Abstract
In contrast to the fast-paced dam construction and river ecosystem alteration which is taking place in the neotropics, ecological knowledge about the effects on fish fauna are still scarce. To contribute with knowledge on the effects of damming on fish fauna, we assessed the response of fish assemblages to the construction of a run-of-the-river dam in an Amazonian river by selecting eight sampling sites along a longitudinal gradient in the vicinity of a new hydroelectric dam. Sites were sampled monthly through a 1-year period before dam closure (2004–2005), 1 year after closure (2006–2007), and 5 years after closure (2011–2012). Following dam closure, there was an initial overall increase in fish abundance and richness across sites. However, despite the initial upsurge, after 5 years, populations were reduced and communities contracted to a level of diversity below that observed prior to dam closure. Respective sites demonstrated distinct ecological responses that were related to the environmental characteristics of their transformed habitats. Important changes in fish assemblages were visible in a short-term period of 5 years after dam closure. Therefore, monitoring fish species assemblages in a longer term is important to assess the consequences of a decrease in diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
River impoundment is a major cause of ecological change in freshwaters (Bunn & Arthington, 2002; Poff & Zimmerman, 2010; Webb et al., 2013). It affects nearly 80% of large river systems in North America and Europe (Nilsson et al., 2005). Tropical river systems are becoming increasingly regulated, and the Amazon region is now being target as a potential source of hydroelectric power (Agostinho et al., 2009). A common consequence of damming is the loss of habitat heterogeneity and a reduction in the diversity of structural features preferred by fish and other aquatic organisms (Poff et al., 2007). It can also fragment river ecosystems, with studies in the Amazon basin showing, for example, that hydroelectric dams halt the long distance upstream migration of catfish and interrupted the downstream migration of their larvae (McAllister et al., 2001).
According to Poff & Hart (2002), different types of dam operations (e.g., storage dams and run-of-the-river dams) lead to different ecological impacts. Large storage dams are associated with the homogenization of flow regimes by attenuating seasonal and annual variation in discharge (Poff & Hart, 2002; Poff et al., 2007). However, the operation and size classifications of dams are important determining factors of their impact, so it is important to consideration respective cases individually. For example, run-of-the-river dams can have whole-reservoir turnover times ranging from a few hours to many weeks and impoundment depths ranging from 1 m to more than 30 m (Poff & Hart, 2002). Much of the research on the impact of hydropower developments on river ecosystems has been carried out on large schemes with the creation of a dam and a reservoir where power generation is not dependent on the natural river flow (Angilletta et al., 2008), while the impacts of run-of-the-river dams on river ecology remain poorly understood (Robson et al., 2011).
Thornton et al. (1981) described three main zones along the reservoir continuum immediately upstream of river dams: lacustrine (reservoir), transition, and fluvial (upstream). The physical and chemical gradients that distinguish these zones are related to hydraulic retention time, sedimentation rates, nutrient concentration, and primary production (Thornton et al., 1996; Pagioro & Thomaz, 2002) and are important determinants of the fish assemblages throughout these new habitats (O’Brien, 1990; Agostinho et al., 1999). During the filling stage, the permanent inundation of terrestrial areas provides an abundant source of allochthonous food and a massive increase in the pelagic environment (Tundisi & Straškraba, 1999). Increases in water transparency, anoxia near the bottom, and decrease in turbulent flow also influence fish community organization. This initial phase usually results in an increased abundance in fish (Baxter, 1977; Agostinho et al., 2007a), but as the new habitats stabilize and release from the reservoir begin, the fish assemblages have to re-adapt (Tundisi & Straškraba, 1999). Over time, there is a decrease in nutrient availability within the reservoir due to sedimentation and exports via discharges, and the food web becomes increasingly autochthonous (Petrere, 1996). This phase is defined as the “depression period,” which leads to a decrease in the fish abundance due to the decrease in food availability and the lack of reproduction conditions for some species (Agostinho et al., 1999).
Understanding the response of fishes to dams in tropical rivers provides valuable knowledge for management aiming to mitigate the detrimental ecological effects. Although recent work has provided important understanding to the phenomena of regulation in tropical rivers (Agostinho et al., 2004a, 2007a; Petesse et al., 2007; Suzuki et al., 2009), the lack of basic, descriptive knowledge provides a major obstacle to strategies of impact mitigation. In contrast to the fast-paced dam construction and river ecosystem alteration which is taking place in the neotropics, taxonomic knowledge remains incomplete, and basic ecological knowledge on most species is lacking (Lucinda et al., 2007).
