Abstract
Lake Tana is one of East Africa’s largest freshwater bodies, yet many of its fishes are migratory and utilize in-flowing tributaries as critical spawning habitat. However, factors such as expanding water resources developments and sand mining along these rivers and streams may disrupt this ecosystem function. We monitored juvenile and adult fish abundance and water quality across five lake tributaries from August 2014 to April 2015 to examine how irrigation schemes and water quality affect assemblage and population structure. Adult assemblages were dominated by Labeobarbus cyprinids and varied between tributaries, albeit without separation by irrigation development or sand mining. Overall, adult abundances of the dominant migratory Labeobarbus species were four-fold higher below the Shini River irrigation weir than upstream. Contrastingly, juvenile abundances were often significantly higher above these structures. Juvenile abundances decreased on average by 46% along the first 1000 m of two irrigation canals, suggesting poor habitat suitability or high mortalities from water withdrawals. Water quality varied more between rivers than sampling times, but without any separation of tributaries by irrigation or sand mining. Conductivity and turbidity-related parameters had the highest correlation with adult assemblage structure and individual species abundances. These findings indicate that Lake Tana tributaries must be managed on a case by case basis, with more focus given to mechanisms allowing fish to bypass irrigation developments and the direct assessment of fish populations between sand mining and other sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lakes hold nearly 90% of the world’s liquid freshwater resources and represent a key ecosystem for aquatic biodiversity (Duker and Borre 2001). Indeed, their relatively isolated nature means that they frequently harbour high levels of aquatic species endemism (Martens and Segers 2009). Lake Tana is one of the largest lakes in East Africa (over 3000 km2) and in the top 32 for conservation priority for Africa and 250 globally (Duker and Borre 2001). It was registered as a UNESCO biosphere reserve in 2015 due to its ecological, religious and socioeconomic importance (Worku 2017). However, in line with many developing tropical regions, the Lake Tana basin is under severe stress from high human population growth and the associated need for increased energy, food and potable water security (Karlberg et al. 2015; Mequanent and Mingist 2019), placing substantial pressure on aquatic ecosystem integrity and biodiversity (Dudgeon et al. 2006).
An estimated 28 fish species inhabit Lake Tana (Getahun and Dejen 2012), including 21 that are endemic to Ethiopia and the last remaining cyprinid (Labeobarbus) species flock globally (Nagelkerke et al. 1994). A number of these species are now registered on the IUCN Red List with assessments ranging from Data Deficient to Endangered. Beyond their biodiversity value, Lake Tana’s Labeobarbus and other fishes contribute substantially to local fishers’ income and household protein although their populations have declined by over 90% since the early 1990s (de Graaf et al. 2004, 2006; Tesfaye and Wolff 2014; Dejen et al. 2017). Despite Lake Tana’s size, its tributaries play a disproportionately large role in shaping the population ecology of the resident fishes, and the most critical threats to species recovery are believed to be those occurring within these tributary environments. Lake Tana is fed by more than 40 small seasonal and seven larger perennial tributaries, although the Blue Nile River is its only outflow (Vijverberg et al. 2009). Quantifying the impact of key threats to fishes during their tributary residency is essential in an attempt to rebuild population strength.
Flow alteration is a key threatening process for riverine ecosystems (Poff et al. 1997). The resulting shifts in flow timing, volume and duration, and the ways in which the associated infrastructures are designed and operated, may have a wide range of ecological consequences (Bunn and Arthington 2002). These can include drying or decline of stream flow and reduced connectivity (Jackson and Marmulla 2001), loss of cues to upstream migration (Thorstad et al. 2007) or time of spawning (Nessler et al. 1988; King et al. 1998), restricted access to floodplain or other nursery habitats (Junk et al. 1989; Kingsford 2000; Humphries et al. 2006), thermal depression and a disconnection between the timing of spawning and peaks in availability of larval prey (Humphries et al. 2013; Rolls et al. 2013), or reduced capacity for downstream larval or juvenile dispersal (O’Connor et al. 2006). A plethora of varying-sized dams along Lake Tana’s tributaries are thought to have caused major disruptions to spawning migration routes, and water withdrawals for upstream irrigation have increased the frequency and duration of cease-to-flow events around the slope and lowland spawning reaches (MoWR 2010; Anteneh et al. 2012; Dejen et al. 2017; Gebremedhin et al. 2017).
Excessive instream sedimentation is also a major challenge for aquatic ecosystems in the Lake Tana basin. Riparian erosion is widespread across most of the region (Steenhuis et al. 2014; Abate et al. 2017), and sand mining in the river channel is expanding along many of the lake’s tributaries (Mingist and Gebremedhin, 2016). The effects of this are likely to be of particular significance to benthic-spawning species like Labeobarbus, including the filling in of deep pools and smothering of gravel beds by mobilized sediment, while the associated suspended material is known to lower the survival of pelagic early life-history stages in other species (Westerberger et al. 1996; Kjelland et al. 2015). Indirect effects may also result from raised levels of nutrients and organic matter and physicochemical parameters such as pH or salinity within the water column. Should such peaks occur during the spawning season, the implications for reproductive success (Sutherland 2007) or assemblage structure (Sutherland et al. 2002) may be substantial and add to those impacts already driven by impoundments.
The present study assessed patterns in the abundance of migratory adult and juvenile fishes across five tributaries of Lake Tana from August 2014 to April 2015. We used a mix of multivariate and univariate approaches to (a) examine how assemblage structure varied over time and between tributaries with and without irrigation dams and in-channel sand mining, (b) directly assess how irrigation infrastructure (dams, diversion canals) influences adult and juvenile abundances, and (c) determine which physicochemical water characteristics are most associated with adult assemblage structure and species abundance within these river systems. Although impacts of weirs on upstream spawning migration have been previously inferred in nearby rivers (e.g. Gebremedhin et al. 2017), we included analyses of adult abundances upstream and downstream of one such weir as a partial reference for the potential impacts on assemblage structure within our study rivers and at the time of our fieldwork. The parallel effects on juvenile abundances, and whether weirs or lowered water quality may be linked to variation in assemblage structure, remain poorly understood for the region.
