Abstract
The cichlid Geophagus sveni, native to the Tocantins-Araguaia basin, was introduced into the floodplain of the upper Paraná River and has achieved great adaptive success, with high abundances there. In order to test whether the enemy release hypothesis is happening in the species invasion process, we collected 29 individuals in the Tocantins River (native range) and 29 in the Paraná River (non-native range) to compare their parasite fauna. In the Tocantins River, 17 fish were parasitized by at least one parasitic species, totaling eight species, comprising 54 individuals in total, while in the non-native fish from the Paraná River, we found only one representative of a parasitic species. We found differences in the weight-length relationship, where individuals from the Paraná River showed a greater investment in weight compared to individuals from the Tocantins River. Furthermore, we observed a significant positive relationship between weight and abundance of parasites in native fish. Our results indicate that the hypothesis of enemy release may be occurring in G. sveni, as the quantitative difference in endoparasites between sites shows that hosts from the Paraná River obtained a competitive advantage when arriving at the site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When fish species are introduced to a new environment, there is a possibility that their parasites will follow (see Taraschewski 2006). If the parasites accompany their host, there are four possibilities of parasite-host association in cases of species introduction, and they will not always be able to adapt successfully to their new environment (Salgado-Maldonado and Pineda-López 2003; Rahel and Olden 2008). The success or failure of parasites in a new environment is determined by various factors such as the parasite's biological traits (specificity, life cycle, and transmission) and the ecological traits of the host (Font 2003). Furthermore, the success rates of parasites are influenced by various biotic and abiotic factors such as the presence or absence of natural enemies, competition with local species for resources, and the climatic conditions of the region and habitat (Vignon and Sasal 2010; Blakeslee et al. 2013).
The enemy release hypothesis explains why non-native species typically exhibit lower parasite intensity and prevalence compared to native species that share the same habitat, as they tend to lose their parasites during the invasion process (Torchin et al. 2003; Goedknegt et al. 2015; Sarabeev et al. 2017; Tierney et al. 2020). The hypothesis is based on the idea that natural enemies, such as parasites, play a critical role in controlling the populations of their host species. These enemies are often specialized to a few host species in their local environment. As a result, when species are introduced to a new area, they typically do not bring their natural enemies with them, leading to a situation where the pressure from natural enemies on the invasive species may be significantly lower than that experienced by native species in the area. This is because the natural enemies in the new area are not adapted to infect the invasive species and are instead specialized to local species (Keane and Crawley 2002; Richardson and Pyšek 2007).
Parasites can pose a threat to their hosts, considering that they can interfere in various ways, directly or indirectly, with the life of the fish they parasitize. It is known that, naturally, parasites require a very high energy demand from their hosts (Sures 2008), which results in a lower energy rate to escape predators and, consequently, survive (Timi and Poulin 2020). In addition, many parasites have the ability to cause behavioral changes and affect growth and nutritional status, and can intervene in the success of intra and interspecific competitions (Minchella and Scott 1991; Iyaji and Eyo 2008; Overstreet 2021). These and other factors, resulting from the action of parasites, directly alter the abundance and diversity of organisms in the environment (Lacerda et al. 2012).
The upper Paraná River floodplain is a diverse but highly invaded aquatic ecosystem in Brazil (Smith et al. 2005; Ota et al. 2018; Bueno et al. 2021), where several non-native species coexist with phylogenetically related native species. The species Geophagus sveni Lucinda, Lucena & Assis, 2010, (Cichlidae), native to the Tocantins-Araguaia sub-basin, is also considered non-native in this environment (Moretto et al. 2008; Lucinda et al. 2010; Gois et al. 2015; Ota et al. 2018; Oliveira and Graça 2020), having its first record in the floodplain in the early 2000s, when it was still erroneously identified as G. proximus (Vidotto and Carvalho 2007; Moretto et al. 2008; Ximenes et al. 2021). Its arrival was through fishkeeping and was facilitated by the reservoirs located upstream of the floodplain, which served as a source of propagules (i.e., stepping stones to invasion). In addition, the high-water transparency, the main variable that explains the abundance of G. sveni, facilitated its dissemination (Graça and Pavanelli 2007; Moretto et al. 2008; Gois et al. 2015; Thomaz et al. 2015).