The aim of this study was to analyze how fish assemblages from a Neotropical river in the Amazon basin were affected by the construction of a run-of-the-river dam in the short term by focusing on two distinct time periods: 1 and 5 years after dam closure. Specifically, we compared fish assemblages before and after dam closure to evaluate the effects on abundance and diversity with respect to (i) space—the longitudinal gradient in fish assemblages that were differentially affected by habitat change immediately downstream of the dam, in the reservoir, in the transition zone, and upstream of the reservoir; and time (ii) the seasonality along this longitudinal gradient and (iii) the difference over time from 1 to 5 years of dam existence.
Materials and methods
Study area
Tocantins River is 2,750 km long and is one of the major tributaries of the lower Amazon River. It has a total drainage area of 767,000 km2, with an average annual mean discharge of about 11,000 m3 s−1. The river basin is characterized by marked rainy and dry seasons with peak flow during the rainy season from October to April (Ribeiro et al., 1995).
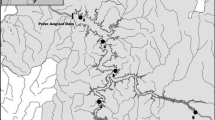
The study area was located at the upper Tocantins River, in the reach affected by the construction of the Peixe Angical Dam (Fig. 1). Most of the seven dams from this river are located in its middle and upper reaches, and Peixe Angical is the fourth in an upstream–downstream sequence. Peixe Angical is a run-of-the-river dam, with constant electricity produced by diverting river flow through turbines before returning the water to the river downstream. In this specific case, water storage is provided, and the river flow is regulated by Serra da Mesa dam, approximately 300 km upstream. Peixe Angical was completed in 2006, it is 39 m high and 6.2 km wide, with an installed capacity of 452 MW. The dam flooded 294 km2 of Cerrado savanna, forming a reservoir 120 km in length with a mean depth of 9.3 m and a water residence time of 18 days (Agostinho et al., 2009).
Environmental data
Environmental variables measured at each sampling site included water depth (m), dissolved oxygen (ml/l), conductivity (µS/cm), transparency (m; the depth at which a Secchi disk was no longer visible) pH, distance from the dam (km), and width (m) (Table 1). Depth was determined using a graduated dip-net pole at the deepest point where the nets were installed. Width (before and after dam closure) and distance from the dam were estimated from satellite images. Dissolved oxygen, water temperature, conductivity, and pH were measured by the multiparameter water quality monitoring system Horiba U-23 and Sonda YSI.
For a period of 5 years pre-dam (2001–2005) and 5 years post-dam (2006–2010), flow data immediately downstream from Peixe Angical Dam were obtained from the Brazilian National Electric Operator—“Operador Nacional do Sistema Elétrico (ONS),” and total precipitation (mm) was obtained from Banco de Dados Meteorológicos para Ensino e Pesquisa (BDMEP), Instituto Nacional de Meteorologia (IMET).
Fish surveys
Survey sites were chosen accordingly to a standardized procedure defined by the fish monitoring programmes followed in this region, where sites were representative of the distinct river stretches differently affected by dam closure (Thornton et al., 1981). Eight permanent sampling sites were defined along the river reach (~95 km; Fig. 1), two sites in each of the four locations corresponding to Downstream—Rio Tocantins Jusante (Site 1), located immediately downstream from the dam; and Rio das Almas (2), located in a nearby tributary; Reservoir—Rio Tocantins Montante (3) and Rio Tocantins Santa Cruz (4) both located where the reservoir was formed; Transition—Rio Tocantins Traçadal (5) and Rio Maranhão Retiro (6) located in a transition zone between the reservoir and the river upstream; and Upstream—Rio Paranã Areia (7) and Rio Palmas Corrente (8), located beyond the direct influence of altered flow associated with the reservoir.
Ecological surveys were conducted monthly during three periods: before dam closure (from October 2004 to September 2005—before), 1 year after dam closure (from October 2006 to September 2007—after 1), and 5 years after dam closure (from February 2011 to January 2012—after 5). At each site, a combination of 12 gill nets of incremental mesh size was used (2.4, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, and 16 cm mesh size). Nets were deployed for a period of 24 h and emptied at 8, 16, and 22 h every day. In order to catch larger individuals in deep water areas, a system of longlines with 40 baited hooks was employed together with a traditional method called “pindá” consisting of five wooden rods with a line, a hook, and fish bait. The combination of gill nets was installed close to the shore during the three time periods (before, after 1, and after 5) at each sampling site. After dam closure (after 1 and after 5 years), sampling nets were also installed at the surface and close to the bottom in locations directly affected by the reservoir (sites 3, 4, 5, and 6), in the newly created habitats. Fish were classified to species level whenever possible according to Nelson (1994), Fink & Fink (1996), McEachran et al. (1996), Johnson & Patterson (1996), and Reis et al. (2003).
