Summary
Aim To compare the efficacy and safety of intermittent every other days 5-dose filgrastim with single pegfilgrastim in patients with breast cancer receiving adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy. Methods In this pilot study, Korean patients who had undergone complete resection for breast cancer and scheduled for adjuvant TAC chemotherapy were enrolled. Patients were randomized to receive either intermittent 5 doses of filgrastim (5 mcg/kg/day) or once-a-cycle pegfilgrastim (6 mg) as primary prophylaxis during the first three cycles of the TAC chemotherapy. Absolute neutrophil count (ANC) was analyzed as well. Results A total of 22 patients were randomly and equally divided into filgrastim or pegfilgrastim arms. Febrile neutropenia (FN) occurred in 1 patient in the pegfilgrastim arm (1 of 33 cycles) and none in the filgrastim arm. G3 neutropenia occurred in 1 patient (1 of 33 cycles) in the filgrastim arm and 2 patients (4 of 33 cycles) in the pegfilgrastim arm (P = 0.476). G4 neutropenia occurred in 11 patients (28 of 33 cycles) in the filgrastim arm and 9 patients (18 of 33 cycles) in the pegfilgrastim arm (P = 0.476). Except for on day 9 in cycle 3, there was no significant difference between the two groups in terms of ANC. Conclusion We observed no significant differences between the two methods of prophylaxis in terms of FN and G3/4 neutropenia incidence in patients receiving adjuvant TAC chemotherapy. Intermittent every other days 5-dose filgrastim may be available alternative to pegfilgrastim.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Six cycles of adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) (75/50/500 mg/m2 every 3 weeks) shows significant improvement in disease-free survival and overall survival in patients with breast cancer. TAC carries advantage over 4 cycles of AC (60/600 mg/m2 every 3 weeks) followed by 4 cycles of docetaxel at 100 mg/m2 every 3 weeks (AC > T) (BCIRG-005) in that TAC has a shorter treatment period than does AC > T while showing similar efficacy and similar incidence of G3/4 neutropenia. However, TAC results in significantly higher incidence of febrile neutropenia (FN) and thrombocytopenia than does AC > T (17.4% vs. 7.7%; P < 0.0001), which prevents widespread use of TAC in breast cancer patients [1].
The use of granulocyte colony-stimulating factors (G-CSFs) brought significant advances in supportive care and improvement of quality of life in cancer patients by significantly reducing the incidence of infectious complications and hospitalization. Moreover, the use of G-CSFs results in preservation of the dose intensity of chemotherapy. A recent review suggested that prophylactic G-CSF decreases the risk of FN and treatment-related deaths including infection-related mortality [2]; however, due to its short half-life, G-CSF needs to be administered multiple times. In this regard, pegfilgrastim (pegylated form of G-CSF), which involves neutrophil elimination, is able to maintain effective plasma concentrations for a longer period than conventional G-CSFs that involves renal elimination [3].
Two studies demonstrated that once a cycle pegfilgrastim on day 2 has the same efficacy and safety profile to multiple daily injections of filgrastim (10–11 daily filgrastim) [4, 5]. Also, randomized studies in patients with breast cancer showed that a single dose of pegfilgrastim produces the same efficacy as 11-daily filgrastim and possibly even more efficacious for preventing FN [6,7,8,9]. The GEPARTRIO study showed that pegfilgrastim alone was more effective than 6-daily filgrastim schedule (day 5–10) in preventing FN (7% vs. 18% of patients, and 2% vs. 5% of cycles, respectively) (P < 0.001) [10].
Nevertheless, for economical and practical reasons (e.g., drug availability, health insurance, patient convenience), a shorter course of treatment would be desirable in situations in which pegfilgrastim use is difficult. Importantly, reports on patients with other types of cancer showed that suboptimal use of G-CSFs may result in unfavorable clinical outcomes [11,12,13]. However, in two randomized studies on breast cancer patients undergoing TAC chemotherapy [10, 14], consecutive 6 or 7-daily filgrastim schedules showed acceptable range of FN incidence (7%–18%).
An area of concern for alternate or intermittent filgrastim administration is that a patient’s absolute neutrophil count (ANC) may significantly fluctuate over time during the course of administration. The half-life of filgrastim is 3.5–3.8 h and daily filgrastim is thus recommended. However, in a previous study [15], clinical outcomes were not significantly different between daily- and intermittent-dose filgrastim schedules.
