Abstract
Identifying the relative importance of various nursery areas is critical for understanding the ecological roles of diverse juvenile habitats, as well as for the sustainable management of fisheries and coastal resources. Recent field collections suggest that a portion of the bonefish (Albula vulpes) population in South Florida may be using nearshore estuarine habitats as nurseries. Nearshore marine habitats are traditionally considered nursery habitat for bonefish. However, the prevalence of their reliance on lower salinity areas is not known. To address this, we used otolith microchemistry using laser ablation inductively-coupled plasma mass spectrometry to examine variation in Strontium (Sr), a marker for salinity, and determine the connectivity between estuarine and marine habitats across the life of bonefish. Sr profiles from otoliths obtained in locations within South Florida (SFL, N = 40) and Southwest Cuba (SCB, N = 10) were compared using a juvenile-migration index (JMI), and change point models to assess the generality of a shift in salinity and thus habitats across life histories. Adult stages of bonefish otoliths collected in SFL and SCB locations showed Sr concentrations connected to marine, high-salinity environments. The JMI showed that a vast majority of individuals (68.4% and 70% in South Florida and Cuba, respectively) moved from low- to high-salinity environments between juvenile and adult stages. The change point models showed that these shifts to high salinity environments occurred suddenly (in 85% of those showing a low to high salinity change), and early in life (after 2 years in South Florida and after 4 years in Cuba) suggesting an ontogenetic habitat change. This study provided evidence that bonefish use low-salinity estuarine environments as juvenile habitats, perhaps more commonly than marine habitats. The reliance on low-salinity environments suggests the potential vulnerability of bonefish nearshore nursery habitats to disturbances associated with coastal freshwater discharges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flats fisheries across the Caribbean Basin are valuable socioeconomic resources that are increasing in popularity as a key component of ecotourism and conservation practices (Ault 2008; Adams et al. 2014; Adams 2017). Even though flats fisheries tend to be largely catch-and-release in various countries (e.g., USA, Puerto Rico, Belize, Bahamas), flats fisheries species are still harvested for subsistence in some regions, and their long-term sustainability is concerning due to signs of stock decline (Adams et al. 2014). Additionally, catch-and-release fisheries that have seen a large increase in recreational fishing effort combined with post-release mortality have pushed the stocks toward an overfished condition (Nelson 2002). In particular, there is increasing evidence of a decline in bonefish (Albula vulpes) over recent decades in South Florida (USA) (Larkin et al. 2010; Frezza and Clem 2015; Santos et al. 2017; Brownscombe et al. 2018). Despite the high economic impact of the bonefish fishery to the South Florida local economy, the availability of stock assessments and bioecological studies are limited. Consequently, key data on spawning and recruitment dynamics, habitat use patterns, and life history remain unknown (i.e., data-limited fishery; Larkin 2011; Adams et al. 2014).
The lack of direct long-term scientific data on bonefish population dynamics in South Florida challenges our ability to identify, with certainty, the main stressors causing the decline of this population (Larkin 2011; Santos et al. 2017; Brownscombe et al. 2018). Particularly, spatiotemporal dynamics in juvenile bonefish abundance, habitat use, and recruitment represent significant knowledge gaps for the South Florida bonefish (Larkin 2011; Brownscombe et al. 2018). Extensive field sampling efforts in South Florida have only produced small collections of bonefish juveniles and larvae, or have observed juveniles from the sympatric cryptic species Albula goreensis, with many collections not distinguishing between the two since this requires genetic testing (Crabtree et al. 1996; Ault et al. 1999; Harnden et al. 1999; Adams et al. 2007; Wallace and Tringali 2010; Larkin 2011). In the Bahamas, juvenile bonefish are common in leeward shallow sand flats (Mojica et al. 1995; Layman and Silliman 2002; Haak et al. 2018). However, other elopomorph species spawn in offshore waters and recruit to estuarine inshore juvenile habitats (Adams et al. 2014), suggesting that bonefish juveniles in South Florida could potentially recruit to nearshore habitats with estuarine conditions (i.e., low and variable salinity) and not exclusively to marine leeward sand flats. This is supported by a handful of recent observations of juvenile A. vulpes in estuarine areas of Florida Bay (Haak and Wallace unpub data; Santos and Rehage unpub data, Fig. 1a).
a Illustration showing the distribution of juvenile and larval bonefish collections in South Florida in relation to low salinity environments (white squares). Shown are genetically confirmed A. vulpes collections in recent (red) and earlier years (yellow). b Bonefish have a pelagic marine larval stage and a benthic adult stage in marine shallow flats habitats. However, it is unclear in South Florida whether post-larvae settles and develop in estuarine or marine habitats (or both), and how much adults use shallow habitats with estuarine salinity conditions. In this study, otolith microchemistry allowed a first estimation of the relative importance of low vs high salinity habitats to the life history of bonefish
How important are these low salinity habitats to the early life history of bonefish (Fig. 1b)? Understanding early life history strategies (e.g., ontogenetic shifts) and recruitment dynamics of bonefish is critical since the quality and the connectivity to nursery habitats are major determinants of the sustainability of adult fish populations (Mumby et al. 2004; Meynecke et al. 2008; Olds et al. 2012; Pittman et al. 2014; Nagelkerken et al. 2015). For marine fishes, recruitment dynamics have a dominant influence on the abundance and distribution of adult populations (Doherty and Fowler 1994; Cowen et al. 2006; Ligas et al. 2011), particularly for bonefish (Klarenberg et al. 2018). In South Florida, it is increasingly important to determine the reliance on nearshore estuarine environment as recruitment and nursery habitats for bonefish (Fig. 1) due to the persistent and drastic alterations to freshwater inputs (Browder et al. 2005; Rudnick et al. 2005; Madden et al. 2009; Stabenau and Kotun 2012). Large portions of nearshore coastal ecosystems have suffered state shifts through altered salinity regimes (i.e., due to changes in the timing, amount and quality of freshwater input), and associated transformations in the submerged aquatic vegetation (i.e., shifts in the composition and dominance of benthic species). These state shifts have been related to the loss in production and recruitment of sportfish species, and reduced quality of nursery grounds (Browder and Ogden 1999; Fourqurean and Robblee 1999; Browder et al. 2005; Rudnick et al. 2005). Thus, the uncertainty about juvenile bonefish recruitment in South Florida, their specific habitat requirements, and sensitivity to habitat loss and degradation (Brownscombe et al. 2018) all highlight the need to assess how the life history strategies of bonefish are connected to estuarine vs. marine habitats (Fig. 1b).
Different technologies such as genetic coding (Selkoe et al. 2008), acoustic telemetry, satellite tracking (Hussey et al. 2015), and isotopic analyses (Layman et al. 2012) have proven invaluable to improving our understanding of habitat use and connectivity, and fish stock structure. Additionally, fish otolith microchemistry is increasingly being used as a tool to improve our understanding of fish populations by identifying movement patterns, habitat connectivity, and essential juvenile habitats, and by allowing us to reconstruct the life history strategies of fishes (Campana 1999; Bath et al. 2000; Mateo et al. 2010; Morat et al. 2014). Strontium (Sr) has been the most important trace element to investigate patterns of fish habitat use, especially to distinguish movement and habitat use between low-salinity environments (e.g., freshwater and estuarine habitats) and high-salinity environments (e.g., offshore and reef habitats, and hyper-salinity basins; Campana 1999; Brown and Severin 2009; Albuquerque et al. 2012; Morat et al. 2014). Studies have shown that otolith Sr levels generally decrease from marine (high) to estuarine (intermediate) and freshwater (low) environments (Campana 1999). Sr has been deemed reliable for habitat use studies because its concentration is relatively stable within, but varies widely among aquatic habitats, and because Sr is metabolically inert (i.e., the newly-deposited material is neither resorbed nor reworked after deposition) and is highly abundant in water and otoliths compared with other trace elements (Campana 1999; Brown and Severin 2009; Reis-Santos et al. 2012).
