Abstract
Large dams disrupt river ecosystems, causing negative effects on fish assemblages. Few studies, however, have investigated how Neotropical fish diversity responds to river regulation in longer temporal scales. In this study, we used a multi-metric approach to investigated temporal changes in taxonomic and functional fish diversity over 15 years-period following the construction of a large dam in the Upper Tocantins River, Amazon. We analyzed biological and environmental data collected in the impounded area before (2000–2002) and after (2003–2014) river regulation. Diversity metrics responded differently to the impoundment. Some metrics were not affected, as they showed little variation over years, such as fish abundance, species, and trait richness. Other metrics changed significantly between periods, such as taxonomic composition, temporal beta diversity, and trait abundance. Species with traits that are sensitive to hydrological alterations declined in the impoundment, while sedentary species were favored. Assemblage trajectories were variable, but community structure developed into a different state in the impoundment. Landscape variables explained changes in fish dissimilarity over time. In general, our study supports the notion that fish diversity in Amazonian rivers is vulnerable to river damming, but also indicates that different metrics show particular responses to this disturbance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Neotropical region is home to the most diverse fish fauna in the world, in taxonomic, functional, and phylogenetic terms (Helfman et al. 2009; Nelson et al. 2016; Toussaint et al. 2016). However, the construction of hydropower dams has disrupted the structure and functioning of river ecosystems (Winemiller et al. 2016), affecting fluvial connectivity (Barbosa et al. 1999; Pompeu et al. 2012), habitat distribution and environmental conditions (Agostinho et al. 2016). Hydropower expansion has significantly threatened Neotropical fish diversity (Pelicice et al. 2017), especially because impounded areas cause permanent changes to limnological conditions, the loss of riverine habitats, and favor species invasions (Agostinho et al. 2007; Pelicice et al. 2015). Impacts on fish assemblages are enhanced when several dams regulate the river course, forming cascades of reservoirs, where lotic stretches are significantly reduced (Barbosa et al. 1999; Santos et al. 2017; Pelicice et al. 2018).
Biological communities may respond abruptly or gradually to disturbances and reassembly patterns may show distinct trajectories over time (Matthews et al. 2013). Regarding dam effects, the direction of assemblage change may vary according to the species present, as well as the conditions, habitats, and resources available in the new ecosystem (Agostinho et al. 2016; Turgeon et al. 2016). In general, long-distance, larger, and long-lived migratory species are negatively affected and tend to decline or disappear (Agostinho et al. 2008; Santos et al. 2017). On the other hand, small and sedentary species find favorable conditions, once they have a high reproductive potential and short longevity (Agostinho et al. 2016). Moreover, some species may persist in very low numbers (Agostinho et al. 2007; Monaghan et al. 2019), making community structure vulnerable to stochastic events. Thus, the structure of fish assemblages in impoundments may show a persistent variation or non-equilibrium dynamics (Loures and Pompeu 2018), although, over longer time scales, a new state may emerge, with a novel structure (Mol et al. 2007; Orsi and Britton 2014; Perônico et al. 2020).
The impacts of dam building on biological communities have been studied since the 1970s (Baxter 1977), covering different facets of fish biodiversity at different spatiotemporal scales (Turgeon et al. 2019; Pereira et al., 2020). Studies carried out in Brazilian watersheds stand out (Agostinho et al. 2016; Arantes et al. 2019; Pereira et al. 2020), particularly those conducted in the Tocantins River, an important Amazonian system. Several studies conducted in this basin have shown that impoundments affect fish diversity in multiples ways (Santos et al. 2004; Agostinho et al. 2009; Mazzoni and Petito 2012; Araújo et al. 2013; Lima et al. 2015), although long-term studies are incipient (e.g., Perônico et al. 2020). Moreover, few studies have investigated assemblage trajectories following river regulation regarding different facets of biodiversity (e.g., taxonomic and functional); there is also a lack of research about the role of environmental factors affecting community reassembly. In this sense, the Tocantins River Basin may serve as a model to investigate temporal variations in fish biodiversity following the construction of large impoundments.
Considering that dams induce permanent changes to limnological conditions and landscape features (Poff et al. 1997; Pringle et al. 2000), affecting different facets of fish diversity, in this paper we evaluated the response of fish assemblages to the construction of Cana Brava Hydropower Dam, Upper Tocantins River, using a multi-metric approach. We investigated long-term trends (15 years) in taxonomic and functional diversity to describe community reassembly, trajectories, and the influence of environmental factors in the impoundment. We seek to answer the following questions: (1) How fish abundance, taxonomic, and functional richness respond to river regulation? (2) How species and trait composition change over time, highlighting changes in the trajectories of the communities, including periods before and after river regulation? (3) How components of beta diversity respond to river regulation? (4) Do limnological and landscape factors explain taxonomic and functional dissimilarity over time?