Data analysis
Species abundance was defined as catch per unit effort (CPUE), expressed as individuals/100 m2 of net/24 h. For classification and ordination, all fish abundance data were log (x + 1) transformed. For parametric analyses, normality and homogeneity of variances were tested by Shapiro–Wilk W-statistic and Levene’s test, respectively.
Environmental variables were reduced by Principal Components Analyses (PCA). Permutational Multivariate Analyses of Variance (PERMANOVA) (bi-factorial) (McCune et al., 2002) was used to test for differences in the environmental variables between locations (downstream, reservoir, transition, and upstream) and the three periods of time considered (before, after 1, and after 5).
To analyze the seasonal difference in fish assemblages between time periods (before, after 1, and after 5 years) in respective locations, abundance data were pooled as rainy season (from October to March) and dry season (from April to September) and tested by Kruskal–Wallis test and the subsequent post-hoc Nemenyi test for pairwise samples.
The number of species and community organization was described by species richness (S, total number of fish species in each sample) and species diversity (H, Shannon–Wiener index). Two-way analysis of variance (ANOVA) was used to test the effects of site location and time periods within each site.
To compare fish communities, the mean abundance for each season (rainy and dry) in each site was used to construct a matrix based on Bray–Curtis distance. The resulting matrix was used to perform a non-metric multidimensional scaling analysis (nMDS). Permutational Multivariate Analyses of Variance (PERMANOVA) (bi-factorial) (McCune et al., 2002) was used to test difference in fish communities between time periods and between site locations. To analyze how communities vary in the same location between the different time periods, a test of dissimilarities percentage (SIMPER) (Clarke & Warwick, 2001) was applied to determine which fish species contributed the most to the differences observed between the groups.
The seasonal abundance matrix and a matrix of associated environmental variables were used to perform a canonical correspondence analysis (CCA) (Ter Braak, 1987) to identify general gradients in ecological and environmental descriptors (ter Braak & Verdonschot, 1995). The multicollinearity of environmental variables was previously tested by Pearson correlations with a threshold of 0.7 (Dormann et al., 2012), and the results were presented as Online Resource 1—Table 1. Eight environmental variables were initially included in the ordination together with the binary dummy variables representing site location in relation to the dam: “Downstream,” “Upstream,” “Reservoir,” and “Transition.” Significant environmental variables were identified via a stepwise addition, with a probability value for entering of 0.05. After the selection of the significant variables, the model was tested a second time through a Monte Carlo global permutation test (999 permutations) (Ter Braak, 1987) to assess the significance of ordination axes. All statistics were computed with R version 2.15.1. (R. Development Core Team, 2012). Vegan package was used for ordination analysis (Oksanen et al., 2013).
Results
Environmental variables
The natural flow regime of the river was modified by the presence of Peixe Angical Dam (Fig. 2). River regulation was associated with a reduction in the magnitude of wet-season peak flow events, which was not related to changes in precipitation.
Seven of the eight environmental variables tested (water temperature, depth, dissolved oxygen, pH, transparency, distance to the dam, and width) explained 57% of variability in the original data (Fig. 3).
Principal components analysis plot of axes 1 and 2. The arrows indicate the environmental variables loadings and geometric forms site loadings. Time periods (before, after 1 year, and after 5 years of dam closure) correspond to different colors, and locations (Downstream, Reservoir, Transition, and Upstream) correspond to different shapes
Both Time (before, after 1, and after 5 years) and Location of sampling points (downstream, reservoir, transition, and upstream) had a significant effect on the variables measured (F 2, 6 = 49.62, R 2 = 0.097, P = 0.01 and F 3, 6 = 218.47, R 2 = 0.647, P = 0.01, respectively). The interaction between time periods and locations was also significant (F 5, 6 = 1.85, R 2 = 0.010, P = 0.043). After 1 and 5 year of dam closure, the water at Reservoir and Transition sites was significantly deeper and more transparent. After 5 years, there was an overall decreased in dissolved oxygen (Table 1).
Temporal and spatial analysis of fish assemblages
A total of 69.343 individual representing 260 fish species, 34 families, and 9 orders were recorded. From these, only two were introduced species: Colossoma macropomum (Couvier, 1816), Piaractus mesopotamicus (Holmberg, 1887). Overall, most species were Characiformes, which were dominated by species belonged to the family Characidae that increased in abundance after dam closure. The number of species in the remaining families from this order varied between the time periods considered but decreasing consistently after dam closure for Curimatidae, Anostomidae, Chilodontidae, and Prochilodontidae and increasing for Erythrinidae, Hemiodontidae, and Serrasalmidae. The order with the next highest number of species overall was the Siluriforms which were fewer after dam closure, mainly due to a decrease in Loricariidae and Pimelodidae.