Materials and methods
Study area
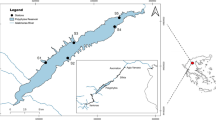
Lake Tana is located at an elevation of approximately 1790 m above sea level, with an estimated surface area of 3050–3600 km2 and a catchment area of approximately 16,500 km2 (Vijverberg et al. 2009). The region’s climate is dominated by a wet season between July and September and a dry season from December to April (Dejen et al. 2017). We selected five tributaries along a 48 km length of the lake’s eastern shoreline, namely the Qimon, Arno, Shini, Chibirna and Guanta rivers (Fig. 1), based on the presence of water resources development works and in-channel sand mining. All have villages or small towns in their close vicinity, and small-scale fish harvesting along their length.
The Qimon River (ca. 22 km) flows directly into Lake Tana, and currently has no in-channel irrigation structures or sand mining activities. The surrounding land uses are predominantly small-scale livestock and crop production. The Arno River (ca. 44 km) also flows directly into Lake Tana but has both irrigation infrastructure and sand mining activities within its channel. Numerous small irrigation diversion weirs are located up to 30 km from its mouth, with a typical headloss of around 1.8 m. Irrigation canals of 1–3 km in length take water withdrawals from above these weirs to small-scale irrigated cropping. Sand mining occurs predominately over a 10 km stretch of the river towards its upper end. Adjacent land use is predominantly small-scale irrigated cropping downstream and non-irrigated cropping and livestock grazing upstream.
The Shini River (ca. 49 km) is a tributary of the Ribb River, with their confluence 8.5 km upstream of the Ribb outflow into Lake Tana. It has both irrigation infrastructure and sand mining activities along its channel. Two irrigation weirs are located 33 and 37 km from its mouth, with a headloss of approximately 2.5 m and irrigation canals of around 1.2 km in length conveying water to small-scale irrigated cropping. Sand mining occurs over approximately 12 km of the river from 7.5 km upstream of the mouth. The Chibirna River (ca. 20 km) is a tributary of the Shini River, with their confluence 7 km upstream of the Ribb River. It has neither in-channel sand mining nor irrigation weirs. Land use along both tributaries is predominantly small-scale irrigated and non-irrigated cropping and livestock grazing.
The Guanta River (ca. 19 km) is a tributary of the Gumara River, with its confluence 28 km upstream of the Gumara outflow. It has an irrigation weir located 4.3 km upstream of its confluence with the Gumara (3 m headloss). Adjacent land use is similar to that of the other rivers.
Field methods
Sampling of fish was done at approximately 30-day intervals from all tributaries on five occasions between August and November 2014, with two trips falling within September. Three sampling sites were selected along a 300 to 1900 m length of each tributary (Fig. 1c), based on ease of channel access, presence of a sufficient sampling area, and availability of expected spawning site characteristics such as sand or gravel beds. We used a combination of two multifilament gillnets with 6, 8, 10 and 12 cm stretched mesh panels and a single monofilament gillnet with 4 and 6 cm mesh panels, each net measuring 25 × 1.5 m. Sampling was limited to between 1000 and 1500 h due to the high evening flow rates following heavy afternoon rains. Multifilament gillnets were set for 4 h and monofilament gillnets were set for 2 h. In August and September 2014, we undertook additional adult fish sampling to compare adult abundances within 50 m upstream and downstream of the Shini River irrigation weir. This used the same sampling gears and soak times as per other adult sampling. For all adult fish sampling, fish were removed from the nets on the bank, identified to species (Nagelkerke and Sibbing 2000; Getahun and Dejen 2012), and their body length (standard length, fork length and total length, all in mm) and wet weight (g) recorded. In all cases, net soak duration was recorded and used to standardize catch per unit effort.
At the same time as each adult fish sampling, six physicochemical water quality parameters were measured: conductivity (μS.cm−1), pH, temperature (°C), dissolved oxygen (mg.L−1) and total dissolved solids (TDS, ppm) using Wagtech Int. portable water quality meters, and Secchi depth (cm) using a standard Secchi Disk.
Juveniles were sampled monthly along the Arno and Shini tributaries from December 2014 to February 2015 and again in April. This sampling period was selected as it spans the main irrigation season. No data were available for March 2015 as some canal sampling sites were dry. Sites were located from approximately 50 m downstream and 30 m upstream of the main irrigation weir on each tributary and within the irrigation canal within 50 m of the canal entrance (site 1 below). Juvenile abundances were also compared along the same irrigation canals, from the head of the canal (site 1) and then a further 500 and 1000 m downstream (sites 2 and 3, respectively). All juvenile sampling used a combination of 25 × 2 m beach seine and 1.8 × 1.6 m mosquito nets with a mesh size of 5 and 2 mm, respectively. Three replicate hauls were taken with each net, along 100 m of the canal and tributary sites, around 20 to 35 m apart. At the upstream and downstream sampling sites, each net haul was 10 m2 in area whereas they were either 7.5 m2 (site 1) or 3.5 m2 (sites 2 and 3) within the canals due to space limitations. Accordingly, juvenile abundance estimates were adjusted to a standardized sampling area of 10 m2 (comparison of tributary sites with canal site 1) or 7.5 m2 (comparison of canal sites 1–3). Apart from L. beso, Labeobarbus juveniles could not be identified any further over the size range encountered, although were easily distinguished from other juveniles including Enteromius species based on the identification key of Anteneh et al. (2013). We also discriminated Garra juveniles from adults by their body shape and length. Accordingly, our juvenile data were grouped as either L. beso, remaining Labeobarbus species, or Garra dembecha, and also all species pooled.