Based on past research conducted in the Tocantins River, we expect: (1) a general decrease in species richness and abundance over the years (e.g., Lima et al. 2015); trait richness may show minor changes, but trait abundance will decline in the impoundment (e.g., Lima et al. 2018); (2) The taxonomic and functional composition will differ between pre and post periods. Following the loss of species and traits, temporal changes in assemblages will exhibit a directional trend and a new assemblage state will emerge in the impoundment (e.g., Perônico et al. 2020); (3) “Richness difference”, rather than “Replacement”, explain community dissimilarity over time (temporal beta diversity), since reservoir assemblages have simplified structure if compared to riverine assemblages (e.g., Araújo et al. 2013; Perônico et al. 2020). Finally, (4) we expect that both limnological and landscape variables explain changes in fish diversity following river regulation.
Materials and methods
Sampling area
The Tocantins River is an important tributary of the Amazon system. It rises in the Brazilian shield and flows 2400 km in the north direction toward Marajó Island (Ribeiro et al. 1995). Its headwaters are located at more than 1000 m of altitude, and the watershed drains an area of 351,000 km2. The Tocantins River Basin has been heavily impacted by river regulation, a process that started with the construction of the Tucuruí Hydropower Dam in the late 1980s (Agostinho et al. 2009). Currently, seven large dams regulate the main course, but dozens of large and small dams affect smaller courses; more dams are planned for the coming years (Agostinho et al. 2009; Brasil, Ministério de Minas e Energia 2015; Winemiller et al. 2016).
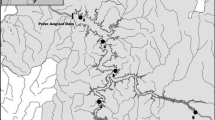
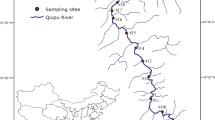
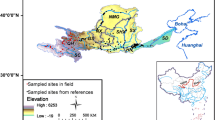
The present study investigated the area affected by the Cana Brava Hydropower Dam, Upper Tocantins Basin (Fig. 1). This is the second large dam in the cascade along the Tocantins River (in the downstream direction). Serra da Mesa Hydropower Dam is located upstream, while São Salvador Hydropower Dam is located immediately downstream (Agostinho et al. 2009). The construction of Cana Brava started in 1998, and the plant started operating in 2002. The dam created a large impoundment (run-of-river type), with a flooded area of 139 km2 (Tractabel-Energia 2017).
Sampling
We collected data from fish fauna inventories and surveys conducted by consulting companies (Tractabel-Energia 2017), made available to the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA) according to Law No. 12,527/2011. These collections comprised 29 independent sampling events conducted over 15 years (at least one collection per year), between 2000 and 2014 (Table S1, supplementary material). We gathered 7 samples before river regulation (P1 to P7) and 22 after (P8 to P29). The sampled sites were distributed along the impoundment (ca. 40 km), covering all zones, i.e. lentic, transition, and lotic. The sampling techniques reported were the same for all periods: gill nets and fishing rods at all sites, and seines, longlines, and traps where environmental conditions were suitable.
During the filling phase (P6 and P7, Table 1 in the supplementary material), fish rescue operations occurred together with regular sampling. Rescued fish were individuals from the phase prior to river regulation that remained in the area, so we considered these samples as pre-dam periods. The number of sampled sites varied between periods (between 2 and 8), so we tested the possible effects of the number of sampled sites and the number of sampled regions (1 to 3: lentic, transition and lotic) on species richness and fish density (see Statistical Analysis). The absence of significant effects allowed us to pool all samples within periods, considering the entire region (≅ 40 km) as a single location. In this sense, we summed all individuals and species collected within periods and investigated temporal variations in the structure of fish assemblages over time.
Taxonomic and functional diversity
To assess taxonomic diversity, we first reviewed species identification using guides, identification keys, and publications related to the studied basin (Santos et al. 2004; Agostinho et al. 2009; Mazzoni and Petito 2012; Lima et al. 2018). Species richness and fish abundance were calculated, respectively, as the absolute number of species and individuals in each period (see Table S2 in the supplementary material). To assess functional diversity, we assigned functional traits to each species, considering aspects related to habitat use, trophic ecology, and reproduction (Tables S3 and S4, supplementary material), since these traits represent main niche dimensions (Winemiller et al. 2015). We obtained information for 86 species, based on Perônico et al. (2020); taxa identified at the genus level were not considered.