Seasonal abundances varied differently between time periods in respective locations. In general, abundance increased significantly at all sites during the rainy season and then decreased after 5 years to levels similar to before dam closure (Fig. 4; Online Resource 1—Table 2). Dry season abundances showed less variation during the first year but increased significantly in Reservoir site 3 and both Upstream sites (7 and 8). After 5 years, dry season abundances decreased to lower levels than before the dam at downstream sites 1 and 2 and the Upstream site 8.
There was no significant difference for species richness (S) between locations (F 3, 12 = 0.58, P = 0.64). Between time periods richness was significantly higher after 1 year and lower after 5 years of dam closure (F 3,12 = 38.44, P < 0.01) (Fig. 5). The interaction between time and location was not significant (F 6, 12 = 0.56, P = 0.75). Shannon–Weiner’s (H′) was not significantly different between locations (F 3, 12 = 0.70, P = 0.57). Between time periods, it was significantly lower after 5 years (F 2, 12 = 41.93, P < 0.001). The interaction between time and location was not significant (F 6, 12 = 1.83, P = 0.20).
Changes in fish composition
Fish communities were significantly different between time periods (F 2, 6 = 15.87, R 2 = 0.34, P = 0.01) and locations (F 3, 6 = 3.83, R 2 = 0.12, P = 0.01). The interaction between locations and time periods was also significant (F 5, 6 = 2.33, R 2 = 0.15, P = 0.01). The nMDS ordination identified three main groups (before, after 1, and after 5 years) with a stress value of 0.13 (Fig. 6). The results of SIMPER analysis that compared the three groups in each location showed that the most significant overall dissimilarities occurred between before and after 5 years after dam closure for all locations (Table 2). The differences between the three groups that constitute the time periods analyzed were explained by species such as Hemiodus unimaculatus (Bloch, 1794), Argonectes robertsi (Langaeni, 1999), and Auchenipterus nuchalis (Spix & Agassiz, 1829) that were more abundant after dam closure at the Reservoir, Transition, and Upstream locations, whereas species such as Hypostomus sp. (Lacépède, 1803) were less abundant. In Downstream sites observed differences were mostly related to a higher abundance of Pimelodus blochii (Valenciennes, 1840) and Pachyurus junki (Soares & Casatti, 2000) during the first years of dam closure and a general decrease in abundance of Caenotropus labyrinthicus (Kner, 1858) and Hemiodus microlepis (Kner, 1858) (Table 3).
The results of canonical correspondence analysis (CCA) of fish species composition and environmental variables are shown in Fig. 7. Gradients in community structure were related to dissolved oxygen (F = 5.6; P = 0.01), Transparency (F = 2.36; P = 0.01), and site location (downstream—F = 2.46; P = 0.02 and upstream—F = 2.18; P = 0.03). The explanatory variables accounted for 40% of the total variation, with the first and second axes representing 69% of that variation (cumulative proportion explained) (Table 4). Higher values of dissolved oxygen were mostly associated with sites sampled before and 1 year after dam closure, while increased transparency was associated with sites sampled after 5 years. The second CCA axis separated sites accordingly to their position in relation to the dam from upstream to downstream locations.
Canonical correspondence analysis (CCA) diagram of seasonal species abundance for each sampling site coded 1–8 and season: r rainy season; d dry season. Time periods are coded by colors: Medium gray before; Light gray after 1 year; Black after 5 years. Environmental variables are represented by arrows that point towards the direction of maximum variation. Eigenvalues were 0.25 for the first axis and 0.08 for the second axis
Discussion
This study indicates that damming was associated with an overall increase in fish abundance during the first year, followed by a decrease after 5 years. Seasonal patterns in abundances were differently affected according to the location of habitats in relation to the dam, indicating a possible consequence of altered seasonal hydrologic regimes. In a period of 5 years, fish communities had contracted to a level of diversity below that observed prior to dam closure and sites located Upstream, outside the zone of direct habitat alteration, also changed. These results are in agreement with other studies in tropical rivers subjected to damming where an increase in richness and abundance in the first year after impoundment is a characteristic response of fish communities that is usually not sustained with reservoir aging (Agostinho et al., 2007a, 2008; Mazzoni et al., 2012). The initial upsurge in fish is consistent with the “trophic upsurge period” (Kimmel & Groeger, 1986; Petrere, 1996) where the newly formed habitat by the reservoir provides a high availability of food as a consequence of increased primary production and allochthonous food resources. Planktonic species such as H. unimaculatus increase, and as consequence of the increase in zooplankton during this initial phase (Rocha et al., 1999), zooplanktonic species like Moenkhausia dichroura (Kner, 1858) also increase in the reservoir habitats. These small fish, with a high fecundity, pelagic eggs, and larvae and often proliferate in the first years after impoundment (Agostinho et al., 2007a; Dias et al., 2005). Representing important prey for larger, predatory fish, they help explain the marked increase in piscivorous species such as Serrasalmus sp. (Lacépède, 1803) (Luz-Agostinho et al., 2006; Agostinho et al., 2007a).