Statistical analyses
Adult fish data were pooled within each tributary x sampling trip combination for final analyses. Non-metric multi-dimensional scaling (NMDS) ordination was first used to display patterns in assemblage structure between tributaries and the five sampling trips, based on the Bray-Curtis similarity resemblance matrix and fourth-root transformed data. One-way analysis of similarities (ANOSIM) was used to test for significant differences between tributary and sampling trip groups using the same similarity matrices, while similarity percentages (SIMPER) analysis was used to examine the relative contribution of individual species to any significant pair-wise comparison of tributaries or sampling trips.
The effect of the Shini River irrigation dam on total adult abundances of the four dominant migratory fishes (Labeobarbus intermedius, L. beso, L. brevicephalus and L. nedgia) was examined for August and September 2014 using simple frequency histograms. Variation in juvenile abundances was then examined between upstream and downstream of the Arno, Shini and Guanta River irrigation weirs and within each diversion canal entrance using two-way analyses of variance (ANOVA). These were performed separately for all species combined, Labeobarbus species combined (excluding L. beso), and both L. beso and G. dembecha on their own, with both Tributary and Site treated as fixed factors. Variation in juvenile abundances was also examined between the mouth and downstream sites along the Arno and Shini irrigation canals using two-way ANOVAs, with Tributary and Site again treated as fixed factors and separate analyses for the four datasets (all species combined, Labeobarbus species excluding L. beso, L. beso, G. dembecha). In both cases, the ANOVA was performed using log (x + 1) transformed data and the nature of any significant differences was determined using Tukey HSD post-hoc tests.
Patterns in water quality across the five tributaries and sampling trips were examined using metric multi-dimensional scaling ordination based on the Euclidian distance resemblance matrix and normalized log (x + 1) transformed data. ANOSIM was then used to test for significant differences between river and trip groups, based on the same similarity matrices, and the variables most responsible for any significant pairwise comparisons were determined using SIMPER analyses.
BIO-ENV (Clarke and Gorley 2015) was used to investigate the influence of water quality variables on adult fish assemblage structure across the five tributaries and sampling trips. This employed Spearman Rank correlation coefficients to test for significant relationships between the adult fish abundance and water quality data matrices. Again, these were based on the Bray-Curtis similarity matrix of the fourth-root (fish) and Euclidean distance matrix of normalized log (x + 1) (water quality) datasets. BIO-ENV results were expressed as the best solution involving up to five water quality variables.
Significant associations between each water quality variable and adult fish abundance were tested using two-tailed Spearman Rank correlations for each of the six most abundant fish species across all tributaries and sampling trips. Due to the number of tests undertaken, significant relationships were accepted at p ≤ 0.10 to reduce the risk of Type II errors.
All univariate analyses were performed using SPSS version 26, while multivariate analyses were undertaken using Primer version 7.0.13.
Results
Adult fish abundance, diversity and assemblage structure
A total of 1302 fish and 13 species were sampled over the five tributaries and five sampling trips (Table 1). These were dominated by Labeobarbus species (9 species, 95.5% of the total catch) while the remaining taxa comprised Enteromius humilis, Clarias gariepinus, Garra dembecha and Oreochromis niloticus. Labeobarbus intermedius and L. brevicephalus were the most abundant species (72.5% overall) and were encountered across all tributaries and on most sampling trips. Labeobarbus nedgia and L. beso were absent from just the Qimon River, while all remaining species were found in one to three tributaries. The total species count per tributary ranged from four in the Guanta to nine in those without in-channel dams or sand extraction (Chibirna and Qimon). Ten species and 407 fish were encountered during the first September trip and either six or eight species and 202 to 251 fish during the remaining trips.
The NMDS of adult abundance data showed considerable variation in assemblage structure both within and between tributaries (Fig. 2a). ANOSIM detected significant differences among tributaries (Table 2, p = 0.001) as well as between all but two of the tributary pairs: comparison of the Shini River with its Chibirna tributary and of the Qimon and Guanta rivers. No consistent separation of irrigation from non-irrigation tributaries was evident, nor of tributaries with in-channel sand mining from those without it. In contrast, no significant differences in adult assemblage structure were detected between sampling trips (Fig. 2b; Table 2).
SIMPER analysis indicated that most of the significant tributary comparisons were primarily driven by differences in the abundance of L. nedgia, L. beso and L. brevicephalus (Table 3). The single exception was variation between the Arno and Qimon rivers, which was mainly due to differences in O. niloticus and L. nedgia abundances. The level of dissimilarity between tributary pairs was highest for the Shini and Qimon (55.6%) and Chibirna and Qimon (52%) rivers and lowest for the Arno and Guanta rivers (35.7%).
Effect of irrigation infrastructure on adult fish
Adults of the four dominant Labeobarbus species in the Shini River were present in much higher abundances downstream than upstream of the sampled irrigation dam during August and September 2014 (Fig. 3). Overall, abundances below the dam were four-fold higher than upstream. Labeobarbus intermedius was the only species captured upstream of the dam in August, while L. nedgia was only encountered downstream in September.
Variation in abundance of the dominant migratory Labeobarbus species between upstream (black bars) and downstream (grey bars) of the Shini River irrigation dam, (a) August and (b) September 2014. L. int, Labeobarbus intermedius; L. brev, Labeobarbus brevicephalus. Data are the total number of each species sampled per site per month
Patterns in juvenile abundances
Juvenile abundances were generally highest in the Shini and Arno rivers and upstream of the irrigation weirs (Fig. 4; Table 4), although we detected a significant interaction between the effects of tributary and site for two of the data sets (all species pooled, Labeobarbus species pooled). In both cases, there was no difference in abundances between sites in the Arno River, but significantly higher abundances either upstream (Shini) or downstream (Guanta) of the irrigation weir. For L. beso on its own, abundances were significantly higher in the Arno and Shini rivers and upstream of the irrigation weir. While no significant site difference was detected for G. dembecha juveniles, their abundances were higher in the Arno and Shini rivers than in the Guanta.