Functional diversity indices were calculated based on a presence/absence matrix, obtained from the original trait matrix using the dummy matrix model. Next, we calculated Functional Richness (FRic), which represents the functional volume occupied by all species in the functional multidimensional space, and Functional Divergence (FDiv), which evaluates the distribution of abundances within the functional space (Villéger et al. 2008). Both indices were calculated with the dbFD function, FD package (Laliberté and Legendre 2010). For tests with functional traits, the Community Weighted Mean (CWM) was also calculated. This index represents the abundance of each trait in the period, calculated as CWM = Σ pi x trait, where: CWM = proportion of the functional trait in a given period; pi = relative abundance of species p; trait = presence (1) or absence (0) of a given trait, or its intensity (in case of continuous values) (Garnier et al. 2004).
Limnological and landscape variables
We obtained data on limnological (local) variables from technical reports made available by the companies responsible for fish monitoring. As with fish data, the number of sites was different among periods, and we investigated if this aspect affected limnological conditions in each period (see Statistical Analysis). We found no significant effect, so we calculated arithmetic means for each variable in each period, considering only samples where fish collections were obtained concomitantly (n = 17). We collected information about 16 limnological variables, but three were discarded due to collinearity (Table 1) (see values in Table S5, supplementary material), based on the Variance Inflation Factor (VIF) < 10, using the VIF function of the faraway package (Zuur et al. 2007; Borcard et al. 2018).
To measure landscape variables (regional) (Table 1), we obtained annual data on land use and cover from the MapBiomas platform v.4. For landscape characterization, we selected 20 km2 buffers along both sides of the river channel. Data is automatically generated in the platform, retrieving the proportion of the area (km2) occupied by each class of objects (see values in Table S6, supplementary material), for each year (2000–2014) (MapBiomas 2019).
Beta diversity and community trajectories
We investigated temporal patterns in beta diversity, comparing dissimilarity trends in taxonomic and functional diversity (incidence and abundance-based) between periods, following Podani and Schmera (2011). We calculated total beta diversity and its components: “richness/abundance differences” and “replacement”. “Total beta diversity” is the composition dissimilarity between samples, and values range between 0 (total similarity) and 1 (total dissimilarity). The “richness/abundance differences” component measures how differences in species/trait richness and abundance affect beta diversity; high values indicate a stronger influence on beta diversity patterns. The “replacement” evaluates how the substitution of species/traits, or species/traits with equivalent abundances, affects beta diversity; high values indicate stronger effects on beta diversity patterns. Beta diversity analyses considered Jaccard and Ruzicka dissimilarity indexes for incidence and abundance data, respectively.
Temporal trajectories in community structure were described and compared with the six hypothetical trajectories suggested by Matthews et al. (2013), which contrast two main dynamics (“gradual” or “saltatory”) split in three types of behavior: non-directional, directional or directional with the return to a previous community state. The “non-directional” trajectory entails temporal changes resulting from stochastic events without a tendency to stabilization. “Directional” trajectory occurs when the changes are progressive, and a new state is reached. “Directional with return” trajectory occurs when a directional trajectory is followed by return to a previous state over time (Matthews et al. 2013) (Fig. S1, supplementary material).
Statistical analysis
Analyses of taxonomic and functional diversity considered incidence and abundance-based data. We chose this approach because these data measure different aspects of community structure (Podani et al. 2013); moreover, river regulation affects the incidence and abundance of species (Agostinho et al. 2007).
As mentioned above, we performed pre-tests to verify if the number of sampling sites and zones affect the abundance, species richness and limnological conditions in each period. We used linear regressions to investigate the effect of the number of sites and zones (predictors) on abundance and richness (response variables), using the lm function of the stats package (Chambers 1992). We found no significant effect on richness (number of sites: b = −0.35, F = 0.1, p = 0.74; number of zones: b = 6.83, F = 1.4, p = 0.24) and density (number of sites: b = −56.38, F = 0.4 p = 0.53; number of zones: b = 449.8, F = 0.96, p = 0.34). This result justified the pooling of samples within their respective period. We also investigated if environmental characteristics varied among sites. We applied a Permutational Multivariate Analysis (PERMANOVA) to investigate if the limnological variables varied between the sampling sites (pre-test mentioned above), using the adonis2 function of the vegan package (Oksanen et al. 2016). Lack of differences (results in Table S7, supplementary material) justified the pooling of samples within periods.