In this study, we applied an otolith microchemistry approach to the life history of bonefish to determine the relative contribution of estuarine nearshore habitats to the life of bonefish across South Florida and Southwest Cuba. Thus, the goal of this study was to examine Sr profiles in otoliths to (1) determine the salinity environments of the adult stage (Sr:Ca concentrations during the adult stage) between different fishing locations in South Florida and Southwest Cuba; (2) quantify changes in the salinity environment across the life of bonefish; and (3) if there is evidence of such salinity changes along Sr profiles, identify the ages at which these possible ontogenic habitat shifts may be occurring. Furthermore, we compared Sr profiles in otoliths of bonefish collected in South Florida and Southwest Cuba to assess general recruitment strategies and juvenile migratory patterns across the Caribbean Basin, and identify variation in life history strategies possibly associated with hydrological alterations and management. The latter takes into consideration that coastal watershed hydrology has been completely transformed in South Florida, but relatively unaltered in Southwest Cuba.
Materials and methods
Study domain
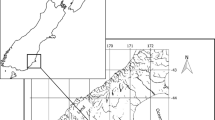
We conducted sampling in two regions in the Caribbean basin: South Florida and Southwest Cuba (Fig. 2, Table 1). In South Florida, the sampling concentrated in Biscayne Bay, Florida Bay, and the Florida Keys (Fig. 2a); three main fishing areas/subregions of the Florida bonefish fishery. Both, Biscayne Bay and Florida Bay are shallow subtropical estuarine lagoons that are influenced by the hydrodynamics of the Greatest Everglades Ecosystem watershed (Browder et al. 2005; Rudnick et al. 2005; Lirman et al. 2008). Biscayne Bay is located adjacent to the city of Miami, and Florida Bay at the southern end of the Everglades National Park (Fig. 2a). Both bays have nearshore, shallow fragmented seagrass seascapes with meso- (5–18 ppt) and poly-haline conditions (18–30 ppt) (Lirman et al. 2008; Larkin 2011; Santos et al. 2011; Hall et al. 2016) that could be suitable for bonefish juveniles. However, altered freshwater flows associated with freshwater management upstream have disturbed the salinity regimes of both bays (Browder et al. 2005; Rudnick et al. 2005; Lirman et al. 2008), potentially putting these nearshore nursery habitats at risk.
Map of the study domain in (a) South Florida and (b) Southwest Cuba. Fishing locations in South Florida included: Biscayne Bay (blue), Florida Bay (light blue), and the Florida Keys (black and including the Upper, Middle and Lower Keys). Fishing locations in Southwest Cuba included: Zapata Swamp (blue) and San Felipe Cays (black)
The sampling in the Florida Keys concentrated in the Upper Florida Keys (Key Largo to Lower Matecumbe Key), Middle Keys (Long Key to the Seven Mile Bridge), and Lower Keys (Seven Mile Bridge to the Marquesas, Fig. 2a). For this study, areas on the ocean (east) side of the Upper and northern part of the Middle Keys were considered part of the Florida Keys, while western areas were considered part of Florida Bay (Frezza and Clem 2015). Unlike Biscayne Bay and Florida Bay, nearshore and shallow habitats (e.g., leeward sand flats) in the Florida Keys suitable for juvenile and adult bonefish have a more stable euhaline marine environment (30–35 ppt; Briceño and Boyer 2012).
Bonefish collected in Southwest Cuba came from fishing locations within San Felipe Keys (Cayo San Felipe) and Zapata Swamp (Cienagas de Zapata; Fig. 2b, Table 1B). San Felipe Keys and Zapata Swamp share some climatic, habitat and fishery conditions with the locations sampled in South Florida. Both locations have marine resources highly valued for tourism (e.g., scuba, hiking) and fishery activities (e.g., commercial, recreational, sport), and which are protected and manage by a National Park system and designated no-take zones (Claro and Lindeman 2003; Perera Valderrama et al. 2017; Goulart et al. 2018). Similar to the influence of the Everglades on Biscayne Bay and Florida Bay ecosystems, the Zapata Swamp complex drainage system, the largest in the Caribbean, influences coastal and marine habitats that support high biodiversity (Perera Valderrama et al. 2017). The Zapata Swamp national park has an enforcement program, and there is a limited bonefish catch-and-release recreational fishery in the Las Salinas lagoon and other portions of Zapata Swamp (Rennert et al. this issue). San Felipe Keys are a chain of islands similar to the Florida Keys, characterized by coastal lagoons associated with mangroves and soft bottom with seagrass, and bordered by fringe and patch reefs (de la Guardia et al. 2018). Bonefish in this region is subject to subsistence fishing pressure (Rennert et al. this issue) and reduced freshwater inputs (Baisre and Arboleya 2006).
Sampling and otolith processing
The otoliths samples used in this study were originally obtained and processed by two previous bonefish aging projects in South Florida (Larkin 2011) and Cuba (Rennert et al. in this issue). Adult and juvenile bonefish specimens were collected by numerous fishermen using several sampling gears. Bonefish in South Florida were obtained from tournaments, and fishers and guides operating in Biscayne Bay, Florida Bay, and the Florida Keys. We obtained bonefish samples collected between 2003 and 2010 (N = 40) (Table 1A), but the majority of the samples were collected on the last 2 years (> 50% of the samples concentrated in 2008–2010, including collections associated with mortalities due to the 2010 cold event, Larkin 2011). In Cuba, we obtained bonefish samples from fishers in local markets (N = 10) between 2016 and 2017, which primarily catch bonefish using gill nets (Table 1B). For all samples, otoliths were removed and stored until sectioned. The left sagittae were then embedded in crystal polyester resin, and transverse sections were cut through the cores using a low-speed blade saw. Once mounted onto glass the cross sections were used to age the specimens by counting the number of annuli from the core (see Larkin 2011; Rennert et al. this issue for additional details on otolith processing and aging). All sample used in our study were genetically confirmed to be A. vulpes.
Otolith microchemistry analysis
Elemental analysis for each otolith was conducted using Laser Ablation Inductively-Coupled Plasma Mass Spectrometry (LA-ICP-MS) at the Florida International University Trace Evidence Analysis Facility (http://teaf.fiu.edu/). An Axiom J200 laser ablation unit (Applied Spectra, Fremont, CA, USA) was utilized to digitally draw transects (core to edge) and set laser settings while an ELAN DRC II ICP-MS (Perkin Elmer, Waltham, MA, USA) was used to identify and quantify elements based on their atomic mass. LA-ICP-MS settings are detailed in Table S1 (Supplementary Materials). The ICP-MS instrument was set to start recording elemental signals 20 s before ablation to account for the background noise that was later removed from the signal strength calculation for each element. Calibration standards from the National Institute of Standards and Technology (NIST), Standard Reference Material (SRM) 610 and NIST SRM 612 (Trace Elements in Glass, NIST, Gaithersburg, MD, USA), were used at the start and conclusion of each lab session to adjust for any instrumental drift. All signals were adjusted to remove the background noise. Instrument sensitivity was calculated following the equation modified by McGowan et al. (2014) to include both SRMs:
Where RAN610 and RAN612 are the average NIST SRM 610 and 612 signals for the element in question; CAN610 and CAN612 represent the values taken from the respective SRM Certificate for the element; RISS is the average value for Calcium; RIS610 and RIS612 are the average NIST SRM 610 and 612 signals for Calcium; CIS610 and CIS612 are the values taken from the respective SRM Certificate for Calcium; and CISS is the concentration of Calcium in the otolith taken from FEBS-1 (National Research Council Canada, Ottawa, Ontario, Canada). Calcium was used for normalization of the analyte in the equation due to the abundance of calcium naturally found in otoliths (McGowan et al. 2014). The Sr concentration was then reported as mmol of Sr over mol of calcium (Ca) (mmol/mol; referred to as Sr:Ca hereafter).