Variation in mean juvenile abundance between sites upstream, downstream and within the irrigation canal of the Arno, Shini and Guanta rivers, December 2014 to April 2015. (a) All species combined, (b) Labeobarbus spp. excluding L. beso, (c) L. beso, and (d) Garra dembecha. Variation between sites is shown separated by tributary for (a) and (b) due to a significant tributary x site interaction term (Table 4). Note varying y-axis scales. Data are shown ±1 standard error
Juvenile abundances did not show any statistical differences along the Arno and Shini irrigation canals for any of the four data sets (Fig. 5, Table 5). Nevertheless, mean abundances were consistently lower at site 3 for all four data sets and both canals, declining from sites 1 to 3 by an average of 46.4% (Fig. 5).
Variation in mean juvenile abundance along the Arno River and Shini River irrigation canals, December 2014 to April 2015. (a) All species combined, (b) Labeobarbus spp. excluding L. beso, (c) L. beso, and (d) Garra dembecha. Numbers shown on each graph indicate the mean percentage decline in abundance between the Mouth and 1000 m sites. Note varying y-axis scales. Data are shown ±1 standard error
Variation in water quality and its influence on adult abundances
Variation in the six water quality parameters across the five tributaries is shown in Tables 6. When examined together, these indicated significant variation in the physicochemical characteristics among tributaries overall (p = 0.001) and between six of the 10 tributary pairs (Fig. 6a; Table 7). However, significant temporal variation was only evident between the first and final two sampling trips (Fig. 6b; Table 7). SIMPER analysis (Table 8) revealed that differences between tributaries and sampling trips were mostly driven by water temperature and the two parameters related to water turbidity (TDS, Secchi depth).
Water physicochemical characteristics appeared to explain some of the variability in adult assemblage structure across the study tributaries and sampling interval (Table 9). The variable most associated with these patterns was conductivity (rs = 0.398 on its own). In combination with TDS, pH and Secchi depth, it explained almost 44% of the variation in assemblage structure. Similarly, the variables most associated with the abundances of individual species were conductivity and TDS (Table 10). Interestingly, these associations were all positive, indicating greater adult abundances under conditions of higher conductivity and turbidity levels.
Discussion
The diversity and uniqueness of Lake Tana’s fishes have now been established for over 20 years, including the systematics and ecomorphological differentiation of members of the Labeobarbus species flock (Nagelkerke et al. 1994; Nagelkerke and Sibbing 2000) and how these sit within the broader assemblage (Getahun and Dejen 2012). However, we now also have a stronger sense of the main threats faced by these species, particularly in conjunction with their tributary habitat requirements and use. Findings from the present study add to this understanding and demonstrate the effects of irrigation infrastructures on adult fish diversity and adult and juvenile abundances within Lake Tana tributaries, as well as how physicochemical water characteristics may shape adult assemblages and abundances.
Our findings particularly indicate the need for a stronger understanding of Lake Tana tributaries as individual fish biodiversity units. Most tributary pairs had a significantly different fish assemblage, and the prominence of tributary variation in physicochemical water characteristics further reinforces this notion. For Labeobarbus species, de Graaf et al. (2005) concluded that there were no species preferences for different tributaries, albeit from having only sampled around river mouths along the Lake Tana shoreline. For practical reasons, past studies of Tana tributary fishes (present study included) have only examined a subset of the lake’s tributaries, and some differences in interpreting patterns may have arisen from the choice of fishing methods or other aspects of the sampling design. Determining how assemblage structures vary across all Tana tributaries using past data and comparable metrics would help establish existing monitoring gaps as well as a stronger sense of any key threat hotspots from planned impoundments or other factors.
Instream barriers can play a role in structuring migrating tropical fish populations (Lorenzen et al. 2007), including across the present study region. We detected higher diversity and abundances of adult fish downstream than upstream of the Shini irrigation dam at the end of the 2014 wet season and onset of the Labeobarbus spawning season. Similarly, when Gebremedhin et al. (2017) compared assemblages between dam and non-dam Tana tributaries, they found significantly higher Labeobarbus diversity downstream of irrigation dams on two tributaries but not along two other undammed streams. Most of our 15 assemblage sampling sites were downstream of irrigation dams and this may have contributed to the lack of an irrigation signal in our NMDS and ANOSIM results for assemblage structure.
In contrast, however, we found a general pattern of juvenile entrainment upstream of the Arno and Shini irrigation dams. This is the first direct demonstration of irrigation dams appearing to hinder downstream fish movement in any East African river system. The capacity for juvenile downstream movement is critical in many fishes for subsequent growth and adult development (O’Connor et al. 2006) and has also been inferred from longitudinal abundance patterns in another Tana tributary, the Gelda River (Anteneh et al. 2011). Yet, juveniles may often be reluctant to navigate instream barriers (Behrmann-Godel and Eckmann 2003) or else sustain lethal and sub-lethal injuries in the process (Bell and DeLacy 1972). Both factors could potentially lead to downstream recruitment deficits in river-spawning species. Improved weir designs (Haro et al. 1998) or options such as installing bypass channels around structures (Gebler 1998; Lorenzen et al. 2007) may help reduce such impediments. In situations where flow velocities can be adjusted to account for the swimming capabilities of small fish, doing so during critical migration points in the lunar or annual cycle may be a cheaper, more practical solution to boost recruitment downstream of existing structures. Unfortunately, Ethiopian instream barriers often have an inflexible, fixed-crest design (authors’ personal observations), and solutions such as bypass channels may have greater efficacy.