To examine the response of taxonomic and functional diversity to river regulation (question 1), we built line graphs based on richness (number of species and FRiq) and abundance data (number of specimens and FDiv). We performed linear regressions to verify if diversity metrics (response variables) varied over periods (predictor, P1 to P29). To evaluate temporal changes in fish composition (species and traits; question 2), we first applied Non-Metric Multidimensional Scaling (NMDS) to ordinate periods according to variations in species and trait composition, using the metaMDS function (Vegan package) (Oksanen et al. 2016). Next, we performed a multivariate permutation analysis (PERMANOVA) to test for significant differences between pre and post periods. We examined NMDS trends to identify community trajectories (question 2), based on trends proposed in Matthews et al. (2013). In order to investigate beta diversity patterns (question 3) and the partition of components, we used the beta.multi function of the BAT package (Podani and Schmera, 2011; Cardoso et al. 2015). A PERMANOVA was applied to investigate differences in the structure of beta diversity (partitioning) before and after river regulation. Additionally, we built a triangular plot (simplex) to check the distributions of pairwise comparisons concerning the partitive components of beta diversity.
Finally, to investigate the influence of limnological and landscape variables on beta diversity between periods (question 4), we performed a Distance-Based Redundancy Analysis (dbRDA’s) (Legendre and Andersson 1999). We related the different matrices resulting from beta diversity partition (response variables) with limnological and landscape variables (predictor variables), using the Forward Selection to retain variables with the best predictive response, using the forward.sel.par function of the adespatial package (Miller and Farr 1971). All analyses were conducted in R environment (R CORE TEAM 2019), and figures were drawn through the function ggplot from the ggplot2 package. Statistical significance implied p < 0.05.
Results
The data set comprised 17,451 individuals, 110 species and 29 periods sampled between 2000 and 2014. We found a rapid increase in richness (taxonomic and functional) and abundance (taxonomic) during the filling/rescue period (P6 and P7). However, we observed no significant variation in richness (Linear Regression: b = 0.26, F = 1.3, p = 0.26), abundance (b = −5.8, F = 0.09, p = 0.76) and functional richness (b = 0.01, F = 0.19, p = 0.66) over periods (Fig. 2). However, functional abundance reduced over periods (b = −0.003, F = 14.7, p < 0.001).
Twenty-one species were collected only before river regulation, while 27 species were exclusive to the impoundment (Table S2, supplementary material), indicating species replacements. Species composition differed between pre and post periods, considering incidence (PERMANOVA; R2-adjust = 0.11, F = 3.41, p < 0.001) and abundance data (R2-adjust =0.11, F = 3.34, p < 0.001). Trait composition based on incidence data showed no difference between periods (R2-adjust = 0.05, F = 1.65, P = 0.11), but functional composition based on abundance differed significantly (R2-adjust = 0.08, F = 2.27, p < 0.04) (Fig. 3).
Non-metric Multidimensional Scaling analysis (NMDS) applied to investigate temporal trajectories in taxonomic and functional assemblage composition in the area impounded by Cana Brava Dam, considering incidence (PA) and abundance (Abund) data. Blue dots: before (1 to 7: 2000–2002); Red dots: after (8 to 29: 2003–2014) river regulation
We recorded the decline of some species with common features that are sensitive to the impoundment (e.g. Prochilodus nigricans, Pimelodus blochii, Raphiodon vulpinus), and the increase of others that have pre-adaptations to this new ecosystem (e.g. Moenkhausia dichroura, Roeboides affinis, Cynodon gibbus, among others) (Table S2, S3s and S4, supplementary material). NMDS results indicated that temporal trajectories in taxonomic composition were similar to the “Directional Saltatory” model, as pre and post periods tended to separate in the ordination space (Fig. 3). Trajectories in functional diversity were similar to the “Non-Directional Saltatory” model (Fig. 3, based on the trajectories of Fig. S1), with considerable variation between periods and overlap between pre and post periods.
Taxonomic beta diversity was greater than functional beta diversity, and the substitution of species and traits was the most important component for the dissimilarity between periods (for both incidence and abundance data) (Table 2, Fig. 4). Total Beta Diversity and the “replacement” component supported this pattern since they differed between periods (Table 3). The “richness/abundance difference” component showed similar values between periods. Landscape variables explained variations in community dissimilarity (Table 4), being watercourse (WC) and urban infrastructure (UI) the main variables (Table S8, supplementary material). Limnological variables, in contrast, were not significant (Table 4).
Simplex analysis (triangular representation) showing the distributions of pairwise comparisons concerning the partitive components of beta diversity, based on incidence and abundance data. Similarity = similarity; diff.Rich = Richness diference; diff.Abund = Abundance difference; Replacement = replacement
Discussion
The construction of Cana Brava Hydropower Dam affected different facets of fish diversity in the Upper Tocantins River, Amazon Basin. We observed significant changes in community structure over a 15-years period, including changes in taxonomic composition, beta diversity patterns, and a decline in trait abundance. However, we recorded little temporal variation in other attributes, such as fish abundance, species, and trait richness. We also observed contrasting temporal trajectories concerning species and trait composition. These results indicate some context-dependency in the response of fish assemblages to river regulation, especially because strong changes in fish diversity have been reported in other impoundments (Araújo et al. 2013; Lima et al. 2015; Perônico et al. 2020). In general, our study supports the notion that fish diversity in Amazonian rivers is vulnerable to river damming, but also indicates that different metrics show particular responses to this disturbance.