The reorganization of fish communities after impoundment is a consequence biological requirement of South American fish, which have evolved predominantly in flowing waters, given the few numbers of natural occurring lakes in this region (Gomes & Miranda, 2001; Agostinho et al., 2007a, 2008). Consequently, although there was an overall increase in abundance and richness during this the first year after dam closure, the increase in abundance was a consequence of few species in respective study locations (e.g., P. blochii at downstream sites and H. unimaculatus and A. nuchalis at Upstream sites). After 5 years, some species had decreased (e.g., P. blochii and Oxydoras niger, Valenciennes, 1821) or had been locally eliminated (e.g., Hypostomus sp. and Geophagus altifrons, Heckel, 1840), while others became more dominant (e.g., Serrasulamus sp. downstream and A. nuchalis in other locations), reflecting similar patterns of colonization and dominance following damming in other tropical rivers (Agostinho et al., 2007a, 2009).
The colonization successes in the first year were probably a consequence of overlapping events connected to reservoir filling that favored the initial development of some species that, in the longer term, were not sustained. The resultant decrease in diversity after 5 years is in agreement with data from previous studies (Agostinho et al., 2007a; Mazzoni et al., 2012). After the period of trophic upsurge, reservoirs often progress into a phase of trophic depletion and low productivity that eventually result in a decrease in fish abundance and richness in the lacustrine area (Agostinho et al., 2008) as many tropical fish species lack the morphological (e.g., locomotion and swimming capacity) and behavior (e.g., reproductive plasticity) characteristics to successfully occupy the pelagic area created by the new reservoir after the filling stage (Gomes & Miranda, 2001).
Our results suggest that the construction of Peixe Angical Dam modified the spatial distribution of fish communities along the Tocantins River (i.e., nMDS and CCA results). Changes in spatial distribution have been documented for Neotropical migratory species (Agostinho et al., 2007b, 2008; Hoeinghaus et al., 2009) and more recently for nonmigratory species (Araújo & Marques, 2013). The previous longitudinal gradient in the river was altered immediately after dam closure. In part, this was due to the colonization of fishes that were not previously present in the areas inundated by the reservoir that migrated to these zones in response to improved feeding and shelter opportunities. The strong hydrological gradient associated with the dam (i.e., lentic conditions in the reservoir and lotic upstream) and consequential decreased in dissolved oxygen and increase in depth and transparency represented a radical transformation to fish habitats. At the same time, river regulation diminished the seasonality variability of river habitats and transformed the rivers energy base and environmental context for food–web interactions. While the dam itself represents an obvious barrier to the long-range migratory species, it is also likely that the habitat alterations had an important influence on the mobility of a wide range of species previously inhabiting this stretch of river. Thus, while analysis identified the statistical significance of the measured variables, these correlates should also be regarded as surrogates for the broad range of environmental changes that resulted from dam closure.
Fish assemblages in downstream sites were also affected by the dam. However, here the ecological response was distinctly different from the other locations. Migratory fish that would normally have migrated upriver past the dam site tend to accumulate in the tailwater area (e.g., P. blochii). Also, lotic fishes are especially vulnerable to discharges of deoxygenated water from the spillways and fish populations can be vulnerable to increased levels of predation associated with high densities of predatory fish (Agostinho et al., 2007a, b). In addition, these zones are often a focus of increased fishing, inspired by the accumulation of large migratory fishes and may be a contributory factor in the decrease in fish diversity at the downstream sites after 5 years. In the long term, other impacts such as the disruption to reproduction and fish recruitment and the lack of access to floodplain resources may cause further declines in diversity (Gomes & Agostinho, 1997; Agostinho et al., 2004b, 2007a; Mérona et al., 2005). However, most studies and monitoring programmes in Brazilian tropical reservoirs lack monitoring points downstream from the dam, focusing exclusively on consequences in the reservoir zone. These data demonstrate that this can be particularly limiting for systems like the Tocantins River, especially when they are subjected to a cascade of dams which cause biotic homogenization (Petesse & Petrere, 2012).