Although we did not detect a statistically significant pattern in juvenile abundances along the Arno and Shini irrigation canals, there were nonetheless substantial numbers of juveniles within them and an average reduction of around 46% in abundance between the canal entrance and 1000 m downstream. Whether irrigation canals represent a supportive habitat extension for Tana tributary juveniles and small fishes to any extent is unclear. However, there are two possible mechanisms underpinning the reduced abundances downstream. The first is that dispersal into canals is mostly limited to their upstream reaches. Roberts and Rahel (2008) found substantial movement by trout within a large canal system using radio-tracking. Nevertheless, extensive entrainment and dispersal of fish have not always been evident along even large canal systems (Daniels 2001; although see King and O’Connor 2007). A radio-tracking or similar approach would be needed to gauge the extent to which varying dispersal rates were responsible for our patterns.
The second potential explanation, however, is declining habitat suitability downstream, particularly in terms of loss of water depth. Roberts and Rahel (2008) found a 77% mortality rate of their radio-tracked fish after flows were diminished at the end of the irrigation season and concluded that dewatering was a major threat to fish survivorship within irrigation systems. We observed large numbers of dead juveniles along the Arno and Shini canals at the end of the irrigation season, and anecdotal reports from nearby farmers were similar (G. Teshome, unpublished data). Entrainment of fish within downstream canal reaches and subsequent mortalities following dewatering may be a significant issue in the present study system. However, irrespective of a canal’s habitat suitability and mortality losses, the best ecological outcome would still likely be the retention of fish within tributary channels. A variety of fish diversion screens have been implemented internationally to minimize fish ingress into irrigation systems (Baumgartner and Boys 2012), although none have yet been tested along Tana tributaries. Exploring these as a mechanism to reduce recruitment losses in the study region would have strong merit.
The present study is the first to directly link tributary water quality with fish assemblage and population measures in the Tana basin. Conductivity and turbidity-related variables (Secchi depth, TDS) had the strongest association with both adult assemblage structure and individual species’ abundance. However, past studies [reviews by Kjelland et al. (2015) and Koehnken et al. (2020)] have tended to find negative associations with the water quality parameter(s), whereas the present study detected positive relationships. One explanation for this may be the use by Tana tributary fishes of conditions around peak flow periods for their cue to shift upstream to spawn (Anteneh et al. 2012). Higher flow rates are often linked with increased turbidity levels (Chen and Chang 2019) although not necessarily conductivity or other solutes (Dzikowski and Jobard 2012; Heppell et al. 2017). In Malawi’s Lake Chilwa basin, Jamu et al. (2003) found that stream discharge, conductivity and suspended solids levels were significant predictors of the timing of spawning migration in two Barbus species. Similar relationships are yet to be modelled for any Tana fishes and represent a substantial knowledge gap, although may clarify patterns observed in the present study.
Studies elsewhere have linked increased instream turbidity and suspended material levels to various anthropogenic sediment disturbances (Sutherland et al. 2002; Jamu et al. 2003; Kjelland et al. 2015; Koehnken et al. 2020) although not necessarily for conductivity (Bayram and Önsoy 2015). Agricultural practices and vegetation clearing have resulted in substantial erosion problems across the Tana region (Steenhuis et al. 2014), and high levels of downstream siltation have been documented in two of our study catchments (Ribb and Gumara: Abate et al. 2017; Zimale et al. 2018). However, fluvial sand and gravel extraction also occurs along most Tana tributaries (Mingist and Gebremedhin 2016). More broadly, such activities typically result in at least channel incision and loss or increased mobilization of gravel and sand bars, as well as exacerbated levels of siltation and parameters such as turbidity downstream (Koehnken et al. 2020).
Mingist and Gebremedhin (2016) is the only published study to have considered the potential impacts of fluvial sand and gravel mining on freshwater fish anywhere within Africa. Yet, quantifying the extent to which aquatic biota in Tana tributaries are being affected by sedimentation and associated reductions in water quality requires more evidence. The main season for sand mining in the Arno and Shini rivers matches the peak timing of Labeobarbus species spawning migrations into the study tributaries (August to October: e.g. Palstra et al. 2004; Gebremedhin et al. 2012; Anteneh et al. 2012, 2013; Teshome et al. 2015). Sedimentation and turbidity over those periods would likely have consequences for both adults (e.g. loss of spawning habitat in lithophilic spawners: Sutherland et al. 2002; Sutherland 2007) and early life-history stages (e.g. lowered egg retention and survival in smothered gravel beds; reduced visual acuity, growth and survival in larvae) in fishes of the Tana tributaries. However, discriminating the effects of instream materials extraction from those due to landscape erosion will be challenging, especially given the diffuse nature of the latter, and require careful, control–impact sampling designs (Underwood 1994).
Conclusions
We found Lake Tana tributaries to comprise a suite of unique fish biodiversity units. From the end of the 2014 wet season and into the dry season, variability in assemblage structure among tributaries per se was stronger than either temporal patterns or between rivers with and without irrigation dams or sand-mining. Nevertheless, the total number of species was highest in the two tributaries without either dams or sand extraction. Effective conservation measures at the lake scale will need to bear in mind that protecting assemblages in only one or more tributaries will not guarantee the conservation of all species.
We agree with previous assertions that irrigation dams disrupt upstream spawning migration in Lake Tana fishes and add evidence that the subsequent downstream movement of juveniles is also substantially impacted. Although irrigation canals off tributaries create additional aquatic habitat, we think they offer very limited ecological functionality and potentially lead to significant population losses following dewatering. We recommend implementing measures that facilitate multidirectional movement around weirs and dams and screening of irrigation intake points to minimize losses of juvenile fish into canals.
Water quality parameters such as turbidity and conductivity were highest during the peak spawning period, possibly due to a coincidence between higher-flow intervals and associated channel disturbance and the Labeobarbus seasonal spawning strategy that maximizes access of the fish to spawning sites and dispersal of propagules. Direct assessments of how poor water quality may be inflated through catchment erosion or sand mining, using robust sampling designs, are urgently needed to develop effective intervention strategies. Establishing a greater understanding of how irrigation infrastructure and water quality impact the early life history stages of fish within tributaries as a priority knowledge gap will help limit future recruitment failures and loss of Lake Tana fish species.