Despite being a well-established species in the floodplain, the parasitic fauna of G. sveni has not been given due consideration when examining the mechanisms and impacts of its invasion. It is noteworthy that parasites are regarded as a critical response variable for assessing ecosystem health, and disregarding them can lead to the loss of over 70% of biological information (Galli et al. 2005; Lymbery et al. 2014; Ortega et al. 2015a, b). In line with the enemy release hypothesis, the absence of parasites and other natural enemies may give certain non-native species a competitive advantage, aiding in their demographic expansion and boosting their likelihood of successful invasion (Torchin et al. 2003; Torchin and Mitchell 2004; Torchin and Lafferty 2009).
Thus, we evaluated the endoparasite fauna of G. sveni populations from the Tocantins River sub-basin and the upper Paraná River floodplain in order to compare them. Considering that G. sveni is a well-established species in the invaded environment, we hypothesize that this success is associated with the process of enemy release, which leads to improved condition of individuals in these locations. Thus, we expect to find differences in endoparasite composition between populations collected in the Tocantins and Paraná rivers, with a higher prevalence of these endoparasites in individuals found in the basin of origin. Furthermore, it is expected that body condition of individuals found in the Paraná River will be greater than that of individuals found in the Tocantins River.
Material and methods
Study area
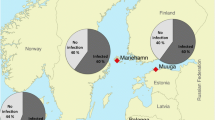
The Tocantins River originates in the state of Goiás, in the central region of Brazil, and flows northward through different sedimentary basins for 2,500 km, passing through the states of Tocantins, Maranhão and Pará (Santos et al. 2004) (Fig. 1). The ichthyofauna is closely related to the Amazon basin, especially in the lower course (Goulding et al. 2003), but the Tocantins River basin is notable for containing a high degree of endemism and high diversity (Santos et al. 2004; Lucinda et al. 2007; Abell et al. 2008; Bertaco and Carvalho 2010; Carvalho et al. 2010) (Fig. 1).
The Paraná River is the main river forming the La Plata Basin and the second largest in length in South America, with approximately 3,089 km (Agostinho et al. 1999). In the upper part, above the Itaipu Hydroelectric Plant, lies the floodplain of the upper Paraná River, and is considered the last remaining stretch of this river (230 km in length) within Brazil (Fig. 1). This floodplain is located on the west bank of the river, composed of different biotopes (microhabitats) such as floodplain lakes, channels, and rivers with distinct degrees of connectivity. In addition, the ichthyofauna of the region presents high diversity, being composed of more than 211 recorded species (Gubiani et al. 2007; Ota et al. 2018).
Collection, processing and identification of fish and parasites
Fish were captured with 2.4; 3; 4; 5; 6; 7; 8; 10; 12; 14 and 16 cm nets between non-adjacent nodes, 10-m trawl nets with 2 mm mesh thickness and rods with 4/0 and 7/0 cm hooks. The nets and hooks remained at each sampling point for a period of 24 h, being searched at dawn (08:00) at dusk (16:00) and at night (22:00). The collected hosts were anesthetized, euthanized, and taxonomically identified. Then, standard length (cm) and total weight (g) were measured. The hosts were sacrificed by medullary sectioning, in accordance with the Ethics Committee on Animal Use (CEUA No. 1420221018) of the State University of Maringá. A total of 29 specimens of G. sveni were collected in the Tocantins River in the city of Porto Nacional (10°42′25.9" S 48°25′14.0" W), where it is native, in 2018, and 29 specimens in the Paraná River in the city of Porto Rico (22°45'S and 53°16'W) in 2018 and 2019.
The fish were gutted and the gastrointestinal tract was analyzed with the aid of the optical stereomicroscope to search for the parasites. Parasite collection, preservation and preparation procedures were carried out according to Eiras et al. (2000) and parasite identification was based on the works of Yamaguti (1961), Travassos et al. (1969), Moravec (1998) and Vicente and Pinto (1999), as well as the use of identification keys, reference guides and updated articles in the area.