Declines in species richness and abundance are common trends in impounded areas (Agostinho et al. 2016), which tend to stabilize at lower levels in the first decade of the impoundment (Agostinho et al. 2007; Orsi and Britton 2014; Lima et al. 2015; Perônico et al. 2020). We expected this pattern in the area affected by Cana-Brava Dam, however, richness and abundance showed similar values over a 15 years-period, including pre- and post-impoundment moments. The response of richness and abundance to river regulation may vary according to several factors, such as impoundment size, age, and morphometry, position in the basin, presence of tributaries, the species pool or the presence of other dams (Agostinho et al. 2016; Santos et al. 2017). In the present study, some factors may explain the absence of effects. For example, the region is located in the upper reach of the basin, where fish diversity is naturally lower and some sensitive groups, such as large-bodied migratory fishes, are less common (Mazzoni and and Petito 2012). In addition, the impoundment affected a short river stretch (ca. 30 km), implying that the area probably harbored a smaller species pool. Moreover, the area was already disturbed by a large dam located a few kilometers upstream (Serra da Mesa Hydropower Dam; Agostinho et al. 2009; Mazzoni and Petito 2012), so fish diversity was already impacted when Cana Brava Dam was closed.
Although species richness and abundance showed little response between periods, the taxonomic composition changed significantly, confirming our expectation and previous studies in the basin (Araújo et al. 2013; Lima et al. 2015; Perônico et al. 2020) and elsewhere (Agostinho et al. 2016). Changes in composition also explained community dissimilarity over time. We predicted that “Richness difference”, rather than “Replacement”, would explain temporal beta diversity, as reservoir assemblages tend to be simplified versions of riverine assemblages (e.g., Araújo et al. 2013; Orsi and Britton 2014). However, richness and abundance showed little variation between periods, while species composition and trait abundance changed significantly, reflecting unique species/trait combinations or species/traits with exclusive abundances (Podani and Schmera 2011) in pre and post periods.
Functional diversity also responded differently to the impoundment. For example, trait richness and composition showed little variation among periods, probably due to the high number of species with redundant traits in the Neotropical region (Nelson et al. 2016; Toussaint et al. 2016). In this case, significant changes in species composition may not result in changes to trait richness and composition. Strong alterations in trait composition have been reported in other impoundments of the basin (Perônico et al. 2020), but in Cana Brava Reservoir, fish assemblages were composed of the similar traits in pre and post periods. However, we recorded significant variation in the abundance of traits, indicating that the impoundment affected positively or negatively some traits. In fact, the new environmental filters created by the impoundment tend to favor species with particular set of traits (Arantes et al. 2019), particularly those with greater behavioral plasticity (e.g., Moenkhausia dichroura, Roeboides affinis, Cynodon gibbus, Serrasalmus eigenmanni, Serrasalmus rhombeus, Plagioscion squamosissimus). These fish are sedentary, with a diet based on algae, fish, and invertebrates (including omnivorous), with high reproductive potential (e.g. small Clupeiformes and Cichlidae) (Agostinho et al. 2007).
In contrast, species with particular traits declined in the impoundment, such as long-distance migrators (e.g., Prochilodus nigricans, Pimelodus blochii, Raphiodon vulpinus) and detritivorous rheophilic fishes (e.g., Loricariidae). In fact, migratory species, whose life cycle depends on the natural flow regime, are more vulnerable and tend to decrease over time (Hoeinghaus et al. 2009; Lima et al. 2018). These results emphasize the importance of assessing multiple facets of biodiversity since communities respond in different ways to disturbances and human impacts. In the area affected by Cana Brava Dam, some metrics showed little response (i.e., species richness, abundance, and trait composition), while others were sensitive to the dam (i.e., composition and trait abundance), indicating the importance of using different approaches to evaluate dam effects.
Long-term changes in assemblage composition (ca. 15 years) evidenced structuring trajectories between pre and post periods. We expected a new community state in the impoundment (e.g., Perônico et al. 2020). However, trajectories were variable, with taxonomic trajectories matching the Saltatory Directional model since pre and post periods showed distinct composition; functional trajectories, on the other hand, followed a Saltatory non-Directional model, as periods overlapped. Fish diversity in impounded areas usually experiences significant variations during the first years after river regulation, mostly in response to abrupt changes in habitat conditions and trophic status (Monaghan et al. 2019). This pattern was clearly observed in the area affected by Cana Brava Dam, as fish richness and abundance peaked during the filling period. After this unstable phase, impoundment assemblages may reach a new stable state (Mol et al. 2007; Orsi and Britton 2014; Perônico et al. 2020), particularly if environmental conditions are stabilized. Some metrics indeed showed decreased variability in the impoundment (e.g., richness and abundance), but trajectories in species/trait composition were highly variable. Therefore, our results suggest that the taxonomic community structure reached a different state in the impoundment, while functional structure seems to follow a more complex dynamic.