From a management perspective, the loss of species with reservoir aging emphasizes the importance of preserving the upper lotic stretches and tributary systems in the Tocantins Rivers that appear to still maintain natural conditions with flowing waters that could sustain nursery habitat for both migratory and non-migratory species (Agostinho et al., 2008; Araújo & Marques, 2013). The fact that Upstream sites showed differences after 5 years might be related to the fact that not only the studied river stretch was limited upstream by another dam (São Salvador) in 2009, but also one of its main tributaries upstream (Manoel Alves River) was regulated in 2008. Under these conditions, we might speculate that the natural flow regime of this river stretch might be compromised, and further analysis would be necessary to assess its effect on migratory and rheophilic fish. The fact that Peixe Angical Dam is part a cascade reservoir system raises important management issues. Petesse & Petrere (2012) reported a tendency towards biotic homogenization among reservoirs in cascade systems as a consequence of the loss of unique native species and the progressive environmental maturation of constituent reservoirs. This suggests that longer term assessment of fish assemblages throughout the Tocantins cascade system is important to achieve a more comprehensive evaluation of the effects of dam closure.
Conclusions
This study provides insight about the effects of river regulation in a tropical river system in a spatial–temporal context. One year after dam closure, there was increase in richness and abundance of fish assemblages that may have been related to increased productivity and greater food availability. After 5 years, however, abundance decreased and communities contracted to diversity levels below those observed prior to dam closure. The changes observed were related to the environmental gradients defined by location relative to the dam, which caused a transformation in fish communities at all sites. The growth of the hydropower industry particularly in tropical regions where little is known about the ecology of fishes poses an evident risk to tropical biodiversity and the provision of ecosystem goods and services to human societies. Our results show that diversity has declined in a period of only 5 years. However, this time period can only show short-term changes in the areas affected by the dam. It will be important to continue monitoring fish species assemblages in a longer term to assess the consequences of a decrease in diversity.
References
Agostinho, A. A., L. E. Miranda, L. M. Bini, L. C. Gomes, S. M. Thomaz & H. I. Suzuki, 1999. Patterns of colonization in reservoirs and prognoses on aging. In Tundisi, J. G. & M. Straskraba (eds), Theoretical reservoir ecology and its applications. International Institute of Ecology, Brazilian Academy of Sciences and Backhuys Publishers, São Paulo: 227–265.
Agostinho, A. A., L. C. Gomes, S. Veríssimo & E. K. Okada, 2004a. Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Reviews in Fish Biology and Fisheries 14: 11–19.
Agostinho, A. A., S. M. Thomaz & L. C. Gomes, 2004b. Threats for biodiversity in the floodplain of the Upper Paraná River: effects of hydrological regulation by dams. Ecohydrology & Hydrobiology 4: 255–256.
Agostinho, A. A., L. C. Gomes & F. M. Pelicice, 2007a. Ecologia e Manejo de Recursos Pesqueiros em Reservatorios do Brasil. EDUEM, Maringá: 512.
Agostinho, A. A., E. E. Marques, C. S. Agostinho, D. A. Almeida, R. J. Oliveira & J. B. Rodrigues, 2007b. Fish ladder of Lajeado Dam: migration on one way routes? Neotropical Ichthyology 5: 121–130.
Agostinho, A. A., F. M. Pelicice & L. C. Gomes, 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68: 1119–1132.
Agostinho, C. S., F. M. Pelicice & E. E. Marques, 2009. Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. Rima, São Paulo: 179.
Angilletta, M. J. J., E. A. Steel, K. K. Bartz, J. G. Kingsolver, M. D. Scheuerell, B. R. Beckman & L. G. Crozier, 2008. Big dams and salmon evolution: changes in thermal regimes and their potential evolutionary consequences. Evolutionary Applications 1: 286–299.
Araújo, E. & E. Marques, 2013. Changes in distance decay relationships after river regulation: similarity among fish assemblages in a large Amazonian river. Ecology of Freshwater Fish 22: 543–552.
Baxter, R., 1977. Environmental effects of dams and impoundments. Annual Review of Ecology and Systematics 8: 255–283.
Bunn, S. E. & A. H. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492–507.
Clarke, K. R. & R. Warwick, 2001. Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth.
Dias, R. M., D. Bailly, R. R. Antonio, H. I. Suzuki & A. A. Agostinho, 2005. Colonization of the Corumba Reservoir (Corumba River, Parana River Basin, Goias State, Brazil) by the “lambari” Astyanax altiparanae (Tetragonopterinae; Characidae). Brazilian Archives of Biology and Technology 48: 467–476.