Data analysis
To compare the parasite fauna of G. sveni individuals from the Tocantins River and the Paraná River we used the metrics of prevalence, mean intensity and mean abundance of parasite infection according to Bush et al. (1997). Prevalence is the number of hosts infected by a given parasite species, divided by the number of hosts analyzed and multiplied by 100 (result expressed as a percentage %). The mean abundance represents the total number of parasites of a given species divided by the total number of fish examined. Finally, the average intensity of infestation is the total number of parasites observed of a given species, divided by the number of hosts infected with this same parasite species.
Since the presence of parasites in the fish can affect the investment of individuals in size, we evaluated the length–weight relationship between the sampled sites using an ANCOVA. For this, we used weight as a response variable, length as a predictor variable and sampled sites (i.e., Rio Tocantins and Rio Paraná) as a covariate. Before performing the analyses, we tested the assumption of parallelism (homogeneous slope between sites) by evaluating the interaction between locations and length (García-Berthou and Moreno-Amich 1993). The absence of interaction between the variables allows the comparison of average weights in a given size between sampled sites.
To assess whether the abundance of parasites affects the weight of G. sveni individuals and whether there is a difference in the weight of individuals between the sampled sites, we used a generalized linear model (GLM) with Gaussian distribution. For this purpose, weight was used as a response variable, and parasite abundance and sampled sites as predictor variables. Statistical procedures were performed in R software version 4.0.4 (R Core Team 2021) with the packages vegan (Oksanen et al. 2019), ggpubr (Kassambara 2020) and ggplot2 (Wickham 2016).
Results
Twenty-nine fish were collected at each site, totaling 58 individuals of Geophagus sveni analyzed. Of these, 17 were parasitized in the Tocantins River and only one in the Paraná River (Table 1). In total, 54 endoparasite specimens were collected from the Tocantins River and one specimen from the Paraná River. Most parasites were represented by adult stages and only one genus in larval stage (Raphidascaris) was found in both collection sites. Consequently, the parasitological indices of prevalence, mean intensity and mean abundance were much higher for the hosts collected in the Tocantins River (Table 1). The parasites of the phyla Nematoda and Acanthocephala stand out because 51.7% and 17.22% of the fish were parasitized by these groups, respectively.
Individuals of Geophagus sveni collected in the Tocantins River had higher mean weight and length (92.07 ± 42.86 g and 14.38 ± 2.37 cm) than individuals collected in the Paraná River (85.7 ± 45.42 g and 13.2 ± 2.67 cm). The assumption of parallelism was met for the ANCOVA analysis, where the interaction between location and standard length did not show a significant effect (i.e., both locations show the same variation in the proportion between weight and length). On the other hand, the single effect of standard length and location showed significant effects. The significant positive effect of standard length on weight is already an expected result, since in the weight-length relationship, larger individuals have greater weight. The significant effect of the sampling site shows that, for a given size, individuals of G. sveni from Paraná have greater weight, compared to individuals from the Tocantins River (i.e., they have a greater investment in growth) (Table 2, Fig. 2).
The results of the GLM showed that the abundance of parasites has a significant negative effect on the weight of individuals of G. sveni, that is, the greater the abundance of parasites, the lower the weight of the individual (Table 3). On the other hand, the location did not show a significant effect, which can be explained by the presence of individuals of greater standard length in the Tocantins River, as mentioned above.
Discussion
The results presented suggest that the enemy release hypothesis is occurring in Geophagus sveni. The difference in parasitological indices, especially the prevalence of endoparasites, recorded between sites, demonstrates that the hosts from the Paraná River may have obtained a competitive advantage upon arrival at the site due to the absence of endoparasites. The low species richness in G. sveni at the invaded site was already recorded by Lehun et al. (2020), since only Ascocotyle sp. and Raphidascaris (Sprentascaris) sp. were found in this host, suggesting that endoparasite species may have been lost in the invasion process. It is important to consider that G. sveni was introduced by the aquarium trade, likely the introduction propagule were treated against parasites (Harms 1996). Another possibility is that the dispersal jump that G. sveni perceived, by chance, only included a small group of individuals that did not have parasites or were less infected.