In this study, we compared the efficacy and safety of intermittent every other days of 5-dose filgrastim (D3–D11) with once-a-cycle pegfilgrastim (D2) in patients receiving adjuvant TAC chemotherapy.
Patients and methods
Patients
This pilot study (NCT02685111) was an open-label, randomized, multicenter study that compared intermittent every other days of 5-dose filgrastim (filgrastim) with once-a-cycle pegfilgrastim (pegfilgrastim) in patients who had undergone surgery for pathologically diagnosed early breast cancer (high-risk stage II or stage III) or completely resected stage IV, and anticipated to undergo adjuvant chemotherapy with the TAC regimen (docetaxel, doxorubicin, and cyclophosphamide).
The inclusion criteria were as follows: 18 to 70 of age, Eastern Cooperative Oncology Group (ECOG) performance 0–1, ANC ≥ 1500/mm3, platelet count ≥100,000/mm3, adequate renal functions (Cr < 1.5 × upper limit of normal [ULN]), adequate liver function (bilirubin <1.5 × ULN, AST/ALT <2.5 × ULN), and adequate cardiac function (ejection fraction ≥50% as measured by MUGA or 2D echocardiography without clinically significant abnormalities). All patients voluntarily participated in this study and provided written informed consent.
The exclusion criteria were as follows: history of immunotherapy or chemotherapy, history of autologous stem cell transplantation or bone marrow transplantation, radiation therapy within 4 weeks after written informed consent, any other concurrent malignancies or history of malignancy within the last 5 years (excluding completely resected stage I early skin cancer), pregnant or lactating women, women of childbearing potential not employing adequate contraception, other serious illness or medical conditions that hinder chemotherapy, unstable cardiac disease (e.g., congestive heart failure, arrhythmia, symptomatic coronary artery disease) despite treatment, myocardial infarction within 6 months prior to study entry, history of significant neurological or psychiatric disorders including dementia and seizure, active uncontrolled infection (viral, bacterial, or fungal), concomitant administration of any other experimental drugs under investigation, concomitant chemotherapy, hormonal therapy, or immunotherapy, history of usage of G-CSFs, and history of HIV (+) or HCV (+). However, HBV (+) patients who undergo primary prophylaxis were eligible.
Six referral hospitals in Korea participated in this study. The protocols of this study were approved by the institutional review boards of Asan Medical Center, Inje University Sanggye Paik Hospital, Yonsei Cancer Center, Ulsan University Hospital, Korea University Guro Hospital and National Cancer Center, and conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. All participants provided written informed consent prior to enrollment.
Drug dosage and study schedule
The TAC chemotherapy was administered once every 3 weeks for 6 cycles. The two prophylactic G-CSF regimens (filgrastim and pegfilgrastim) were compared during the first 3 cycles of TAC chemotherapy (study period); during the subsequent 3 cycles of TAC chemotherapy (practice period), the patients were treated as clinical routine practice. Each TAC cycle consisted of doxorubicin 50 mg/m2 followed by cyclophosphamide 500 mg/m2 and docetaxel 75 mg/m2 given intravenously on day 1. To prevent fluid retention, patients received premedication with dexamethasone (six doses of 8 mg per os from the night before chemotherapy and until the evening of the day after chemotherapy).
Filgrastim (Neupogen®) was administered on D3, D5, D7, D9, and D11 of each cycle during the study period. A dose of 5 μg/kg/day filgrastim was administered subcutaneously as a bolus injection into the outer upper arm, abdomen (except within 5 cm from the navel), front middle thigh, or the upper outer buttocks area. Pegfilgrastim (Neulasta®) was administered at D2 of each cycle during the study period. A dose of 6 mg once a cycle was administered subcutaneously 24 (± 2) hours after the completion of chemotherapy. Both filgrastim and pegfilgrastim were administered at home by the patients themselves.
Complete blood count (CBC) was examined at D1, D7, D9, and D14 at every cycle of TAC chemotherapy. Safety profiles were evaluated by physical examination, vital signs, performance status, CBC with differential counts, and serum chemistry using NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 before every cycle of TAC chemotherapy. The schedule of this study is shown in Table 2.