Data processing and statistical analysis
The first step in analyses was to relate distance in the otolith to age. The distance-from-core of the ablation laser transect (otolith radius) was related to the estimated age of each bonefish (obtained from the previous aging studies, Suppl. Mat. Fig. S1). A generalized additive model (GAM) with a log link function and Gaussian error distribution was used to estimate the bonefish age as a function of the otolith radius (age-otolith radius model hereafter) separately for the 40 South Florida and 10 Cuban samples. The model was later used to estimate the expected age of habitat shifts, and to classify the Sr:Ca concentration profile into age classes. Based on the age and growth analyses performed in Florida (Crabtree et al. 1996) and Cuba (Rennert et al. this issue), the age at maturity used in this study was 4 years for Florida otoliths, and 1 year for Cuba otoliths.
The Sr:Ca concentrations during the adult stage were compared among sampling locations (within each region) through analysis of variance (One-way ANOVA). A Tukey post-hoc test was used to assess pairwise Sr:Ca differences among the South Florida locations, and a t-test (Welch two sample t-test) to compare the two locations in Cuba. Thus, these tests were performed to detect distinct patterns of habitat occupancy for adult bonefish between South Florida and Southwest Cuba based on salinity. Residuals diagnostic plots were used to assess the assumptions of ANOVA and t-test, and the response variable was log (+1) transformed to approach and conform to the normal distribution assumption (Suppl. Mat. Fig. S2-S3).
We then performed analyses to assess changes in the salinity environment over the life of bonefish based on significant changes in the otolith Sr:Ca profiles. First, to identify significant salinity changes (i.e., estuarine ↔ marine habitats, Fig. 2b), the change of Sr:Ca concentration from core (larvae/juvenile stage) to edge (adult stage) (i.e., the slope of the Sr:Ca profile) was assessed by fitting a linear model using generalized least squares (GLS). An AR-1 autocorrelation structure was added to the models to control for autocorrelation and avoid the violation of independence due to the proximity and sequential nature of the data points (see Zuur et al. 2009 for details). We used the GLS model output for each otolith to create a juvenile migratory index (JMI, sensu Albuquerque et al. 2012). For each bonefish, a JMI was calculated by identifying the significance (α = 0.01 - i.e., a larger alpha value considered to account for a type 1 error) and direction of the slope coefficient (β1) (Suppl. Mat. Tables S2-S3). If the coefficient in the model was not significant (p > 0.01), the JMI was classified as “No Change”. If significant then, the JMI was classified as either “Positive” or “Negative” depending on the direction of the slope coefficient (± β1). We were interested in the Sr:Ca trends between the juvenile and adult stage; thus, positive and negative values of JMI were presumed to indicate ontogenic habitat changes toward higher and lower salinities, respectively (i.e., juveniles moving from estuarine to marine conditions, or adults moving to estuarine habitats, Fig. 2b). A Chi-squared contingency table test (χ2 test) was then performed to assess for variation in the frequency of JMI scores among locations and determine variation in these possible habitat shifts in Cuba vs. South Florida.
Last, we used a breakpoint analysis to identify ontogenic habitat shifts by quantifying abrupt changes on the Sr:Ca profiles of those individuals classified with either a positive or negative JMI. The breakpoint analysis helped to determine the distance-from-core at which individuals presented major abrupt changes in Sr:Ca signatures; interpreted here, as the age where bonefish showed shifts between estuarine and marine habitats. The breakpoint analysis was based on a change point model (CPM) with a sequential change detection approach (i.e., observations are processed sequentially; Ross 2015). A generalized likelihood ratio test statistic (GLR) was used to identify the significant breakpoints (Ross 2015). We used the CPM results to quantify the proportion of individuals with the presence or absence of a significant breakpoint in their Sr:Ca profiles (e.g., indicative of abrupt or complete vs. gradual or partial habitat changes).
Following the CPMs, we created a mean distribution of distance-from-core values identified by the breakpoint analysis (i.e., the distance-from-core where a major shift in Sr:Ca is expected to occur) to identify the age at which these breakpoints were observed. Due to the limited sample size, we constructed these mean distributions based on a bootstrapping procedure with 1000 replicates and with replacement (Davison and Hinkley 1997; Canty and Ripley 2016). These bootstrapped means (and upper and lower 95% confidence levels) were then used to estimate the ages of shifts in Sr:Ca concentrations by using the age-otolith radius model (Suppl. Mat. Fig. S1).
All statistical analyses were performed in R v3.2.5 (R Core Team 2017). The GLS linear model, change point model and the bootstrapping were performed respectively with the nlme (Pinheiro et al. 2017), cpm (Ross 2015) and boot (Canty and Ripley 2016) R packages.
Results
Adult Sr:Ca concentrations
Strontium concentrations measured in all otoliths were above the detection limits, except for two South Florida samples that we eliminated from further analyses. Thus, we were able to use temporal trends in Sr:Ca concentrations (Sr:Ca profiles) as a tool to assess ontogenic habitat shifts in 38 South Florida and 10 Southwest Cuban A. vulpes. Across all fishing locations and regions, Sr:Ca concentrations at the adult stage were above 1.0 mmol/mol (Fig. 3). However, there were significant differences in adult Sr:Ca concentrations between the fishing locations sampled in South Florida (ANOVA, F2; 33,964 = 17.66, p = 2.31 × 10−8) and Cuba (Welch t-test, t1; 1292 = 13.57, p = 2.2 × 10−16). In Florida, fish collected in both Florida Bay and the Florida Keys showed a significantly higher Sr:Ca concentration than in Biscayne Bay (Fig. 3a, Tukey test, p = 1 × 10−6 and p = 3 × 10−6). In Cuba, the mean Sr:Ca concentration of adults in Zapata Swamp, where freshwater inputs are greater, was significantly higher than the mean concentration in the San Felipe Keys (Welch t-test, t1; 1292 = 13.57, p = 2.2 × 10−16, Fig. 3b).
Mean Sr:Ca concentrations (mmol/mol ± SE) in bonefish otoliths for adults compared across (a) South Florida fishing locations (Florida Bay, Biscayne Bay, Florida Keys) and (b) Southwest Cuba fishing locations (Zapata Swamp and San Felipe). The letters mark significant groupings within the South Florida fishing locations, while the asterisk demarks a significant difference between Cuban fishing locations
Juvenile migration index
The JMI was used to assess the direction of salinity and thus habitat change (i.e., low ↔ high salinity; estuarine ↔ marine habitats) between juvenile and adult stages. Positive values of JMI were more frequent (68.4% and 70% in Florida and Cuba, respectively) than negative or neutral (no change) values across all fishing locations and regions (Fig. 4, Table 2), indicating a dominant pattern of individuals shifting from low to high-salinity environments between the juvenile and adult stage. However, based on the chi-squared test, this proportion toward positive values was not significantly different across fishing locations (Table 2, χ2 = 0.2–3.57; p < 0.05), probably due to a low count in some of the JMI scores (more than 20% of cells have an expected count less than 5). A negative change between the juvenile and adult stage was not detected in any of the locations (Table 2, Fig. 4), indicating a lack of movement from high to low salinity environments.