According to Gendron et al. (2012), studies on the enemy release hypothesis have typically quantified the phenomenon by comparing levels of species parasitism between native and introduced areas (such as in studies by Torchin et al. 2001; Kvach and Stepien 2008; Blakeslee et al. 2009). These studies suggest that differences in parasitism may be influenced by a range of factors, including local habitat characteristics, community structure, and other environmental variables (Colautti et al. 2004). The duration of the enemy release phenomenon is another factor that may impact its level of impact. As time passes, new associations may form between parasites and hosts in the invaded range, resulting in a gradual recruitment of parasites by introduced hosts (Cornell and Hawkins 1993; Krakau et al. 2006). Therefore, the release of parasites in this study may be a transient and temporary situation. Additionally, Roche et al. (2010) note that a decrease in the number of parasite species could lead to reduced competition among different parasite species within individual hosts, resulting in higher abundances of the remaining parasite species.
Introduced species may indeed accumulate parasites, but the number of taxa recruited within the period that is assessed may be less than half the number found in their native range (Torchin and Mitchell 2004; Kvach and Skóra 2007). Following this hypothesis, introduced species are expected to accumulate native parasites over time: the longer the invader is established, the more native parasites it should acquire (Blaustein et al. 1983; Torchin and Lafferty 2009). Torchin et al. (2001) report that the rate of accumulation of a parasite species can occur every hundred years in introduced populations of Carcinus maenas. However, Gendron et al. (2012) provides additional empirical evidence in support of the release release hypothesis by demonstrating that Neogobius melanostomus, while experiencing a loss of its parasites, this reduction in parasitism rate in a non-native species may be short-lived.
The parasites found in this study are intestinal and the decrease in the richness of endoparasites of G. sveni in the Paraná River can be explained by the fact that they have complex life cycles, making them dependent on more than one host to finish it and, consequently, reproduce. It may be that, intermediate and definitive hosts do not occur in the new environment (Kvach and Stepien 2008), moreover, native parasites do not share a coevolutive history with the introduced host, being considered unsuitable to complete their cycle. We assume that helminth species are less aggregated in communities in an invasive population because the host individual has an approximately equal negative effect on any parasite species, due to the fact that native parasites do not have specific adaptations to the new host.
The results indicated that the weight-length relationship of fish from the Tocantins River was significantly lower than that from the Paraná River. The abundance of parasitic species found in native fish reinforces that the absence of parasites in the introduced location benefited non-native fish and that they managed to overcome one of the biotic barriers in the invasion. High water transparency (as is the case in the Paraná River) may also have played an important role in G. sveni physiology and/or fitness in its introduced range, as this cichlid tends to be visually oriented during food acquisition and in the processes surrounding its reproductive cycle (Moretto et al. 2008; Gois et al. 2015). This abiotic characteristic of the place also favored the colonization of other invasive fish, such as the Cichla kelberi, which obtained advantages in the search for food and reproductive partners (Espínola et al. 2010).
The absence or drastic decrease of parasites, causes the hosts to be able to direct energy to another factor that favors them (Robar et al. 2011), especially during the process of sojourn and establishment to the new environment. We found that the individuals collected in the Paraná River showed higher investment in weight, compared to individuals from the Tocantins River, possibly, this may be a result of the adaptive success of the species. Gois et al. (2015) in a study conducted in the Paraná River, suggest that there may be a niche overlap between G. sveni and Satanoperca pappaterra (native cichlid of the region), given the phylogenetic and morphological proximity of the species, in addition to the similarity in the diet, therefore, it is likely that the two species show similar behaviors in resource acquisition, causing strong competitive interactions to develop under such a scenario. Furthermore, it was observed that the strong competitive interactions between G. sveni and S. pappaterra, indicate that the invasive species may exhibit a more effective foraging strategy relative to the native, which resulted in a lack of niche packing (change in resource use to avoid competition) by S. pappaterra. One of the assumptions of the enemy release hypothesis holds that, if proven, the host may benefit from the situation by reaching larger sizes, both in length and population density, when compared to individuals present in the native area (Ondračková et al. 2010; Torchin et al. 2001).