Data availability
All data are available from the corresponding author upon request.
References
Abate M, Nyssen J, Moges MM, Enku T, Zimale FA, Tilahun SA, Adgo E, Steenhuis TS (2017) Long-term landscape changes in the Lake Tana Basin as evidenced by delta development and floodplain aggradation in Ethiopia. Land Degrad Dev 28:1820–1830. https://doi.org/10.1002/ldr.2648
Anteneh W, Getahun A, Dejen E (2011) Assessment of downstream dispersal of juveniles of the migratory riverine spawning Labeobarbus species of Lake Tana (Ethiopia). Proc Ethiop Fish Aquat Sci Assoc 2:198–206
Anteneh W, Getahun A, Dejen E (2013) Spawning migrations of Lake Tana Labeobarbus spp. (Teleostei: Cyprinidae) in the Ribb River, Ethiopia. Afr J Aquat Sci 38(Suppl):61–68. https://doi.org/10.2989/16085914.2013.776942
Anteneh W, Getahun A, Dejen E, Sibbing FA, Nagelkerke LA, de Graaf M, Wudneh T, Vijverberg J, Palstra AP (2012) Spawning migrations of the endemic Labeobarbus (Cyprinidae, Teleostei) species of Lake Tana, Ethiopia: status and threats. J Fish Biol 81:750–765. https://doi.org/10.1111/j.1095-8649.2012.03362.x
Baumgartner LJ, Boys C (2012) Reducing the perversion of diversion: Applying world-standard fish screening practices to the Murray-Darling Basin. Ecol Manag Restor 13:135–143. https://doi.org/10.1111/j.1442-8903.2012.00655.x
Bayram A, Önsoy H (2015) Sand and gravel mining impact on the surface water quality: a case study from the city of Tirebolu (Giresun Province, NE Turkey). Environ Earth Sci 73:1997–2011. https://doi.org/10.1007/s12665-014-3549-2
Behrmann-Godel J, Eckmann R (2003) A preliminary telemetry study of the migration of silver European eel (Anguilla anguilla L.) in the River Mosel, Germany. Ecol Freshw Fish 12:196–202. https://doi.org/10.1034/j.1600-0633.2003.00015.x
Bell M, DeLacy A (1972) A compendium on the survival of fish passing through spillways and conduits. Fisheries Engineering Research Program, US Army Engineers Division, North Pacific Corps of Engineers, Portland
Bunn SJ, Arthington AH (2002) Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manag 30:492–507. https://doi.org/10.1007/s00267-002-2737-0
Chen J, Chang H (2019) Dynamics of wet-season turbidity in relation to precipitation, discharge, and land cover in three urbanizing watersheds, Oregon. River Res Appl 35:892–904. https://doi.org/10.1002/rra.3487
Clarke KR, Gorley RN (2015) Primer v7: User Manual / Tutorial. Primer-E, Plymouth
Daniels RA (2001) Untested assumptions: the role of canals in the dispersal of sea lamprey, alewife, and other fishes in the eastern United States. Environ Biol Fish 60:309–329. https://doi.org/10.1023/A:1011032907484
de Graaf M, Machiels MAM, Wudneh T, Sibbing FA (2004) Declining stocks of Lake Tana’s endemic Barbus species flock (Pisces; Cyprinidae): natural variation or human impact? Biol Conserv 116:277–287. https://doi.org/10.1016/S0006-3207(03)00198-8
de Graaf M, Nentwich D, Osse JWM, Sibbing FA (2005) Lacustrine spawning: is this a new reproductive strategy among ‘large’ African cyprinid fishes? J Fish Biol 66:1214–1236. https://doi.org/10.1111/j.0022-1112.2005.00671.x
de Graaf M, van Zwieten PAM, Machiels MAM, Lemma E, Wudneh T, Dejen E, Sibbing FA (2006) Vulnerability to a small-scale commercial fishery of Lake Tana’s (Ethiopia) endemic Labeobarbus compared with African catfish and Nile tilapia: an example of recruitment-overfishing? Fish Res 82:304–318. https://doi.org/10.1016/j.fishres.2006.05.011
Dejen E, Anteneh W, Vijverberg J (2017) The decline of the Lake Tana (Ethiopia) fisheries: causes and possible solutions. Land Degrad Dev 28:1842–1851. https://doi.org/10.1002/ldr.2730
Duker L, Borre L (2001) Biodiversity conservation of the World’s lakes: a preliminary framework for identifying priorities. LakeNet Report Series, Number 2. Monitor International, Annapolis, Maryland
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DI, Lévêque C, Naiman RJ, Prieur-Richard A-H, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182. https://doi.org/10.1017/S1464793105006950
Dzikowski M, Jobard S (2012) Mixing law versus discharge and electrical conductivity: relationships: application to an alpine proglacial stream. Hydrol Process 26:2724–2732. https://doi.org/10.1002/hyp.8366
Gebler RJ (1998) Examples of near-natural fish passes in Germany: drop structure conversions, fish ramps and bypass channels. In: Jungwirth M, Schmutz S, Weiss S (eds) Fish migration and fish bypasses. Blackwell Science, London, pp 403–419
Gebremedhin S, Getahun A, Anteneh W, Gedif B, Gashu B, Tefera B, Berhanie Z, Alemaw D (2017) Effect of large weirs on abundance and diversity of migratory Labeobarbus species in tributaries of Lake Tana, Ethiopia. Afr J Aquat Sci 42:367–373. https://doi.org/10.2989/16085914.2017.1411774
Gebremedhin S, Mingist M, Getahun A, Anteneh W (2012) Spawning migration of Labeobarbus spp. (Pisces: Cyprinidae) of Lake Tana to Arno-Garno River, Lake Tana sub-basin, Ethiopia. SINET: Ethiopian J Sci 35:95–106
Getahun A, Dejen E (2012) Fishes of Lake Tana: A guidebook. Addis Ababa University Press, Addis Ababa