Functional composition trajectory showed an intermediate pattern between equilibrium and nonequilibrium, matching with the loose equilibrium model, which predicts temporal changes but without directionality (DeAngelis et al. 1985). The study area is highly affected by river regulation, and dam operation and reservoir drawdown episodes may induce stochastic variations. Other stochastic factors may affect the trajectory of impoundment assemblages, especially the construction of new dams, changes in land cover, and species invasions, which together may impede the community to reach a stable state in the long-term (e.g., Loures and Pompeu 2018).
We expected that limnological and landscape variables would explain changes in fish diversity following river regulation (Agostinho et al. 2007; Poff and Zimmerman 2010), but most variables showed no significant effect. Only “watercourse” and “urban infrastructure” correlated with variations in community dissimilarity. The variable “watercourse” corresponded to the flooded area, which changed abruptly after 2002 (periods 6–7, see Table S6 supplementary material). This variable works as a proxy for changes in water flow and the creation of lentic conditions, a major disturbance to riverine fish diversity, affecting habitat availability, dispersion dynamics, and environmental filtering processes (Pelicice et al. 2015). “Urban Infrastructure” is related to socio-economic development in the area, which may have been fueled after the formation of the reservoir (Tilt et al. 2009), causing changes to the landscape. This variable may affect other factors, such as fishery activities (Agostinho et al. 2007; Philippsen et al. 2019), primary productivity, and the trophic status of the reservoir (Vanni et al. 2005, 2006; Monaghan et al. 2019). We expected that changes in limnological conditions would affect fish diversity, as impoundments transform the local environment, especially in the filling phase and in the first years of the impoundment (Agostinho et al. 2007). However, limnological variables produced no effect on fish diversity, probably because these conditions stabilized in the impoundment, creating a homogeneous environment. Moreover, the studied stretch is regulated by another dam upstream, so environmental conditions were already affected (Barbosa et al. 1999; Santos et al. 2017). Therefore, alterations in the landscape induced by the impoundment explained changes in fish assemblage dissimilarity in the study area.
Conclusion
Fish assemblages in the Upper Tocantins River showed a diversity of responses to the construction of Cana Brava Hydropower Dam, indicating that dams disrupt community structure in complex ways. In general, the impoundment changed fish beta diversity patterns in taxonomic and functional terms, with temporal variation in species composition being more predictable. Impoundment assemblages are relatively stable because dams regulate the natural flow regime (Poff et al. 1997; Agostinho et al. 2007), but they are vulnerable to changes in the terrestrial and aquatic environments. In this sense, fish diversity may show some changes in the coming years, as assemblages continue adapting to the novel environment, and eventual factors may induce stochastic variations. Our study emphasizes the need for multi-metric approaches, as they reveal how disturbances affect different facets of diversity, offering a more complete view of community change. Most studies, related to temporal impacts of dams on ichthyofauna, are still based on simple and more intuitive metrics, like abundance and species richness, but they may not reveal impacts in some circumstances, requiring more inclusive approaches.