The data presented in this paper correspond to the first study to test an ecological theory of invasion (sensu Keane and Crawley 2002) for G. sveni, comparing its endoparasitic fauna in two locations, native and introduced, in Brazil. The results obtained from the study provide potential explanation for the success and abundance of species founded by the Paraná River, due to the low prevalence of parasites, considered natural enemies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author.
References
Abell R, Thieme ML, Revenga C et al (2008) Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58:403–414. https://doi.org/10.1641/B580507
Agostinho AA, Júlio-Jr HF, Lowe-McConnell RH (1999) Estudos ecológicos de comunidades de peixes tropicais. Edusp, Sao Paulo, pp 374–400
Bertaco VA, Carvalho FR (2010) New species of Hasemania (Characiformes: Characidae) from central Brazil, with comments on the endemism of upper rio Tocantins basin, Goiás State. Neotrop Ichthyol 8:27–32
Blakeslee AMH, Keogh CL, Byers JE et al (2009) Differential escape from parasites by two competing introduced crabs. Marine Ecol Prog Ser 393:83–96. https://doi.org/10.3354/meps08225
Blakeslee AMH, Fowler AE, Keogh CL (2013) Marine invasions and parasite escape: updates and new perspectives. Adv Mar Biol 66:87–169. https://doi.org/10.1016/B978-0-12-408096-6.00002-X
Blaustein AR, Kuris AM, Alio JJ (1983) Pest and parasite species richness problems. Am Nat 122:556–566
Bueno ML, Magalhães ALB, Andrade Neto FR et al (2021) Alien fish fauna of southeastern Brazil: species status, introduction pathways, distribution and impacts. Biol Invasions 23:3021–3034. https://doi.org/10.1007/s10530-021-02564-x
Bush AO, Lafferty KD, Lotz JM et al (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Carvalho FR, Bertaco VA, Jerep FC (2010) Hemigrammus tocantinsi: a new species from the upper rio Tocantins basin, Central Brazil (Characiformes: Characidae). Neotrop Ichthyol 8:247–254
Colautti R, Ricciardi A, Grigorovitch IA (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733. https://doi.org/10.1111/j.1461-0248.2004.00616.x
Cornell HV, Hawkins BA (1993) Accumulation of native parasitoid species on introduced herbivores: a comparison of “hosts-as-natives” and “host-as-invaders.” Am Nat 141:847–865
Eiras JDC, Takemoto RM, Pavanelli GC (2000) Métodos de estudo e técnicas laboratoriais em parasitologia de peixes, pp 171–171
Espínola LA, Minte-Vera CV, Júlio HF (2010) Invasibility of reservoirs in the Paraná Basin, Brazil, to Cichla kelberi Kullander and Ferreira, 2006. Biol Invasions 12:1889. https://doi.org/10.1007/s10530-009-9657-3
Font WF (2003) The global spread of parasites: what do Hawaiian streams tell us? Bioscience 53:1061–1067. https://doi.org/10.1641/0006-3568(2003)053[1061:TGSOPW]2.0.CO;2
Galli P, Stefani F, Benzoni F et al (2005) Introduction of alien host–parasite complexes in a natural environment and the symbiota concept. Hydrobiologia 548:293–299. https://doi.org/10.1007/s10750-005-3645-0
García-Berthou E, Moreno-Amich R (1993) Multivariate analysis of covariance in morphometric studies of the reproductive cycle. Can J Fish Aquat Sci 50:1394–1399. https://doi.org/10.1139/f93-159
Gendron AD, Marcogliese DJ, Thomas M (2012) Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St. Lawrence Basin. Biol Invasions 14:367–384. https://doi.org/10.1007/s10530-011-0083-y
Goedknegt MA, Feis ME, Wegner KM et al (2015) Parasites and marine invasions: ecological and evolutionary perspectives. J Sea Res 113:11–27. https://doi.org/10.1016/j.seares.2015.12.003
Gois KS, Pelicice FM, Gomes LC et al (2015) Invasion of an Amazonian cichlid in the upper Paraná River: facilitation by dams and decline of a phylogenetically related species. Hydrobiologia 746:401–413. https://doi.org/10.1007/s10750-014-2061-8