Juvenile migration index (JMI) across fishing locations in (a) South Florida and (b) Southwest Cuba. ‘Positive’ (black; indicative of an increase in salinity through life) denotes significant positive slope coefficients in the Sr:Ca profiles, while ‘No change’ (grey; indicative of constant or variable salinity through life) denotes the number of nonsignificant slopes. No otoliths with negative changes were observed (indicative of a decrease in salinity through life)
Ontogenic habitat shifts
To identify habitats shifts, we considered only individuals with a positive JMI in the breakpoint analysis (N = 33, Florida = 26, Cuba = 7). The majority of these individuals showed a significant break point in their Sr:Ca signatures (84.6% of Florida and 85.7% of Cuban samples respectively; Table 3). On average, the breakpoint in Sr:Ca signatures on bonefish otoliths in South Florida was detected closer to the otolith core (Table 4A, Fig. 5a), and at lower Sr:Ca concentrations (Table 4A). However, there was an overlap between the mean distributions of distance-from-core identified by the breakpoint analysis, indicating similar Sr:Ca breakpoints along the otolith.
a Mean distribution of distance-from-core indicating a significant positive shift in Sr:Ca signatures and interpreted as ontogenetic habitat shifts in South Florida (red) and Southwest Cuba (black). Vertical lines indicate the mean distance-from-core identified by the breakpoint analysis. We determined (b) the age of this ontogenetic habitat shift in South Florida (red) and Southwest Cuba (black)
Combining this information with the age-otolith radius model, we observed that the majority of individuals manifested a habitat shift from a low to high salinity environment after 2 (95% CI [1, 3]) years of age in South Florida, and after 4 (95% CI [2, 8]) years of age in Southwest Cuba (Table 4A, Fig. 5b). However, we could not discern a significant difference in the age of habitat shifts between Florida and Cuba as told by the overlapping 95% confidence intervals (Fig. 5). Similarly in South Florida, the shift in Sr:Ca signatures did not differ across fishing locations (Fig. 6, Table 4B). We saw a trend in the mean of ontogenetic habitats shifts, which occurred after 1 (95% CI [1, 2]) year of age in Florida Bay, after 2 (95% CI [1, 2]) years of age in Biscayne Bay and after 3 (95% CI [1, 6]) years of age in the Florida Keys, but overlapping confidence indicated no significant differences among these values (Fig. 6b).
a Mean distribution of distance-from-core indicating a significant positive shift in Sr:Ca signatures and interpreted as ontogenetic habitat shifts in South Florida fishing locations: Florida Bay (black), Biscayne Bay (dark grey) and the Florida Keys (grey). Vertical lines indicate the mean distance-from-core identified by the breakpoint analysis. We determined (b) the age of these shifts lower to higher salinity in each location
Discussion
Juveniles of many marine fish species often recruit to nearshore habitats where they reside and develop for some extended time (months to years) before moving and joining adult populations at offshore or marine habitats (Beck et al. 2001; Gillanders et al. 2003; Mumby et al. 2004). Thus, identifying the relative importance of various nursery areas is critical for the understanding of the ecological roles of diverse habitats as well as for the sustainable management of fisheries and other coastal resources (Mumby et al. 2004; Mateo et al. 2010; Nagelkerken et al. 2015). However, for many marine fish species, the life history strategies and ontogenic shifts between nursery and adult habitats are unknown, including for highly-valued recreational species such as the bonefish (Larkin 2011; Adams et al. 2014; Brownscombe et al. 2018). In our study, we used otolith microchemistry to reconstruct the life history strategies of bonefish and assess the relative importance of estuarine habitats for recruitment (i.e., nursery areas). Strontium concentrations of adult stages on the bonefish otoliths were different across all locations in South Florida and Southwest Cuba, but consistently higher than the juvenile stage Sr:Ca concentrations. A juvenile-migration index showed that a vast majority of individuals (68.4% and 70% in South Florida and Southwest Cuba, respectively) moved from low- to high-salinity environments between the juvenile and adult stage. The breakpoint analysis of the Sr:Ca profiles showed that the in most samples (85%), the increase in salinity was marked and occurred early in life, consistent with an ontogenetic habitat shift. This shift was detected between 2 and 4 years of age in both South Florida and Southwest Cuba. These findings point to the reliance of bonefish on two types of nursery habitats, estuarine and marine nearshore habitats, but a greater reliance on the estuarine habitats as juveniles.
These results increase our understanding of the possible bonefish paths of recruitment, and provide the first quantitative evidence of the connectivity of juvenile nearshore estuarine habitats with adult marine habitats for this species in the Caribbean Basin. Our findings follow on a number of studies that have shown the utility of otoliths Sr tracers to assess the life history strategies and habitat linkages in fishes (Campana 1999; Brown and Severin 2009; Albuquerque et al. 2012; Morat et al. 2014). For instance, Morat et al. (2014) used Sr (along with Barium and isotopic analyses) to identify two type of nurseries for the common sole in the Mediterranean (brackish and marine/hypersaline environments), and argue for habitat-integrated management of fisheries. Albuquerque et al. (2012) used Sr profiles to establish the estuarine-dependency of Whitemouth croaker early in its life history (for 71% of marine-sampled fish), and to identify the timing of estuarine egress at 2–4 years of age. Also, similar to our study, Sr profiles of Pomatomus saltarix showed a variety of life history patterns and plasticity in the use of coastal and estuarine habitats in Australia (Schilling et al. 2018).
While otolith chemistry is known to be reflective of the surrounding water chemistry, including salinity, other factors such as temperature, growth rate, and ontogeny can potentially influence Sr uptake into the otoliths (Brown and Severin 2009; Webb et al. 2012; Izzo et al. 2018). All processes affecting ion transport have the potential to affect otolith chemistry (Izzo et al. 2018). To date, however, no laboratory experiments have been performed to disentangle the importance of these factors on bonefish otoliths Sr uptake. Several reared laboratory experiments have consistently shown a positive correlation between otolith Sr and salinity (Campana 1999; Rooker et al. 2004; Chen et al. 2008; Izzo et al. 2018). Still, laboratory experiment results could be influenced by the duration of the experiment (i.e., exposition time), and the condition and growth rate of specimens (Izzo et al. 2018). The opportunistic nature of our sampling design also hindered our ability to compare the otolith Sr:Ca concentrations to field salinity measurements to confirm their relationship in bonefish otoliths. At the same time, our Sr:Ca (mol/mmol) values were lower than those of other studies that have used this trace element to assess ontogenetic habitat shifts between estuarine and marine habitats (Suppl. Mat. Table S4). This inconsistency highlights the need for follow-up studies to address how Sr in bonefish otoliths respond to salinity and how other physiological or ontogeny (e.g., diet shifts) factors may influence the Sr-salinity relationship. However, our results do match Sr:Ca values reported by a study on a South Atlantic sciaenid, which demonstrated estuarine to marine habitat shifts over ontogeny (Albuquerque et al. 2012; Suppl. Mat. Table S4).
Despite these potential unaccounted sources of Sr variation in bonefish otoliths, the overall Sr:Ca profile pattern is consistent with other studies’ findings that have identified ontogenic habitat shifts using this trace element. In our study, 85% of the individuals showed an abrupt change in their Sr:Ca from low to high values. As observed in our study, the predominance of abrupt changes (i.e., breakpoint) from low to higher Sr:Ca concentrations have been successfully used by previous work to determine habitat shifts between estuarine and marine habitats, and determine the period of reliance on estuarine habitats (Fablet et al. 2007; Brown and Severin 2009; Tabouret et al. 2011; Payne Wynne et al. 2015; Rohtla and Vetemaa 2016). For instance, Payne Wynne et al. (2015) used a regime shift analysis based on Sr:Ca profiles to identify various migratory patterns of blueback herring (Alosa aestivalis) in the coast of Maine (USA). In addition, a recent review and meta-analysis by Izzo et al. (2018) concluded that the Sr:Ca in fish otoliths is mostly correlated with the element concentrations of the surrounding water, with the strength and variation of this relationship being influenced by other intrinsic factors (e.g., growth rate, sexual maturation, body condition). Thus, the positive JMI and abrupt changes observed in our study are consistent with what would be expected if bonefish switch from estuarine to marine habitats (Fig. 2b). Moreover, the consistency of the JMI patterns across the locations and regions, and the observations of juvenile bonefish in nearshore habitats with estuarine conditions (Fig. 2a), support the premise that the Sr:Ca patterns observed here are related to habitat changes across a salinity gradient and not to other environmental or physiological factors.