Dormann, C. F., J. Elith, S. Bacher, C. Buchmann, G. Carl, G. Carré, J. R. G. Marquéz, B. Gruber, B. Lafourcade, P. J. Leitão, T. Münkemüller, C. McClean, P. E. Osborne, B. Reineking, B. Schröder, A. K. Skidmore, D. Zurell & S. Lautenbach, 2012. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 35: 001–020.
Fink, S. V. & W. L. Fink, 1996. Interrelationships of ostariophysan fishes (Teleostei). In Stiassny, M. L., L. R. Parenti & G. D. Johnson (eds), Interrelationships of Fishes. Academic Press, New York: 209–250.
Gomes, L. C. & A. A. Agostinho, 1997. Influence of the flooding regime on the nutritional state and juvenile recruitment of the curimba, Prochilodus scrofa, Steindachner, in Upper Paraná River, Brazil. Fisheries Management and Ecology 4: 263–274.
Gomes, L. & L. Miranda, 2001. Riverine characteristics dictate composition of fish assemblages and limit fisheries in reservoirs of the Upper Paraná River Basin. Rivers Research & Management 76: 67–76.
Hoeinghaus, D. J., A. A. Agostinho, L. C. Gomes, F. M. Pelicice, E. K. Okada, J. D. Latini, E. A. L. Kashiwaqui & K. O. Winemiller, 2009. Effects of river impoundment on ecosystem services of large tropical rivers: embodied energy and market value of artisanal fisheries. Conservation Biology 3: 1222–1231.
Johnson, G. D. & C. Patterson, 1996. Relationships of lower euteleostean fishes. In Stiassny, M. L., L. R. Parenti & G. D. Johnson (eds), Interrelationships of Fishes. Academic Press, San Diego: 251–332.
Kimmel, B. L. & A. W. Groeger, 1986. Limnological and ecological changes associated with reservoir aging. In Hall, G. E. & M. J. Van Den Avyle (eds), Reservoir fisheries management: strategies for the 80’s. A National Symposium on Managing Reservoir Fishery Resources, Wiley Interscience: 103–109.
Lucinda, P. H. F., I. S. Freitas, A. B. Soares, E. E. Marques, C. S. Agostinho & R. J. Oliveira, 2007. Fish, Lajeado Reservoir, rio Tocantins drainage, state of Tocantins, Brazil. Check List 3: 70–83.
Luz-Agostinho, K. D. G., L. M. Bini, R. Fugi, A. A. Agostinho & H. F. Júlio Júnior, 2006. Food spectrum and trophic structure of the ichthyofauna of Corumba reservoir, Paraná river Basin, Brazil. Neotropical Ichthyology 4: 61–68.
Mazzoni, R., E. P. Caramaschi & R. Iglesias-Rios, 2012. Usina Hidrelétrica de Serra da Mesa: 15 anos de Estudos da Ictiofauna do Alto Tocantins. FURNAS, Rio de Janeiro.
McAllister, D. E., J. F. Craig, N. Davidson, S. Delany & M. Seddon, 2001. Biodiversity Impacts of Large Dams. Background Paper No. 1 Prepared for IUCN/UNEP/WCD.
McCune, B., J. B. Grace & D. L. Urban, 2002. Analysis of Ecological Communities. MjM Software Design, Gleneden Beach, OR.
McEachran, J. D., K. A. Dunn & T. Miyake, 1996. Interrelationships of the batoid fishes (Chondrichthyes: Batoidea). In Stiassny, M. L. J., L. R. Parenti & G. D. Johnson (eds), Interrelationships of Fishes. Academic Press, San Diego: 63–84.
Mérona, B., R. Vigouroux & F. L. Tejerina-Garro, 2005. Alteration of fish diversity downstream from Petit-Saut Dam in French Guiana. Implication of ecological strategies of fish species. Hydrobiologia 551: 33–47.
Nelson, J. S., 1994. Fishes of the World. Wiley, New York: 600.
Nilsson, C., C. Reidy & M. Dynesius, 2005. Fragmentation and flow regulation of the worlds large river systems. Science 308: 405–408.
O’Brien, W. J., 1990. Perspectives on fish in reservoir limnology. In Thornton, K. W., B. L. Kimmel & F. E. Payne (eds), Reservoir Limnology: Ecological Perspectives. Wiley Interscience, New York: 209–225.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. Henry, H. Stevens & H. Wagner, 2013. Vegan: Community Ecology Package. R package version 2.0-10. http://CRAN.R-project.org/package=vegan.