References
Agostinho AA, Gomes LC, Pelicice FM (2007) Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil UEM:501p
Agostinho AA, Gomes LC, Santos NC, Ortega JC, Pelicice FM (2016) Fish assemblages in Neotropical reservoirs: colonization patterns, impacts and management. Fish Res 173:26–36
Agostinho AA, Pelicice FM, Gomes LC (2008) Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 68(4):1119–1132
Agostinho CS, Pelicice FM, Marques EE (2009) Reservatório de peixe angical: bases ecológicas para o manejo da ictiofauna. RiMa, São Carlos 188p
Arantes CC, Fitzgerald DB, Hoeinghaus DJ, Winemiller KO (2019) Impacts of hydroelectric dams on fishes and fisheries in tropical rivers through the lens of functional traits. Curr Opin Environ Sustain 37:28–40
Araújo ES, Marques EE, Freitas IS, Neuberger AL, Fernandes R, Pelicice FM (2013) Changes in distance decay relationships after river regulation: similarity among fish assemblages in a large Amazonian river. Ecol Freshw Fish 22(4):543–552
Barbosa FAR, Padisák J, Espíndola ELG, Borics G, Rocha O (1999) The cascading reservoir continuum concept (CRCC) and its application to the river Tietê-basin, São Paulo State, Brazil 425-439p
Baxter RM (1977) Environmental effects of dams and impoundments. Annu Rev Ecol Syst 8(1):255–283
Borcard D et al. (2018) Numerical ecology with R. Springer. https://doi.org/10.1007/978-1-4419-7976-6
Brasil, Ministério de Minas e Energia (2015) Plano Decenal de Expansão de Energia 2024. Empresa de Pesquisa Energética. Brasília: MME/EPE. 467p
Cardoso P, Rigal F, Carvalho JC (2015) BAT–biodiversity assessment tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol Evol 6(2):232–236
Chambers JM (1992) Linear models. In: Chambers JM, Hastie TJ (eds) Wadsworth & Brooks/Cole
DeAngelis DL, Waterhouse JC, Post WM, O'Neill RV (1985) Ecological modelling and disturbance evaluation. Ecol Model 29(1–4):399–419
Garnier E, Cortez J, Billès G, Navas ML, Roumet C, Debussche M, Neill C (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85(9):2630–2637
Helfman G, Collette BB, Facey DE, Bowen BW (2009) The diversity of fishes: biology, evolution, and ecology. John Wiley & Sons. 737p
Hoeinghaus DJ, Agostinho AA, Gomes LC, Pelicice FM, Okada EK, Latini JD et al (2009) Effects of river impoundment on ecosystem services of large tropical rivers: embodied energy and market value of artisanal fisheries. Conserv Biol 23(5):1222–1231
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91(1):299–305
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69(1):1–24
Lima AC, Agostinho CS, Sayanda D, Pelicice FM, Soares AM, Monaghan KA (2015) The rise and fall of fish diversity in a neotropical river after impoundment. Hydrobiologia 763(1):207–221
Lima AC, Sayanda D, Agostinho CS, Machado AL, Soares AM, Monaghan KA (2018) Using a trait-based approach to measure the impact of dam closure in fish communities of a Neotropical River. Ecol Freshw Fish 27(1):408–420
Loures RC, Pompeu PS (2018) Long-term study of reservoir cascade in South-Eastern Brazil reveals spatio-temporal gradient in fish assemblages. Mar Freshw Res 69(12):1983–1994
MapBiomas P (2019) Coleção 4 da Série Anual de Mapas de Cobertura e Uso de Solo do Brasil. Available from: https://www.mapbiomas. org. Accessed 6 July 2019
Matthews WJ, Marsh-Matthews E, Cashner RC, Gelwick F (2013) Disturbance and trajectory of change in a stream fish community over four decades. Oecologia 173(3):955–969
Mazzoni R, Petito J (2012) Distribuição, Abundância e estrutura das populações de peixes antes e depois do represamento in Usina Hidrelétrica de Serra da Mesa: 15 anos de estudos da ictiofauna do Alto Tocantins. Rio de Janeiro: Furnas. 139–167
Miller JK, Farr SD (1971) Bimultivariate redundancy: a comprehensive measure of interbattery relationship. Multivar Behav Res 6(3):313–324
Mol JH, Mérona BD, Ouboter PE, Sahdew S (2007) The fish fauna of Brokopondo reservoir, Suriname, during 40 years of impoundment. Neotropical Ichthyol 5(3):351–368
Monaghan KA, Agostinho CS, Pelicice FM, Soares AM (2019) The impact of a hydroelectric dam on Neotropical fish communities: a spatio-temporal analysis of the trophic upsurge hypothesis. Ecol Freshw Fish
Nelson JS, Grande TC, Wilson MV (2016) Fishes of the world. John Wiley & Sons, p 756
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D et al (2016) Vegan: community ecology package. R Package Version 2:4–1
Orsi ML, Britton JR (2014) Long-term changes in the fish assemblage of a neotropical hydroelectric reservoir. J Fish Biol 84(6):1964–1970
Pelicice FM, Azevedo-Santos VM, Esguícero ALH, Agostinho AA, Arcifa MS (2018) Fish diversity in the cascade of reservoirs along the Paranapanema River, Southeast Brazil. Neotropical Ichthyology 16(2)
Pelicice FM, Azevedo-Santos VM, Vitule JR, Orsi ML, Lima Junior DP, Magalhães AL et al (2017) Neotropical freshwater fishes imperilled by unsustainable policies. Fish Fish 18(6):1119–1133
Pelicice FM, Pompeu PS, Agostinho AA (2015) Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish Fish 16(4):697–715