The prevalence of positive slopes in Sr:Ca profiles and the breakpoint analysis suggested that bonefishes often observed on marine flats spend time as juveniles in nearshore habitats with lower salinity conditions (i.e., estuarine habitats) and experienced marked ontogenetic habitats switches. Other elopomorph species related to bonefish (e.g., Tarpon, Megalops atlanticus and Ladyfish, Elops saurus) also display ontogenic habitat shifts involving nearshore habitats. For example, studies on M. atlanticus otoliths and scales Sr:Ca concentrations have successfully shown migratory patterns across estuarine gradients, and the settlement and habitat use in environments with diverse salinity conditions (e.g., freshwater, brackish, marine and hypersalinity conditions; Brown and Severin 2008; Seeley et al. 2015; Rohtla and Vetemaa 2016).
The JMI patterns across all of the locations in South Florida and Southwest Cuba (both “No Change” and “Positive” JMI) also supported the notion that, like many other species in the Caribbean, bonefish relay on different nearshore habitats for nursery functions (Nagelkerken et al. 2000, 2015; Grol et al. 2014). For instance, our results indicated that a large portion of the bonefish populations uses environments with lower salinity at early ages (~70%) but, a considerable fraction (~30%) of the populations use environments with similar salinity conditions throughout their entire life-history (Fig. 2b). Other otolith microchemistry studies have also observed similar intra-specific variability in juvenile habitat use and ontogenic habitat shifts (Brown and Severin 2009; Morat et al. 2014; Rohtla and Vetemaa 2016; Schilling et al. 2018). Multi-patterns in habitat use and recruitment may indicate a bet-hedging strategy – i.e., a form of diversification of activities having the purpose of reducing risk through averaging independent random events (Lauck et al. 1998). Spatial asynchrony through bet-hedging may ensure that some recruits are distributed across various spatial and temporal states of environmental suitability (Lauck et al. 1998; Hsieh et al. 2006; Coates and Hovel 2014). This strategy may be critical for the resilience of bonefish populations, especially in South Florida, where nearshore environments are dynamic and highly influenced by natural and anthropogenic disturbances.
The higher Sr:Ca values in the otolith adult stage agree with observed habitat use patterns of adult bonefishes in marine environments (Adams et al. 2007, 2014; Murchie et al. 2013). However, there were significant differences between adult Sr:Ca values across locations in South Florida and Southwest Cuba that may be associated with distinct hydrological and habitat use patterns. For instance, the adult Sr:Ca values in Biscayne Bay were lower than those of other locations. A portion of the bonefishing grounds in Biscayne Bay is in shallow nearshore areas influenced by freshwater canals (Larkin 2011), which may explain these lower concentrations. In contrast, high salinity regimes are prevalent in large portions of Florida Bay, including recurrent, seasonal hypersalinity conditions (Stabenau and Kotun 2012). In Southwest Cuba, the Sr:Ca signatures were higher in the adult stage of the individuals assigned to the Zapata Swamp Region, an area with high freshwater discharge (Perera Valderrama et al. 2017). The different locations where fishers collected the samples or intra-specific fitness related to bonefish habitat use are factors that could contribute to the unexpected differences observed between the Zapata and Felipe Keys adults Sr:Ca. Intra-specific variability in Sr:Ca could be influenced by differences in diet and fitness of individuals as discussed earlier (Izzo et al. 2018). This could be a possibility for bonefish, knowing that the quality and quantity of freshwater discharges have been associated with major transformations of estuarine and coastal habitats by inducing habitat loss and fragmentation, and by changing nutrient pathways and physical properties of the water column (Browder et al. 2005; Rudnick et al. 2005; Orth et al. 2006; Lirman et al. 2014; Santos et al. 2015).
There is growing evidence that growth and reproductive development, in conjunction with salinity, can also exert a major influence in the variance of otolith element incorporation (Campana 1999; Stanley et al. 2015; Sturrock et al. 2015). An age-length study performed in Cuba determined that bonefish sexually mature approximately at 1–2 years of age, and determined that individuals grow to smaller sizes and mature earlier than in South Florida (Rennert et al. this issue). Also, within South Florida, there are differences in bonefish size distribution among fishing locations, potentially illustrating a growth rate differentiation at the local scale. The trend for regional and local variability in the timing of ontogenetic habitat shifts detected could have been associated with this difference at the local and regional scale in growth rate and age/size at maturity. Differences in maturity and growth rate can be influenced by fisheries-induced evolution (Olsen et al. 2004) and by environmental controls on the quality and quantity marine habitats (McManus and Travis 1998; Froeschke and Stunz 2012; Yeager et al. 2012). Furthermore, these life history parameters can influence how long fish species depend on or benefit from nursery habitats and areas use for shelter (i.e., exposure time; Gillanders et al. 2003; Claudet et al. 2010; Grol et al. 2014; Nagelkerken et al. 2015), and the extent of protein synthesis that influence the amount of the trace elements that is incorporated into the blood (Campana 1999).
The expected age of egress from habitat with lower salinity observed in our study points to a suite of potential risk factors associated with estuarine nearshore disturbances. Especially, risk factors associated with freshwater discharges such as nutrients, pollution, and contaminants. Restoration efforts in South Florida are ongoing to reestablish natural flows and minimize algal blooms, hypersalinity, and seagrass die-off events in coastal habitats (Browder et al. 2005; Rudnick et al. 2005; Kelble et al. 2013; Hall et al. 2016). Estuarine habitat conditions associated with freshwater deliveries may influence bonefish, especially juveniles, by affecting factors that control individual fitness (e.g., quality and quantity of food), shelter quality (e.g., patch complexity, habitat fragmentation), and predation rate and seascape of risk (Grol et al. 2011; Santos et al. 2011, 2018; Nagelkerken et al. 2015). We believed that the results of our study are important to lay the foundation for future research studies on bonefish ecology. Specifically, the results provide evidence that the health of bonefish fisheries in South Florida (and in the Caribbean) may be strongly linked to freshwater management and Everglades restoration efforts than previously anticipated.
In summary, this study provided evidence that bonefish in South Florida and Southwest Cuba use low-salinity waters associated with estuarine conditions, especially during juvenile and subadult stages. The majority of studies performed to assess the distribution of bonefish post-larvae and juveniles have concentrated on leeward sand flats and shallow marine lagoons (Larkin 2011; Brownscombe et al. 2018). However, the results of this study highlight the importance of including nearshore estuarine habitats in future sampling efforts for a complete assessment of bonefish nursery habitats and recruitment events. Future steps of this study should consider other trace elements, such as Barium and Zinc, that also indicate habitat shifts (Morat et al. 2014; Sturrock et al. 2015; Izzo et al. 2018), and contrast otoliths trace elements between young-of-the-year and adults collected across salinity environments (e.g., Sr:Ca profile analysis of young-of-the-year vs adults; Tabouret et al. 2011; Albuquerque et al. 2012; Morat et al. 2014). In addition, future steps should identify distinct water fingerprints using trace elements to resolve the importance and identity estuarine habitats, and examine how different habitats are proportionally used across bonefish age classes and sub-populations (e.g., Lara et al. 2007; Mateo et al. 2010; Morat et al. 2014). The spatial discrimination between adult and juvenile bonefish habitats should be a priority for the conservation of this species; especially in South Florida, where the quality of the flats fisheries and the stability of nearshore habitats are highly interconnected with freshwater management decisions associated with the confounding pressure of population growth and climate change.