Pagioro, T. A. & S. M. Thomaz, 2002. Longitudinal patterns of sedimentation in a deep, monomictic subtropical reservoir (Itaipu, Brazil–Paraguay). Archiv für Hydrobiologie 154: 515–528.
Petesse, M. L. & J. M. Petrere, 2012. Tendency towards homogenization in fish assemblages in the cascade reservoir system of the Tiete river basin, Brazil. Ecological Engineering 48: 109–116.
Petesse, M. L., M. Petrere & R. J. Spigolon, 2007. The hydraulic management of the Barra Bonita reservoir (SP, Brazil) as a factor influencing the temporal succession of its fish community. Brazilian journal of Biology 67: 433–445.
Petrere, J. M., 1996. Fisheries in large tropical reservoirs in South America. Lakes & Reservoirs: Research and Management 2: 111–133.
Poff, N. L. & D. D. Hart, 2002. How dams vary and why it matters for the emerging science of dam removal. BioScience 52: 659–668.
Poff, N. L. & J. K. H. Zimmerman, 2010. Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55: 194–205.
Poff, N. L., J. D. Olden, D. M. Merritt & D. M. Pepin, 2007. Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences of the United States of America 104: 5732–5737.
R. Development Core Team, 2012. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Reis, R. E., S. O. Kullander & C. F. Ferraris-Jr, 2003. Check List of the Freshwater Fishes of South and Central America. Editora PUCRS, Porto Alegre.
de Ribeiro, M. C. L. B., M. Petrere & A. A. Juras, 1995. Ecological Integrity and Fisheries Ecology of the Araguaia-Tocantins River Basin, Brazil. Regulated Rivers: Research and Management 11: 325–350.
Robson, A., I.G. Cowox & J.P. Harvey, 2011. Impact of run-of-river hydro-schemes upon fish populations. Phase 1. Literature review. WFD114: Project Final Report. Scotland and Northern Ireland Forum for Environmental Research (SNIFFER), Edinburgh, Scotland.
Rocha, O., E. L. G. Matsumura-Tundisi, T. Espíndola, K. F. Roche & A. C. Rietzler, 1999. Ecological theory applied to reservoir zooplankton. In Tundisi, J. & M. Straskraba (eds), Theoretical Reservoir Ecology and its Applications. International Institute of Ecology, Brazilian Academy of Sciences and Backhuys Publishers, São Paulo: 457–476.
Suzuki, H. I., A. A. Agostinho, D. Bailly, M. F. Gimenes, H. F. Júlio & L. C. Gomes, 2009. Inter-annual variations in the abundance of young-of-the-year of migratory fishes in the Upper Paraná River floodplain: relations with hydrographic attributes. Brazilian Journal of Biology 69: 649–660.
Ter Braak, C., 1987. CANOCO—A Fortran Program for Canonical Community Ordination by Correspondence Analysis, Principal Correspondence Analysis and Redundancy Analysis. TNO Institute of Applied Computer Science, Wageningen.
ter Braak, C. J. F. & P. F. M. Verdonschot, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences 57: 255–289.
Thornton, K. W., R. H. Kennedy, J. H. Carroll, W. W. Walker, R. C. Gunkel & S. Ashby, 1981. Reservoir sedimentation and water quality: an heuristic model. In Stefan, H. G. (ed.), Proceedings of the Symposium on Surface Water Impoundments. American Society of Civil Engineers, New York: 654–661.
Thornton, K. W., B. L. Kimmel & F. E. Payne, 1996. Reservoir Limnology: Ecological Perspectives. Wiley, New York.
Tundisi, J. & M. Straškraba, 1999. Theoretical Reservoir Ecology and Its Applications. Backhuys Publishers, São Carlos: 592 pp
Webb, A. J., K. A. Miller, E. L. King, S. C. de Little, M. J. Stewardson, J. K. H. Zimmerman & N. LeRoy Poff, 2013. Squeezing the most out of existing literature: a systematic re-analysis of published evidence on ecological responses to altered flows. Freshwater Biology 58: 2439–2451.
Acknowledgements
This study was supported by National Foundation for Science and Technology (FCT) with a grant to ACL (SFRH/BD/51408/2011) and CESAM funding (UID/AMB/50017/2013). The authors would like to thank ENERPEIXE S.A. for providing the data which made this study possible and anonymous editor and reviewers for valuable comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Odd Sandlund
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lima, A.C., Agostinho, C.S., Sayanda, D. et al. The rise and fall of fish diversity in a neotropical river after impoundment. Hydrobiologia 763, 207–221 (2016). https://doi.org/10.1007/s10750-015-2377-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2377-z