Goulding M, Barthem FEJG, Ferreira EJG (2003) The Smithsonian atlas of the Amazon. Smithsonian books, Washington DC
Graça JW, Pavanelli SC (2007) Peixes da planície de inundação do alto Rio Paraná e áreas adjacentes. Maringá, Eduem, pp 210–212
Gubiani EA, Gomes LC, Agostinho AA et al (2007) Persistence of fish populations in the upper Paraná River: effects of water regulation by dams. Ecol Freshw Fish 16:191–197. https://doi.org/10.1111/j.1600-0633.2006.00211.x
Harms CA (1996) Treatments for parasitic diseases of aquarium and ornamental fish. Semin Avian Exot Pet Med 5:54–63. https://doi.org/10.1016/S1055-937X(96)80018-1
Iyaji FO, Eyo JE (2008) Parasites and their freshwater fish host. Biol Res 6:328–338
Kassambara A (2020) Ggpubr: 'ggplot2' Based publication ready plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Krakau M, Thieltges DW, Reise K (2006) Native parasites adopt introduced bivalves of the North Sea. Biol Invasions 8:919–925. https://doi.org/10.1007/s10530-005-4734-8
Kvach Y, Skóra KE (2007) Metazoa parasites of the invasive round goby Apollonia melanostoma (Neogobius melanostomus) (Pallas) (Gobiidae: Osteichthyes) in the Gulf of Gdańsk, Baltic Sea, Poland: a Comparison with the Black Sea. Parasitol Res 100:767–774. https://doi.org/10.1007/s00436-006-0311-z
Kvach Y, Stepien CA (2008) Metazoan parasites of introduced round and tubenose gobies in the Great Lakes: support for the “enemy release hypothesis.” J Great Lakes Res 34:23–35. https://doi.org/10.3394/0380-1330(2008)34[23:MPOIRA]2.0.CO;2
Lacerda ACF, Takemoto RM, Poulin R et al (2012) Parasites of the fish Cichla piquiti (Cichlidae) in native and invaded brazilian basins: release not from the enemy, but from its effects. Parasitol Res 112:279–288. https://doi.org/10.1007/s00436-012-3135-z
Lehun AL, Hasuike WT, Silva JOS et al (2020) Checklist of parasites in fish from the upper Paraná River floodplain: an update. Rev Bras Parasitol Vet 29:e008720. https://doi.org/10.1590/S1984-29612020066
Lucinda PHF, Freitas IS, Soares AB et al (2007) Fish, Lajeado reservoir, rio Tocantins drainage, state of Tocantins, Brazil. Check List 3:70–83. https://doi.org/10.15560/3.2.70
Lucinda PHF, Lucena CAS, Assis NC (2010) Two new species of cichlid fish genus Geophagus Heckel from the Rio Tocantins drainage (Perciformes: Cichlidae). Zootaxa 2429:29–42. https://doi.org/10.11646/zootaxa.2429.1.2
Lymbery AJ, Morine M, Kanani HG et al (2014) Co-invaders: the effects of alien parasites on native hosts. Int J Parasitol Parasites Wildl 3:171–177. https://doi.org/10.1016/j.ijppaw.2014.04.002
Minchella DJ, Scott ME (1991) Parasitism: a cryptic determinant of animal community structure. Trends Ecol Evol 6:250–254. https://doi.org/10.1016/0169-5347(91)90071-5
Moravec F (1998) Nematodes of freshwater fishes of the Neotropical region. In: (ed) List of nematodes of freshwater fishes in the Neotropical Region. Academia, Publishing House of the Academy of Sciences of the Czech Republic, pp 49
Moretto EM, Marciano FT, Velludo MR et al (2008) The recent occurrence, establishment and potential impact of Geophagus proximus (Cichlidae: Perciformes) in the Tietê River reservoirs: an Amazonian fish species introduced in the Paraná Basin (Brazil). Biodivers Conserv 17:3013–3025. https://doi.org/10.1007/s10531-008-9413-5
Oksanen J, Blanchet FG, Friendly M et al (2019) Vegan: Community ecology package. Retrieved from https://CRAN.R-project.org/package=vegan
Oliveira RC, da Graça WJ (2020) Encephalon gross morphology of the cichlid Geophagus sveni (Cichlidae: Geophagini): comparative description and ecological perspectives. J Fish Biol 97:1363–1374. https://doi.org/10.1111/jfb.14495