References
Adams AJ (2017) Guidelines for evaluating the suitability of catch and release fisheries: lessons learned from Caribbean flats fisheries. Fish Res 186:672–680. https://doi.org/10.1016/J.FISHRES.2016.09.027
Adams AJ, Wolfe RK, Tringali MD et al (2007) Rethinking the status of Albula spp. Biology in the Caribbean and Western Atlantic. Biol Manag World Tarpon Bonefish Fish 203. https://doi.org/10.1201/9781420004250.ch15
Adams AJ, Horodysky AZ, Mcbride RS et al (2014) Global conservation status and research needs for tarpons (Megalopidae), ladyfishes (Elopidae) and bonefishes (Albulidae). Fish Fish 15:280–311. https://doi.org/10.1111/faf.12017
Albuquerque CQ, Miekeley N, Muelbert JH, Walther BD, Jaureguizar AJ (2012) Estuarine dependency in a marine fish evaluated with otolith chemistry. Mar Biol 159:2229–2239. https://doi.org/10.1007/s00227-012-2007-5
Ault JS (2008) Biology and management of the world tarpon and bonefish fisheries. CRC Press, Boca Raton
Ault JS, Diaz GA, Smith SG, Luo J, Serafy JE (1999) An efficient sampling survey design to estimate pink shrimp population abundance in Biscayne Bay, Florida. North Am J Fish Manag 19:696–712. https://doi.org/10.1577/1548-8675(1999)019<0696:AESSDT>2.0.CO;2
Baisre JA, Arboleya Z (2006) Going against the flow: effects of river damming in Cuban fisheries. Fish Res 81:283–292. https://doi.org/10.1016/J.FISHRES.2006.04.019
Bath GE, Thorrold SR, Jones CM, Campana SE, McLaren JW, Lam JWH (2000) Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim Cosmochim Acta 64:1705–1714. https://doi.org/10.1016/S0016-7037(99)00419-6
Beck MW, Heck KL Jr, Able KW et al (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nurse. Bioscience 51:555–556. https://doi.org/10.1641/0006-3568(2001)051
Briceño HO, Boyer JN (2012) 2012 annual report of the water quality monitoring project for the water quality protection program of the Florida Keys National Marine Sanctuary. 1–79
Browder JA, Ogden JC (1999) The natural South Florida system II: predrainage ecology. Urban Ecosyst 3:245–277. https://doi.org/10.1023/A:1009504601357
Browder JA, Alleman R, Markley S, Ortner P, Pitts PA (2005) Biscayne Bay conceptual ecological model. Wetlands 25:854–869. https://doi.org/10.1672/0277-5212(2005)025[0854:BBCEM]2.0.CO;2
Brown RJ, Severin KP (2008) A preliminary otolith microchemical examination of the diadromous migrations of Atlantic tarpon Megalops atlanticus. Biol Manag World Tarpon Bonefish Fish Taylor Fr Group CRC Ser Mar Biol Boca Raton, Florida 259–274
Brown RJ, Severin KP (2009) Otolith chemistry analyses indicate that water Sr:ca is the primary factor influencing otolith Sr:ca for freshwater and diadromous fish but not for marine fish. Can J Fish Aquat Sci 66:1790–1808. https://doi.org/10.1139/F09-112
Brownscombe JW, Danylchuk AJ, Adams AJ, Black B, Boucek R, Power M, Rehage JS, Santos RO, Fisher RW, Horn B, Haak CR, Morton S, Hunt J, Ahrens R, Allen MS, Shenker J, Cooke SJ (2018) Bonefish in South Florida: status, threats and research needs. Environ Biol Fish:1–20. https://doi.org/10.1007/s10641-018-0820-5
Campana S (1999) Chemistry and composition of fish otoliths:pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297. https://doi.org/10.3354/meps188263
Canty A, Ripley B (2016) Boot: bootstrap R (S-plus) functions
Chen H, Shen K, Chang C, Iizuka Y, Tzeng WN (2008) Effects of water temperature, salinity and feeding regimes on metamorphosis, growth and otolith Sr:ca ratios of Megalops cyprinoides leptocephali. Aquat Biol 3:41–50. https://doi.org/10.3354/ab00062
Claro R, Lindeman KC (2003) Spawning aggregation sites of snapper and grouper species (Lutjanidae and Serranidae) on the insular shelf of Cuba. Gulf Caribb Res 14:91–106. https://doi.org/10.18785/gcr.1402.07
Claudet J, Osenberg CW, Domenici P, Badalamenti F, Milazzo M, Falcón JM, Bertocci I, Benedetti-Cecchi L, García-Charton JA, Goñi R, Borg JA, Forcada A, de Lucia GA, Pérez-Ruzafa Á, Afonso P, Brito A, Guala I, Diréach LL, Sanchez-Jerez P, Somerfield PJ, Planes S (2010) Marine reserves: fish life history and ecological traits matter. Ecol Appl 20:830–839. https://doi.org/10.1890/08-2131.1
Coates JH, Hovel KA (2014) Incorporating movement and reproductive asynchrony into a simulation model of fertilization success for a marine broadcast spawner. Ecol Model 283:8–18. https://doi.org/10.1016/j.ecolmodel.2014.03.012
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science (80- ) 311:522–527. https://doi.org/10.1126/science.1122039
Crabtree RE, Harnden CW, Snodgrass D, Stevens C (1996) Age, growth, and mortality of bonefish, Albula vulpes, from the waters of the Florida keys. Fish Bull 94:442–451
Davison A, Hinkley D (1997) Bootstrap methods and their applications. Cambridge University Press, Cambridge
de la Guardia E, Giménez-Hurtado E, Defeo O et al (2018) Indicators of overfishing of snapper (Lutjanidae) populations on the southwest shelf of Cuba. Ocean Coast Manag 153:116–123. https://doi.org/10.1016/j.ocecoaman.2017.12.006
Doherty P, Fowler T (1994) An empirical test of recruitment limitation in a coral reef fish. Science 263:935–939. https://doi.org/10.1126/science.263.5149.935
Fablet R, Daverat F, De Pontual H (2007) Unsupervised Bayesian reconstruction of individual life histories from otolith signatures: case study of Sr:ca transects of European eel (Anguilla anguilla) otoliths. Can J Fish Aquat Sci 64:152–165. https://doi.org/10.1139/f06-173
Fourqurean J, Robblee M (1999) Florida bay: a history of recent ecological changes. Estuaries 22:345–357
Frezza PE, Clem SE (2015) Using local fishers’ knowledge to characterize historical trends in the Florida bay bonefish population and fishery. Environ Biol Fish 98:2187–2202. https://doi.org/10.1007/s10641-015-0442-0
Froeschke JT, Stunz GW (2012) Hierarchical and interactive habitat selection in response to abiotic and biotic factors: the effect of hypoxia on habitat selection of juvenile estuarine fishes. Environ Biol Fish 93:31–41. https://doi.org/10.1007/s10641-011-9887-y
Gillanders BM, Able KW, Brown JA, Eggleston DB, Sheridan PF (2003) Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Mar Ecol Prog Ser 247:281–295. https://doi.org/10.3354/meps247281
Goulart F, Galán ÁL, Nelson E, Soares-Filho B (2018) Conservation lessons from Cuba: connecting science and policy. Biol Conserv 217:280–288. https://doi.org/10.1016/j.biocon.2017.10.033
Grol MGG, Nagelkerken I, Rypel AL, Layman CA (2011) Simple ecological trade-offs give rise to emergent cross-ecosystem distributions of a coral reef fish. Oecologia 165:79–88. https://doi.org/10.1007/s00442-010-1833-8
Grol MGG, Rypel AL, Nagelkerken I (2014) Growth potential and predation risk drive ontogenetic shifts among nursery habitats in a coral reef fish. Mar Ecol Prog Ser 502:229–244. https://doi.org/10.3354/meps10682