Statistical analysis
To compare the two prophylactic regimens, continuous variables were analyzed using the independent T-test or Mann-Whitney U test. Categorical data and proportions were compared with Fisher’s exact test. All statistical analyses were performed using SPSS for Windows version 24.0 (IBM Corp., Armonk, NY, USA). All analyses were two-sided, and statistical significance was set at P values <0.05.
Results
Patients characteristics
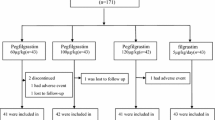
Between July 22, 2016 and December 18, 2017, 25 patients were enrolled, 3 of whom withdrew their consent prior to randomization. Finally, 22 patients were randomly assigned to either the filgrastim arm or once a cycle pegfilgrastim arm. The patient characteristics of this study are shown in Table 1. The median age of the patients was 53.5 (range, 37–69). The ECOG PS was 0 in 18 patients and 1 in 4 patients. Eleven patients were pre-menopausal. Pathologic findings showed that 19 patients had invasive ductal carcinoma (IDC) and 3 patients had invasive lobular carcinoma (ILC). A total of 17 patients were positive for estrogen receptor (ER), 15 were positive for progesterone receptor (PR), and 5 were triple negative breast cancer. All patients were negative for HER-2. One patient showed the presence of ductal carcinoma in situ (DCIS). Regional lymph node involvement was absent in 3 patients. Filgrastim, pegfilgrastim, and chemotherapy were given without omission or delay (Table 2).
Efficacy and safety
Incidence of hematologic toxicities
During the study period, 253 of 264 CBCs (95.8%) were collected. The mean ANCs at each time point are shown in Table 3. Except on D9 of cycle 3, there was no significant differences in mean ANC between the two groups.
Incidence of FN, grade 3 and grade 4 neutropenia per patients and per cycles are shown in Table 4.
All 11 patients in the filgrastim arm had experienced one or more G4 neutropenia. Nine of 11 patients (81.8%) in the pegfilgrastim arm had experienced G4 neutropenia, and in the rest of the patients, G3 neutropenia was the worst grade. In the filgrastim arm, no FN was observed, and one patient in the pegfilgrastim arm experienced FN in cycle 3. During the entire study period, there was 1 case of G3 neutropenia and 28 cases of G4 neutropenia in the filgrastim arm, and 4 cases of G3 neutropenia and 18 cases of G4 neutropenia in the pegfilgrastim arm. In the pegfilgrastim arm, G2 neutropenia was noted as the worst grade experienced in two cases. Overall, there were no significant differences in the incidence of G3 neutropenia, G4 neutropenia, and FN per patients between the two groups (P = 0.476, P = 0.476, and P = 1.000, respectively); during cycles 1 to 3 or each cycle of cycle 1–3, except for G4 neutropenia (P = 0.015), there were no significant differences in the incidence of G3 neutropenia, G3/4 neutropenia, and FN (P = 0.355, P = 0.150, and P = 1.000, respectively). G3 thrombocytopenia occurred in 4 patients in the pegfilgrastim arm and none in the filgrastim arm. The trend of ANCs during the study period is shown in Fig. 1.
Incidence of non-hematologic toxicities
Grade 3 or higher adverse events were rare in both prophylactic regimen arms, and G3 hyperglycemia occurred in 1 of 11 patients (9.1%) in both groups. One patient in the filgrastim arm experienced G3 syncope. There were no significant differences in the number of patients who experienced G1/2 bone pain and G1/2 myalgia between the filgrastim arm and the pegfilgrastim arm (6 of 11 patients vs. 6 of 11 patients, P = 1.000, and 5 of 11 patients vs. 9 of 11 patients, P = 0.183, respectively), as well as in the incidence of G1/2 bone pain and G1/2 myalgia during the entire cycles (12 of 33 cycles vs. 9 of 33 cycles, P = 0.598, and 8 of 33 cycles vs. 16 of 33 cycles, P = 0.072, respectively). However, the incidence of G1 myalgia between two prophylaxes group were significantly different between the filgrastim arm and the pegfilgrastim arm (3 of 33 cycles vs. 12 of 33 cycles, P = 0.017). There was no serious adverse event in both arms during the entire study period. The incidences of non-hematologic toxicities that occurred in two or more patients are shown in Table 5.
Discussion
We report that in a pilot study involving 22 patients with breast cancer receiving adjuvant TAC chemotherapy, there were no significant difference between those who received intermittent every other days of 5-dose filgrastim and those who received once-a-cycle pegfilgrastim in terms of incidence of FN and G4 neutropenia. The two groups also did not show significant difference in the mean ANC at most time points.
