chemicals: Fish, Early-life Stage Toxicity Test. OECD Publishing, Paris
Olson OP, Watabe N (1980) Studies on formation and resorption of fish scales. Cell Tissue Res 211:303–316
Power DM, Llewellyn L, Faustino M, Nowell MA, Björnsson BT, Einarsdottir IE, Canario AVM, Sweeney GE (2001) Thyroid hormones in growth and development of fish. Comp Biochem Physiol C Toxicol Pharmacol 130:447–459
Quigley IK, Parichy DM (2002) Pigment pattern formation in zebrafish: a model for developmental genetics and the evolution of form. Microsc Res Tech 58:442–455
Raimondo S, Hemmer BL, Goodman LR, Cripe GM (2009) Multigenerational exposure of the estuarine sheepshead minnow (Cyprinodon variegatus) to 17β-estradiol. II. population-level effects through two life cycles. Environ Toxicol Chem 28:2409–2415
Rakers S, Gebert M, Uppalapati S, Meyer W, Maderson P, Sell AF, Kruse C, Paus R (2010) ‘Fish matters’: the relevance of fish skin biology to investigative dermatology. Exp Dermatol 19:313–324
Schnitzler J, Klaren PM, Mariavelle E, Das K (2016) The thyroid gland and thyroid hormones in sheepshead minnow (Cyprinodon variegatus) during early development and metamorphosis. Fish Physiol Biochem 42:607–616
Shiao J-C, Wu S-M, Hwang Y-P, Wu D-P, Hwang P-P (2008) Evaluation of thyroid-mediated otolith growth of larval and juvenile tilapia. J Exp Biol 211:1919–1926
Sims K, Eble DM, Iovine MK (2009) Connexin43 regulates joint location in zebrafish fins. Dev Biol 327:410–418
Szisch V, Papandroulakis N, Fanouraki E, Pavlidis M (2005) Ontogeny of the thyroid hormones and cortisol in the gilthead sea bream, Sparus aurata. Gen Comp Endocrinol 142:186–192
Yamano K (2005) The role of thyroid hormone in fish development with reference to aquaculture. JARQ 39:161–168
Yoo JH, Takeuchi T, Seikai T (2000) Sensitivity of the metamorphic events and morphogenesis of Japanese flounder Paralichthys olivaceus during larval development to thyroxine. Fish Science 66:846–850
Acknowledgements
Schnitzler, J. and Frédérich, B. were postdoctoral researchers at F.R.S-FNRS (Fonds de la recherche scientifique). Das, K. is an F.R.S-FNRS Research Associate. This study was partly funded by FRFC no. 2.4635.11. The authors would like to thank the anonymous reviewers for their helpful and constructive comments that greatly contributed to improving the final version of the paper. MARE is the Interfacultary Centre for Marine Research of the University of Liège. AFFISH-RC is the Applied and Fundamental Fish Research Centre of the University of Liège. This paper is a MARE publication no. 329
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Schnitzler, J.G., Dussenne, M., Frédérich, B. et al. Post-embryonic development of sheepshead minnow Cyprinodon variegatus: a staging tool based on externally visible anatomical traits. Ichthyol Res 64, 29–36 (2017). https://doi.org/10.1007/s10228-016-0534-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0534-7