Haak CR, Power M, Cowles GW, Danylchuk AJ (2018) Hydrodynamic and isotopic niche differentiation between juveniles of two sympatric cryptic bonefishes, Albula vulpes and Albula goreensis. Environ Biol Fish:1–17. https://doi.org/10.1007/s10641-018-0810-7
Hall MO, Furman BT, Merello M, Durako MJ (2016) Recurrence of Thalassia testudinum seagrass die-off in Florida bay, USA: initial observations. Mar Ecol Prog Ser 560:243–249. https://doi.org/10.3354/meps11923
Harnden CW, Crabtree RE, Shenker JM (1999) Onshore transport of elopomorph leptocephali and glass eels (Pisces: Osteichthyes) in the Florida keys. Gulf Mex Sci 17:17–26
Hsieh C-H, Reiss CS, Hunter JR, Beddington JR, May RM, Sugihara G (2006) Fishing elevates variability in the abundance of exploited species. Nature 443:859–862. https://doi.org/10.1038/nature05232
Hussey NE, Kessel ST, Aarestrup K et al (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science 348:1255642. https://doi.org/10.1126/science.1255642
Izzo C, Reis-Santos P, Gillanders BM (2018) Otolith chemistry does not just reflect environmental conditions: a meta-analytic evaluation. Fish Fish 19:441–454. https://doi.org/10.1111/faf.12264
Kelble CR, Loomis DK, Lovelace S, Nuttle WK, Ortner PB, Fletcher P, Cook GS, Lorenz JJ, Boyer JN (2013) The EBM-DPSER conceptual model: integrating ecosystem services into the DPSIR framework. PLoS One 8:e70766. https://doi.org/10.1371/journal.pone.0070766
Klarenberg G, Ahrens R, Shaw S, Allen M (2018) Use of a dynamic population model to estimate mortality and recruitment trends for bonefish in Florida bay. Environ Biol Fish. https://doi.org/10.1007/s10641-018-0805-4
Lara MR, Jones DL, Chen Z, Lamkin JT, Jones CM (2007) Spatial variation of otolith elemental signatures among juvenile gray snapper (Lutjanus griseus) inhabiting southern Florida waters. Mar Biol 153:235–248. https://doi.org/10.1007/s00227-007-0799-5
Larkin MF (2011) Assessment of South Florida’ s bonefish stock. These Diss 214
Larkin MF, Ault JS, Humston R, Luo J (2010) A mail survey to estimate the fishery dynamics of southern Florida’s bonefish charter fleet. Fish Manag Ecol 17:254–261. https://doi.org/10.1111/j.1365-2400.2009.00718.x
Lauck T, Clark C, Mangel M, Munro G (1998) Implementing the precautionary principle in FIsheries management through marine reserves. Ecol Appl 8:72–78. https://doi.org/10.1890/1051-0761(1998)8[S72:ITPPIF]2.0.CO;2
Layman CA, Silliman BR (2002) Preliminary survey and diet analysis of juvenile fishes of an estuarine creek on Andros Island, Bahamas. Bull Mar Sci 70:199–210
Layman CA, Araujo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR, Matich P, Rosenblatt AE, Vaudo JJ, Yeager LA, Post DM, Bearhop S (2012) Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol Rev 87:545–562. https://doi.org/10.1111/j.1469-185X.2011.00208.x
Ligas A, Sartor P, Colloca F (2011) Trends in population dynamics and fishery of Parapenaeus longirostris and Nephrops norvegicus in the Tyrrhenian Sea (NW Mediterranean): the relative importance of fishery and environmental variables. Mar Ecol 32:25–35. https://doi.org/10.1111/j.1439-0485.2011.00440.x
Lirman D, Deangelo G, Serafy J, Hazra A, Smith Hazra D, Herlan J, Luo J, Bellmund S, Wang J, Clausing R (2008) Seasonal changes in the abundance and distribution of submerged aquatic vegetation in a highly managed coastal lagoon. Hydrobiologia 596:105–120. https://doi.org/10.1007/s10750-007-9061-x
Lirman D, Thyberg T, Santos R, Schopmeyer S, Drury C, Collado-Vides L, Bellmund S, Serafy J (2014) SAV communities of Western Biscayne Bay, Miami, Florida, USA: human and natural drivers of seagrass and macroalgae abundance and distribution along a continuous shoreline. Estuar Coasts 37:1243–1255. https://doi.org/10.1007/s12237-014-9769-6
Madden CJ, Rudnick DT, McDonald AA et al (2009) Ecological indicators for assessing and communicating seagrass status and trends in Florida Bay. Indic Everglades Restor 9:S68–S82. https://doi.org/10.1016/j.ecolind.2009.02.004
Mateo I, Durbin EG, Appeldoorn RS, Adams AJ, Juanes F, Kingsley R, Swart P, Durant D (2010) Role of mangroves as nurseries for French grunt Haemulon flavolineatum and schoolmaster Lutjanus apodus assessed by otolith elemental fingerprints. Mar Ecol Prog Ser 402:197–212. https://doi.org/10.3354/meps08445
McGowan N, Fowler AM, Parkinson K, Bishop DP, Ganio K, Doble PA, Booth DJ, Hare DJ (2014) Beyond the transect: an alternative microchemical imaging method for fine scale analysis of trace elements in fish otoliths during early life. Sci Total Environ 494–495:177–186. https://doi.org/10.1016/j.scitotenv.2014.05.115
McManus MG, Travis J (1998) Effects of temperature and salinity on the life history of the sailfin molly (Pisces: Poeciliidae): lipid storage and reproductive allocation. Oecologia 114:317–325. https://doi.org/10.1007/s004420050453
Meynecke JO, Lee SY, Duke NC (2008) Linking spatial metrics and fish catch reveals the importance of coastal wetland connectivity to inshore fisheries in Queensland, Australia. 141:981–996. https://doi.org/10.1016/j.biocon.2008.01.018
Mojica R, Shenker JM, Harnden CW, Wagner DE (1995) Recruitment of bonefish, Albula vulpes, around Lee Stocking Island, Bahamas. Fish Bull 93:666–674
Morat F, Letourneur Y, Dierking J, Pécheyran C, Bareille G, Blamart D, Harmelin-Vivien M (2014) The great melting pot common sole population connectivity assessed by otolith and water fingerprints. PLoS One 9. https://doi.org/10.1371/journal.pone.0086585
Mumby PJ, Edwards AJ, Ernesto Arias-González J, Lindeman KC, Blackwell PG, Gall A, Gorczynska MI, Harborne AR, Pescod CL, Renken H, C. C. Wabnitz C, Llewellyn G (2004) Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427:533–536. https://doi.org/10.1038/nature02286
Murchie KJ, Cooke SJ, Danylchuk AJ, Danylchuk SE, Goldberg TL, Suski CD, Philipp DP (2013) Movement patterns of bonefish (Albula vulpes) in tidal creeks and coastal waters of Eleuthera, the Bahamas. Fish Res 147:404–412. https://doi.org/10.1016/j.fishres.2013.03.019
Nagelkerken I, Dorenbosch M, Verberk WCEP, Cocheret de la Morinière E, van der Velde G (2000) Importance of shallow-water biotopes of a Caribbean bay for juvenile coral reef fishes: patterns in biotope association, community structure and spatial distribution. Mar Ecol Prog Ser 202:175–192. https://doi.org/10.3354/meps202175
Nagelkerken I, Sheaves M, Baker R, Connolly RM (2015) The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish 16:362–371. https://doi.org/10.1111/faf.12057
Nelson R (2002) Catch-and-release: a management toolfor Florida. In: Lucy J, Studholme AL (eds) Catch and Release in Marine Recreational Fisheries. American Fisheries Society, pp 11–14