Pereira HR, Gomes LF, Oliveira Barbosa H, Pelicice FM, Nabout JC, Teresa FB, Vieira LCG (2020) Research on dams and fishes: determinants, directions, and gaps in the world scientific production. Hydrobiologia 847(2):579–592
Perônico PB, Agostinho CS, Fernandes R, Pelicice FM (2020) Community reassembly after river regulation: rapid loss of fish diversity and the emergence of a new state. Hydrobiologia 847(2):519–533
Philippsen JS, Minte-Vera CV, Coll M, Angelini R (2019) Assessing fishing impacts in a tropical reservoir through an ecosystem modeling approach. Rev Fish Biol Fish 29(1):125–146
Podani J, Schmera D (2011) A new conceptual and methodological framework for exploring and explaining pattern in presence–absence data. Oikos 120(11):1625–1638
Podani J, Ricotta C, Schmera D (2013) A general framework for analyzing beta diversity, nestedness and related community-level phenomena based on abundance data. Ecol Complex 15:52–61
Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD et al (1997) The natural flow regime. BioScience 47(11):769–784
Poff NL, Zimmerman JK (2010) Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshw Biol 55(1):194–205
Pompeu PDS, Agostinho AA, Pelicice FM (2012) Existing and future challenges: the concept of successful fish passage in South America. River Res Appl 28(4):504–512
Pringle CM, Freeman MC, Freeman BJ (2000) Regional effects of hydrologic alterations on riverine macrobiota in the new world: tropical-temperate comparisons: the massive scope of large dams and other hydrologic modifications in the temperate New World has resulted in distinct regional trends of biotic impoverishment. While neotropical rivers have fewer dams and limited data upon which to make regional generalizations, they are ecologically vulnerable to increasing hydropower development and biotic patterns are emerging. BioScience 50(9):807–823
R Development Core Team. 2019. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available from: http://www.R-project.org. Accessed 8 December 2019
Ribeiro MCLDB, Petrere M, Juras AA (1995) Ecological integrity and fisheries ecology of the Araguaia—Tocantins River basin, Brazil. Regul Rivers: Res Manag 11(3–4):325–350
Santos GM, Mérona BD, Juras AA, Jégu M (2004) Peixes do Baixo Rio Tocantins: 20 anos depois da usina hidrelétrica Tucurui. In: Eletrobras, 2s16p
Santos NCL et al (2017) Environmental filters predict the trait composition of fish communities in reservoir cascades. Hydrobiologia 802(1):245–253
Tilt B, Braun Y, He D (2009) Social impacts of large dam projects: a comparison of international case studies and implications for best practice. J Environ Manag 90:S249–S257
Toussaint A, Charpin N, Brosse S, Villéger, S (2016) Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread Scientific Reports, 6
Tractabel-Energia. 2017. Relatório de Monitoramento da UHE Cana Brava. Available from:http://licenciamento.ibama.gov.br/Hidreletricas/Cana%20Brava/Relatorio%20Monitoramento. Accessed 1 July 2019
Turgeon K, Solomon CT, Nozais C, Gregory-Eaves I (2016) Do novel ecosystems follow predictable trajectories? Testing the trophic surge hypothesis in reservoirs using fish. Ecosphere 7(12):e01617
Turgeon K, Turpin C, Gregory-Eaves I (2019) Boreal river impoundments caused nearshore fish community assemblage shifts but little change in diversity: a multiscale analysis. Can J Fish Aquat Sci 76(5):740–752
Vanni MJ, Arend KK, Bremigan MT, Bunnell DB, Garvey JE, Gonzalez MJ et al (2005) Linking landscapes and food webs: effects of omnivorous fish and watersheds on reservoir ecosystems. BioScience 55(2):155–167
Vanni MJ, Bowling AM, Dickman EM, Hale RS, Higgins KA, Horgan MJ et al (2006) Nutrient cycling by fish supports relatively more primary production as lake productivity increases. Ecology 87(7):1696–1709
Villéger S, Mason NW, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89(8):2290–2301
Winemiller KO, Fitzgerald DB, Bower LM, Pianka ER (2015) Functional traits, convergent evolution, and periodic tables of niches. Ecol Lett 18(8):737–751
Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S et al (2016) Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 351(6269):128–121
Zuur AEN, Ieno GM (2007) Analyzing ecological data. Springer, New York 672
Acknowledgments
Thank you for all the suggestions made by the professors from the Programa de Pós Graduação em Ciências Ambientais-PPGCA of Universidade de Brasília-UnB. This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES (Financing Code 001), a Brazilian public institution for the promotion of research. FBT, FMP and LCGV were supported by productivity fellowships of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 114 kb)
Rights and permissions
About this article
Cite this article
Pereira, H.R., Gomes, L.F., Soares, P.T. et al. Long-term responses of fish diversity to river regulation: a multi-metric approach. Environ Biol Fish 104, 71–84 (2021). https://doi.org/10.1007/s10641-021-01056-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01056-0