Ondračková M, Francová K, Dávidová M et al (2010) Condition status and parasite infection of Neogobius kessleri and N. melanostomus (Gobiidae) in their native and non-native area of distribution of the Danube River. Ecol Res 25:857–866. https://doi.org/10.1007/s11284-010-0716-0
Ortega JCG, Júlio HF Jr, Gomes LC et al (2015a) Fish farming as the main driver of fish introductions in Neotropical reservoirs. Hydrobiologia 746:147–158. https://doi.org/10.1007/s10750-014-2025-z
Ortega N, Price W, Campbell T et al (2015b) Acquired and introduced macroparasites of the invasive Cuban treefrog, Osteopilus septentrionalis. Int J Parasitol Parasites Wildl 4:379–384. https://doi.org/10.1016/j.ijppaw.2015.10.002
Ota RR, Deprá GC, Graça WJ et al (2018) Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotrop Ichthyol 16. https://doi.org/10.1590/1982-0224-20170094
Overstreet RM (2021) Parasitic diseases of fishes and their relationship with toxicants and other environmental factors. In: (ed) Pathobiology of marine and estuarine organisms, CRC press, pp 111–156
R Core Team (2021) R: A language and environment for statistical computing
Rahel FJ, Olden JD (2008) Assessing the effects of climate change on aquatic invasive species. Conserv Biol 22:521–533. https://doi.org/10.1111/j.1523-1739.2008.00950.x
Richardson DM, Pyšek P (2007) Elton, CS 1958: The ecology of invasions by animals and plants. London: Methuen. Prog Phys Geogr 31:659-666. https://doi.org/10.1177/0309133307087089
Robar N, Murray DL, Burness G (2011) Effects of parasites on host energy expenditure: the resting metabolic rate stalemate. Can J Zool 89:1146–1155. https://doi.org/10.1139/z11-084
Roche DG, Leung B, Franco EFM et al (2010) Higher parasite richness, abundance and impact in native versus introduced cichlid fishes. Int J Parasitol 40:1525–1530. https://doi.org/10.1016/j.ijpara.2010.05.007
Salgado-Maldonado G, Pineda-López RF (2003) The Asian fish tapeworm Bothriocephalus acheilognathi: a potential threat to native freshwater fish species in Mexico. Biol Invasions 5:261–268. https://doi.org/10.1023/A:1026189331093
Santos GMD, Juras AA, Mérona BD et al (2004) Peixes do baixo rio Tocantins. 20 anos depois da Usina Hidrelétrica Tucuruí.
Sarabeev V, Balbuena JÁ, Morand S (2017) Testing the enemy release hypothesis: abundance and distribution patterns of helminth communities in grey mullets (Teleostei: Mugilidae) reveal the success of invasive species. Int J Parasitol 47:687–696. https://doi.org/10.1016/j.ijpara.2017.05.006
Smith WS, Espíndola ELG, Rocha O (2005) As introduções de espécies de peixes exóticos e alóctones em bacias hidrográficas brasileiras. In: Rocha O, Espíndola ELG, Fenerich-Verani N et al (eds) Espécies Invasoras de Águas Doces: estudo de caso e propostas de manejo. Editora da Universidade Federal de São Carlos, São Carlos, pp 25–44
Sures B (2008) Host-parasite interactions in polluted environments. J Fish Biol 73:2133–2142. https://doi.org/10.1111/j.1095-8649.2008.02057.x
Taraschewski H (2006) Hosts and parasites as aliens. J Helminthol 80:99–128. https://doi.org/10.1079/JOH2006364
Thomaz SM, Kovalenko KE, Havel JE et al (2015) Aquatic invasive species: general trends in the literature and introduction to the special issue. Hydrobiologia 746:1–12. https://doi.org/10.1007/s10750-014-2150-8
Tierney PA, Caffrey JM, Matthews SM, Costantini E, Holland CV (2020) Evidence for enemy release in invasive common dace Leuciscus leuciscus in Ireland: a helminth community survey and systematic review. J Helminthol 94:1–10. https://doi.org/10.1017/S0022149X20000759
Timi JT, Poulin R (2020) Why ignoring parasites in fish ecology is a mistake. Int J Parasitol 50:755–761. https://doi.org/10.1016/j.ijpara.2020.04.007