Olds AD, Connolly RM, Pitt KA, Maxwell PS (2012) Habitat connectivity improves reserve performance. Conserv Lett 5:56–63. https://doi.org/10.1111/j.1755-263X.2011.00204.x
Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U (2004) Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428:932–935. https://doi.org/10.1038/nature02430
Orth RJ, Carruthers TJB, Dennison WC et al (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996. https://doi.org/10.1641/0006-3568(2006)56[987:agcfse]2.0.co;2
Payne Wynne ML, Wilson KA, Limburg KE, Gillanders B (2015) Retrospective examination of habitat use by blueback herring (Alosa aestivalis) using otolith microchemical methods. Can J Fish Aquat Sci 72:1073–1086. https://doi.org/10.1139/cjfas-2014-0206
Perera Valderrama S, Hernández Ávila A, González Méndez J, Moreno Martínez O, Cobián Rojas D, Ferro Azcona H, Milián Hernández E, Caballero Aragón H, Alcolado PM, Pina Amargós F, Hernández González Z, Espinosa Pantoja L, Rodríguez Farrat LF (2017) Marine protected areas in Cuba. Bull Mar Sci. https://doi.org/10.5343/bms.2016.1129
Pinheiro J, Bates D, DebRoy S, Sarkar D (2017) nlme: Linear and nonlinear mixed effects models. R Dev Core Team 1–97
Pittman SJ, Monaco ME, Friedlander AM, Legare B, Nemeth RS, Kendall MS, Poti M, Clark RD, Wedding LM, Caldow C (2014) Fish with chips: tracking reef fish movements to evaluate size and connectivity of Caribbean marine protected areas. PLoS One 9:e96028. https://doi.org/10.1371/journal.pone.0096028
R Core Team (2017) R: A language and environment for statistical computing
Reis-Santos P, Gillanders BM, Tanner SE, Vasconcelos RP, Elsdon TS, Cabral HN (2012) Temporal variability in estuarine fish otolith elemental fingerprints: implications for connectivity assessments. Estuar Coast Shelf Sci 112:216–224. https://doi.org/10.1016/j.ecss.2012.07.027
Rohtla M, Vetemaa M (2016) Otolith chemistry chimes in: migratory environmental histories of Atlantic tarpon (Megalops atlanticus) caught from offshore waters of French Guiana. Environ Biol Fish 99:593–602. https://doi.org/10.1007/s10641-016-0501-1
Rooker JR, Kraus RT, Secor DH (2004) Dispersive behaviors of black drum and red drum: is otolith Sr:ca a reliable indicator of salinity history? Estuaries 27:334–341. https://doi.org/10.1007/BF02803389
Ross G (2015) Parametric and nonparametric sequential change detection in R: The cpm package. J Stat Softw 66:forthcoming. https://doi.org/10.18637/jss.v066.i03
Rudnick DT, Ortner PB, Browder JA, Davis SM (2005) A conceptual ecological model of Florida Bay. Wetlands 25:870–883. https://doi.org/10.1672/0277-5212(2005)025[0870:ACEMOF]2.0.CO;2
Santos RO, Lirman D, Serafy JE (2011) Quantifying freshwater-induced fragmentation of submerged aquatic vegetation communities using a multi-scale landscape ecology approach. Mar Ecol Prog Ser 427:233–246. https://doi.org/10.3354/meps08996
Santos RO, Lirman D, Pittman SJ (2015) Long-term spatial dynamics in vegetated seascapes: fragmentation and habitat loss in a human-impacted subtropical lagoon. Mar Ecol 37:200–214. https://doi.org/10.1111/maec.12259
Santos RO, Rehage JS, Adams AJ, Black BD, Osborne J, Kroloff EKN (2017) Quantitative assessment of a data-limited recreational bonefish fishery using a time-series of fishing guides reports. 12:e0184776. https://doi.org/10.1371/journal.pone.0184776
Santos RO, Lirman D, Pittman SJ, Serafy JE (2018) Spatial patterns of seagrasses and salinity regimes interact to structure marine faunal assemblages in a subtropical bay. Mar Ecol Prog Ser 594:21–38. https://doi.org/10.3354/meps12499
Schilling H, Reis-Santos P, Hughes J, Smith JA, Everett JD, Stewart J, Gillanders BM, Suthers IM (2018) Evaluating estuarine nursery use and life history patterns of Pomatomus saltatrix in eastern Australia. Mar Ecol Prog Ser 598:187–199. https://doi.org/10.3354/meps12495
Seeley M, Miller N, Walther B (2015) High resolution profiles of elements in Atlantic tarpon (Megalops atlanticus) scales obtained via cross-sectioning and laser ablation ICP-MS: a literature survey and novel approach for scale analyses. Environ Biol Fish 98:2223–2238. https://doi.org/10.1007/s10641-015-0443-z
Selkoe KA, Henzler CM, Gaines SD (2008) Seascape genetics and the spatial ecology of marine populations. Fish Fish 9:363–377. https://doi.org/10.1111/j.1467-2979.2008.00300.x
Stabenau E, Kotun K (2012) Salinity and hydrology of Florida bay: status and trends 1990-2009
Stanley RRE, Bradbury IR, DiBacco C, Snelgrove PVR, Thorrold SR, Killen SS (2015) Environmentally mediated trends in otolith composition of juvenile Atlantic cod (Gadus morhua). ICES J Mar Sci J du Cons 72:2350–2363. https://doi.org/10.1093/icesjms/fsv070
Sturrock AM, Hunter E, Milton JA, EIMF, Johnson RC, Waring CP, Trueman CN (2015) Quantifying physiological influences on otolith microchemistry. Methods Ecol Evol 6:806–816. https://doi.org/10.1111/2041-210X.12381
Tabouret H, Lord C, Bareille G, Pécheyran C, Monti D, Keith P (2011) Otolith microchemistry in Sicydium punctatum: indices of environmental condition changes after recruitment. Aquat Living Resour 24:369–378. https://doi.org/10.1051/alr/2011137
Wallace EM, Tringali MD (2010) Identification of a novel member in the family Albulidae (bonefishes). J Fish Biol 76:1972–1983. https://doi.org/10.1111/j.1095-8649.2010.02639.x
Webb SD, Woodcock SH, Gillanders BM (2012) Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature. Mar Ecol Prog Ser 453:189–199. https://doi.org/10.3354/meps09653
Yeager LA, Acevedo C, Layman CA (2012) Effects of seascape context on condition, abundance, and secondary production of a coral reef fish, Haemulon plumierii. Mar Ecol Prog Ser 462:231–240. https://doi.org/10.3354/meps09855
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer-Verlag, New York
Acknowledgments
We are grateful to the anglers, guides and our Cuban colleagues who assisted in the collection of samples. We would like to thank the International Forensic Research Institute at Florida International University for allowing the use of the Trace Evidence Analysis Facility. Funding was provided by Bonefish and Tarpon Trust and by an FIU Tropical Conservation Internship to R. Schinbeckler. The project was developed in collaboration with the FCE LTER program (NSF DEB-1237517). This is contribution #x from the Center for Coastal Oceans Research in the Institute of Water and Environment at Florida International University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 617 kb)
Rights and permissions
About this article
Cite this article
Santos, R.O., Schinbeckler, R., Viadero, N. et al. Linking bonefish (Albula vulpes) populations to nearshore estuarine habitats using an otolith microchemistry approach. Environ Biol Fish 102, 267–283 (2019). https://doi.org/10.1007/s10641-018-0839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0839-7