The occurrence of FN often calls the need for hospitalization and treatment with intravenous antibiotics, and may be fatal in serious cases. Moreover, chemotherapy delay and dose reduction may be required in the cycles following the occurrence of FN. Primary prophylactic use of G-CSFs is useful in this regard in that they significantly decrease the incidence of FN and other hematologic and non-hematologic toxicities. The effectiveness of G-CSF and pegfilgrastim had been demonstrated in two studies [16, 17], and recent reviews have shown that prophylactic G-CSFs decrease not only the risk of FN but also treatment-related deaths and infection-related mortality. Accordingly, the American Society of Clinical Oncology (ASCO) guidelines recommend the use of primary prophylaxis with G-CSFs in the first and subsequent chemotherapy cycles for patients receiving chemotherapy regimens with a high risk of FN (> 20%) [18]. Similarly, the European Organization for Research and Treatment of Cancer [19] and the National Comprehensive Cancer Network [20] guidelines also recommend the use of G-CSFs in such patients.
An increasing body of evidence supports the use of chemotherapeutic agents at full and planned doses when administered with curative intent. Notably, clinical results from 30 years of follow-up in patients with breast cancer showed that patients receiving at least 85% of the planned chemotherapy dose had significantly higher rate of relapse-free survival and overall survival [21]. The use of G-CSFs results in significantly higher quality-of-life scores in patients treated with TAC [14]; however, there is a lack of data concerning the impact of G-CSFs on disease-free and overall survival [2]. Active chemotherapy regimens such as TAC often entail significant risk of FN, and its high incidence of first-cycle FN supports the need to start G-CSF and pegfilgrastim from the first cycle. A recent cost-minimization analysis showed that first-cycle use of pegfilgrastim may be cost-neutral in patients in whom the predicted risk of FN is less than 20% [22]. The incidence of FN in breast cancer patients treated with TAC ranges from 24.7% to 38% [23,24,25], which are higher than the 20%-cutoff value for indication of primary prophylactic G-CSF use according to ASCO guidelines. Therefore, FN substantially affects chemotherapy outcomes, patient quality of life, and treatment costs by delaying chemotherapy or reducing the dose thereof.
According to the manufacturer’s recommendations (Amgen: Neupogen™), filgrastim are started at 24 h after the last dose of chemotherapy and continued either until ANC has recovered to within the normal range or for 14 days. Several clinical studies have shown that 10–11 days of filgrastim treatment is required for optimal prophylaxis against FN [8, 26]. However, for economic reasons and patient convenience, it has been common practice to initiate filgrastim at a later day in the cycle or to use a shorter course of treatment [11, 12, 27, 28]. In the BCIRG 004 Trial that compared filgrastim and leridistim for the prevention of neutropenic complications in patients with advanced or metastatic breast cancer receiving TAC chemotherapy, the incidence of FN was 7% in the filgrastim arm and grade 4 neutropenia occurred in 85% of patients (53% of cycles); in this trial, patients received G-CSFs on D2 following the administration of TAC until the post-nadir ANC exceeded 1500 cells/mm3 [29]. In the GEICAM 9805 Trial on adjuvant TAC, the percentage of patients who experienced FN in one or more cycles by the NCI-CTC definition were 27.2% and 7.5% in TAC-pre (ciprofloxacin only) and TAC-post groups (filgrastim + ciprofloxacin), respectively [14]. In the GEPARTRIO study that compared pegfilgrastim ± ciprofloxacin vs. filgrastim or ciprofloxacin in patients receiving neoadjuvant TAC chemotherapy, the incidence rates of FN in daily filgrastim arm and ciprofloxacin were 18% and 22% of patients, respectively (5% and 5% of cycles, respectively), and the incidence rates of grade 4 neutropenia were 58% and 62%, respectively (27% and 33% of cycles, respectively) [10]. Notably, whereas the GEICAM Trial used 7-daily filgrastim at days 4–10, the GEPARTRIO study used 6-daily filgrastim at days 5–10 regardless of the manufacturer’s recommendation. These two studies, which used consecutive 6 or 7-daily filgrastim schedules, reported incidence of FN (7%–18%)— this was comparable with the result of the BCIRG 004 trial that used single G-CSF on D2 and continued it until the post-nadir ANC exceeded 1500 cells/mm3. In light of these results, we used short-course filgrastim instead of full-course with the expectation that the short-course would show similar efficacy to pegfilgrastim.