Torchin ME, Lafferty KD (2009) Escape from parasites. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems. Springer, Berlin, pp 203–214
Torchin ME, Mitchell CE (2004) Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ 2:183–190. https://doi.org/10.1890/1540-9295(2004)002[0183:PPAIBP]2.0.CO;2
Torchin ME, Lafferty KD, Kuris AM (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3:333–345. https://doi.org/10.1023/A:1015855019360
Torchin ME, Lafferty KD, Dobson AP et al (2003) Introduced species and their missing parasites. Nature 421:628–630. https://doi.org/10.1038/nature01346
Travassos L, Freitas JF, Kohn A (1969) Trematódeos do Brasil. Mem Inst Oswaldo Cruz 67:1–886
Vicente JJ, Pinto RM (1999) Nematóides do Brasil: nematóides de peixes: atualização: 1985–1998. Rev Bras De Zool 16:561–610. https://doi.org/10.1590/S0101-81751999000300001
Vidotto AP, Carvalho ED (2007) Composition and structure of fish community in a stretch of the Santa Bárbara River influenced by Nova Avanhandava Reservoir (low Tietê River, Sao Paulo State, Brazil). Acta Limnol Bras Zool 19:233–245
Vignon M, Sasal P (2010) Multiscale determinants of parasite abundance: A quantitative hierarchical approach for coral reef fishes. Int J Parasitol 40:443–451. https://doi.org/10.1016/j.ijpara.2009.09.010
Wickham H (2016) Programming with ggplot2. In: ggplot2. Springer, Cham 241–253. https://doi.org/10.1007/978-3-319-24277-4_12
Ximenes AM, Bittencourt PS, Machado VN et al (2021) Mapping the hidden diversity of the Geophagus sensu stricto species group (Cichlidae: Geophagini) from the Amazon basin. PeerJ 9:e12443. https://doi.org/10.7717/peerj.12443
Ximenes AM, Bittencourt PS, Machado VN et al (2021) Mapping the hidden diversity of the Geophagus sensu stricto species group (Cichlidae: Geophagini) from the Amazon basin. PeerJ 9:e12443
Acknowledgements
We are grateful to everyone who assisted us in the field and laboratory during the collection of fish, especially Dr. Eliane da Silva Fernandes and the entire team at the Federal University of Tocantins: Dr. Elineide Eugênio Marques, Dr. Thiago Nilton Alves Pereira and MsC. Alice Ferreira Araujo.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq with a Scientific Initiation scholarship and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES with two scholarships (one for a master's degree and one for a doctorate). Author GM received research support from CNPq (process: 118649/2017–5) and authors ALL and CMM received research support from CAPES (processes: 88882.344478/2019–01 and 88881.361907/2019–01, respectively).
Author information
Authors and Affiliations
Contributions
Gabriela Michelan: Host collection and screening, analysis of parasite, general structure of the manuscript and discussion of results. Atsler Luana Lehun: General structure of the manuscript, statistical analysis and discussion of the results. Carolina Mendes Muniz: statistical analysis. Ricardo Massato Takemoto: supervised the research and contributed to the discussion and text review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures followed the guidelines for capture, handling, and care of animals of the Ethics Committee on Animal/ Universidade Estadual de Maringá (CEUA Nº 5073090620).
Financial interests
There is no financial interest.
Competing Interests
The authors have no financial interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michelan, G., Lehun, A.L., Muniz, C.M. et al. Absence of parasites in non-native fish from a Neotropical floodplain: evidence for the enemy release hypothesis. Environ Biol Fish 106, 1879–1888 (2023). https://doi.org/10.1007/s10641-023-01463-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01463-5