Haro A, Odeh M, Noreika J, Castro-Santos T (1998) Effect of water acceleration on downstream migratory behaviour and passage of Atlantic salmon smolts and juvenile American shad at surface bypasses. T Am Fish Soc 129:351–380
Heppell CM, Binley A, Trimmer M, Darch T, Jones A, Malone E, Collins AL, Johnes PJ, Freer JE, Lloyd CEM (2017) Hydrological controls on DOC: nitrate resource stoichiometry in a lowland, agricultural catchment, southern UK. Hydrol Earth Syst Sci 21:4785–4802. https://doi.org/10.5194/hess-21-4785-2017
Humphries P, Cook RA, Richardson AJ, Serafini LG (2006) Creating a disturbance: manipulating slackwaters in a lowland river. River Res Appl 22:525–542. https://doi.org/10.1002/rra.920
Humphries P, Richardson A, Wilson G, Ellison TL (2013) River regulation and recruitment in a protracted-spawning fish. Ecol Appl 23:208–225. https://doi.org/10.1890/11-2255.1
Jackson DC, Marmulla G (2001) The influence of dams on river fisheries. In: Marmulla G (ed) Dams, fish and fisheries. Opportunities, challenges and conflict resolution. FAO Fisheries Technical Paper No. 419. FAO, Rome, pp 1–44
Jamu DM, Chimphamba JB, Brummett RE (2003) Land use and cover changes in the Likangala catchment of the Lake Chilwa basin, Malawi: implications for managing a tropical wetland. Afr J Aquat Sci 28:123–135. https://doi.org/10.2989/16085910309503777
Junk WJ, Bayley PB, Sparks RE (1989) The flood-pulse concept in river-floodplain systems. Can J Fish Aquat Sci Spec Publ 106:110–127
Karlberg L, Hoff H, Amsalu T, Andersson K, Binnington T, Flores-López F, de Bruin A, Gebrehiwot SG, Gedif B, Zur Heide F, Johnson O, Osbeck M, Young C (2015) Tackling complexity: understanding the food-energy-environment nexus in Ethiopia’s Lake Tana sub-basin. Water Altern 8:710–734
King AJ, O’Connor JP (2007) Native fish entrapment in irrigation systems: A step towards understanding the significance of the problem. Ecol Manag Restor 8:32–37. https://doi.org/10.1111/j.1442-8903.2007.00329.x
King JM, Cambray JA, Impson DN (1998) Linked effects of dam-released floods and water temperature on spawning of the Clanwilliam yellowfish Barbus capensis. Hydrobiologia 384:245–265. https://doi.org/10.1023/A:1003481524320
Kingsford RT (2000) Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecol 25:109–127. https://doi.org/10.1046/j.1442-9993.2000.01036.x
Kjelland MF, Woodley CM, Swannack TM, Smith DL (2015) A review of the potential effects of suspended sediment on fishes: potential dredging-related physiological, behavioural and transgenerational implications. Environ Syst Decis 35:334–350. https://doi.org/10.1007/s10669-015-9557-2
Koehnken L, Rintoul MS, Goichot M, Tickner D, Loftus A-C, Acreman MC (2020) Impacts of riverine sand mining on freshwater ecosystems: A review of the scientific evidence and guidance for future research. River Res Appl 36:362–370. https://doi.org/10.1002/rra.3586
Lorenzen K, Smith L, Nguyen Khoa S, Burton M, Garaway C (2007) Guidance Manual: management of impacts of irrigation development on fisheries. The WorldFish Center, Penang, and International Water Management Institute, Colombo
Marten K, Segers HH (2009) Endemism in inland waters. In: Likens GE (ed) Encyclopedia of Inland Waters, Volume 1. Elsevier, Oxford, pp. 423–430
Mequanent D, Mingist M (2019) Potential impact and mitigation measures of pump irrigation projects on Lake Tana and its environs, Ethiopia. Heliyon 5:e03052. https://doi.org/10.1016/j.heliyon.2019.e03052
Mingist M, Gebremedhin S (2016) Could sand mining be a major threat for the declining endemic Labeobarbus species of Lake Tana, Ethiopia? Singapore J Trop Geo 37:195–208. https://doi.org/10.1111/sjtg.12150
MoWR (Ministry of Water Resources) (2010) Environmental and social impact assessment of about 20,000 ha irrigation and drainage schemes at Megech pump (Seraba), Ribb and Anger dam environmental and social impact assessment of the Ribb irrigation and drainage project, Volume 2/2: Annexes (Final version). Ethiopian Ministry of Water Resources, Addis Ababa
Nagelkerke LAJ, Sibbing FA (2000) The large barbs (Barbus spp., Cyprinidae, Teleostei) of Lake Tana (Ethiopia), with a description of a new species, Barbus osseensis. Neth J Zool 50:179–214. https://doi.org/10.1163/156854200X00072
Nagelkerke LAJ, Sibbing FA, van den Boogaart JGM, Lammens EHRR, Osse JWM (1994) The barbs (Barbus spp.) of Lake Tana: a forgotten species flock? Environ Biol Fish 39:1–22. https://doi.org/10.1007/bf00004751
Nessler TP, Muth RT, Wasowicz AF (1988) Evidence for baseline flow spikes as spawning cues for Colorado squawfish in the Yampa River, Colorado. Am Fish Soc Symp 5:68–79
O’Connor JP, O’Mahony DJ, O’Mahony JM, Glenane TJ (2006) Some impacts of low and medium head weirs on downstream fish movement in the Murray–Darling Basin in southeastern Australia. Ecol Freshw Fish 15:419–427. https://doi.org/10.1111/j.1600-0633.2006.00162.x419
Palstra AJ, de Graaf M, Sibbing FA (2004) Riverine spawning and reproductive segregation in a lacustrine cyprinid species flock, facilitated by homing? Anim Biol 54:393–415
Poff NL, Allan JD, Bain MB, Karr JR, Prestergaard KL, Richter BD, Sparks RE, Stromberg JC (1997) The natural flow regime. BioScience 47:769–784. https://doi.org/10.2307/1313099
Roberts JJ, Rahel FJ (2008) Irrigation canals as sink habitat for trout and other fishes in a Wyoming drainage. T Am Fish Soc 137:951–961. https://doi.org/10.1577/T07-058.1
Rolls RJ, Growns IO, Khan TA, Wilson GG, Ellison TL, Prior A, Waring CC (2013) Fish recruitment in rivers with modified discharge depends on the interacting effects of flow and thermal regimes. Freshw Biol 58:1804–1819. https://doi.org/10.1111/fwb.12169
Steenhuis TS, Tilahun SA, Tessema ZK, Tebedu TY, Moges M, Zimale FA, Worqlu AW, Alemu ML, Ayana EK, Mohamed YA (2014) Soil erosion and discharge in the Blue Nile Basin: Trends and challenges. In: Assefa MM, Abtew W, Shimelis GS (eds) Nile River Basin: Ecohydrological Challenges, Climate Change and Hydropolitics. Springer, Switzerland, pp 133–147. https://doi.org/10.1007/978-3-319-02720-3_8
Sutherland AB (2007) Effects of increased suspended sediment on the reproductive success of an upland crevice spawning minnow. T Am Fish Soc 136:416–422. https://doi.org/10.1577/T06-046.1
Sutherland AB, Meyer JL, Gardiner EP (2002) Effects of land cover on sediment regime and fish assemblage structure in four southern Appalachian streams. Freshw Biol 47:1791–1805. https://doi.org/10.1046/j.1365-2427.2002.00927.x
Tesfaye G, Wolff M (2014) The state of inland fisheries in Ethiopia: a synopsis with updated estimates of potential yield. Ecohydrol Hydrobiol 14:200–209. https://doi.org/10.1016/ecohyd.2014.05.001
Teshome G, Getahun A, Mingist M, Anteneh W (2015) Spawning migration of Labeobarbus species to some tributary rivers of Lake Tana, Ethiopia. Ethiopian J Sci Technol 8:37–50. https://doi.org/10.4314/ejst.v8i1.4
Thorstad EB, Økland F, Aarestrup K, Heggberget TG (2007) Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts. Rev Fish Biol Fisher 18:345–371. https://doi.org/10.1007/s11160-007-9076-4
Underwood AJ (1994) Beyond BACI: Sampling designs that might reliably detect environmental disturbance. Ecol Appl 4:3–15. https://doi.org/10.2307/1942110
Vijverberg J, Sibbing FA, Dejen E (2009) Lake Tana: Source of the Blue Nile. In: Dumont HJ (ed) The Nile: Origin, environments, limnology and human use. Monographiae Biologicae, vol 89. Springer Science + Business Media B.V, Dordrecht, pp 163–192. https://doi.org/10.1007/978-1-4020-9726-3_9
Westerberger H, Rönnbäck P, Frimansson H (1996) Effects of suspended sediments on cod eggs and larvae and on the behaviour of adult herring and cod. ICES CM Report 1996/E:26. International Council for Exploration of the Sea, Thünen-Institut, Rostock
Worku M (2017) Lake Tana as Biosphere Reserve: Review. J Tourism Hospit 6:310. https://doi.org/10.4172/2167-0269.1000310
Zimale FA, Moges MM, Alemu ML, Ayana EK, Demissie SS, Tilahun SA, Steenhuis TS (2018) Budgeting suspended sediment fluxes in tropical monsoonal watersheds with limited data: the Lake Tana basin. J Hydrol Hydromech 66:65–78. https://doi.org/10.1515/johh-2017-0039
Acknowledgements
The authors thank The Rufford Foundation (Grant No. 10137-2) and the Blue Nile Water Institute at Bahir Dar University for their financial support of this study. The Bahir Dar Fish and Other Aquatic Life Research Center loaned us sampling gears, and we especially thank B. Hailu for assistance in the field and with fish species identification. Constructive feedback from F. Taddese helped improve the manuscript prior to submission.
Code availability
Not applicable.
Funding
Financial support was provided by The Rufford Foundation (Grant No. 10137–2 to AG) and the Blue Nile Water Institute at Bahir Dar University.
Author information
Authors and Affiliations
Contributions
GT, AG, MM and WA conceived and designed the study, which was undertaken with funding obtained by AG. GT performed the fieldwork, and GW analysed the data. GT and GW wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All sampling was undertaken in accordance with animal ethics approval from Bahir Dar University’s Animal Ethics and Experimentation Committee.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved of the manuscript prior to its submission.
Competing interests
This manuscript is original work carried out by the authors. This work has not been published previously and is not under consideration for publication elsewhere. The authors declare that they have no known competing financial interests, personal relationships or conflicts of interest that could have appeared to influence the subject matter or materials reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teshome, G., Getahun, A., Mingist, M. et al. Influence of irrigation infrastructures and water quality on fish assemblages in Lake Tana tributaries, north-west Ethiopia. Environ Biol Fish 104, 653–673 (2021). https://doi.org/10.1007/s10641-021-01101-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01101-y




 Chibirna, ■ Arno,
Chibirna, ■ Arno,  Qimon, ▲ Guanta) and (b) five sampling trips (▲ 1, ■ 2,
Qimon, ▲ Guanta) and (b) five sampling trips (▲ 1, ■ 2,  3,
3,  4, ● 5), August to November 2014
4, ● 5), August to November 2014



 Chibirna, ■ Arno,
Chibirna, ■ Arno,  Qimon, ▲ Guanta) and (b) five sampling trips (▲ 1, ■ 2,
Qimon, ▲ Guanta) and (b) five sampling trips (▲ 1, ■ 2,  3,
3,  4, ● 5), August to November 2014
4, ● 5), August to November 2014