It may be possible that there are wide fluctuations in patients’ ANC over time during alternate or intermittent filgrastim administration. However, there was no significant difference in the clinical outcomes between daily- or intermittent-dose filgrastim schedules in a previous literature [15]; therefore, the intermittent every other days 5-dose of filgrastim schedule can be presumed to have clinical outcomes comparable with those of consecutive 6 or 7-daily filgrastim schedules in terms of the coverage of ANC nadir [10, 14]. This justifies the aim of this pilot study in comparing the intermittent every other days of 5-dose of filgrastim with once-a-cycle pegfilgrastim.
In our current study, FN occurred in one case in the pegfilgrastim arm and none in the filgrastim arm, and no significant difference in clinical outcomes was found between the two arms. However, the incidence of G4 neutropenia per cycle was significantly more frequent in the filgrastim arm than in the pegfilgrastim arm (84.8% [28 of 33 cycles] vs. 54.5% [18 of 33 cycles], P = 0.015). This result seems to suggest that the short-course of filgrastim or intermittent every other day of filgrastim is somewhat suboptimal for use in adjuvant TAC chemotherapy. However, it should be noted that the incidence rates of G4 neutropenia are generally higher than those of previous studies on filgrastim (100% in the current study vs. 58–85% in previous studies, and 84.8% in current study vs. 27–53% in previous studies) [10, 29] and pegfilgrastim (72.8% in current study vs. 37% in a previous study, and 54.5% in current study vs. 15% in a previous study) [10]. Considering that we employed the same kind regimen for filgrastim and pegfilgrastim, the high incidence of G4 neutropenia in both filgrastim and pegfilgrastim arm in this study may reflect the limitation of the nature of a pilot study involving a small number of patients. Therefore, the difference in the incidence of G4 neutropenia between the two regimens in this study should be interpreted with caution, and confirmed by large-sized future studies.
Our study is limited in that due to funding issues, the study period only spanned the first 3 cycles of adjuvant chemotherapy and could not cover the whole 6 cycles as originally planned. Also, when this study was designed, pegfilgrastim was not covered by National Health Insurance in Korea. However, during the course of study, it has been possible that the pegfilgrastim receives the National Health insurance benefits. Therefore, there was no reason for patients to participate in this research as socioeconomic advantage was gone away.
In conclusion, through a pilot study on 22 patients with breast cancer, we found that the two prophylactic regimens (intermittent every other days of 5-dose filgrastim and once-a-cycle pegfilgrastim) show comparable outcomes in the incidence of FN and G4 neutropenia as well as mean ANC. Intermittent every other days 5-dose of filgrastim may be a viable alternative to pegfilgrastim when the use of pegfilgrastim is hindered due to economic reasons.
References
Eiermann W, Pienkowski T, Crown J et al (2011) Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 29:3877–3884. https://doi.org/10.1200/jco.2010.28.5437
Kuderer NM, Dale DC, Crawford JLyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167. https://doi.org/10.1200/jco.2006.08.8823
Molineux G (2003) Pegylation: engineering improved biopharmaceuticals for oncology. Pharmacotherapy 23(8 Pt 2):3s–8s. https://doi.org/10.1592/phco.23.9.3S.32886
Crawford J (2003) Once-per-cycle pegfilgrastim (Neulasta) for the management of chemotherapy-induced neutropenia. Semin Oncol 30(4 Suppl 13):24–30. https://doi.org/10.1016/S0093-7754(03)00314-2
Molineux G (2004) The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta). Curr Pharm Des 10:1235–1244. https://doi.org/10.2174/1381612043452613
Holmes FA, O'Shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20:727–731. https://doi.org/10.1200/jco.2002.20.3.727
Siena S, Piccart MJ, Holmes FA, Glaspy J, Hackett JRenwick JJ (2003) A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily Filgrastim in patients with stage II-IV breast cancer. Oncol Rep 10:715–724
Green MD, Koelbl H, Baselga J et al (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35. https://doi.org/10.1093/annonc/mdg019
Park KH, Sohn JH, Lee S et al (2013) A randomized, multi-center, open-label, phase II study of once-per-cycle DA-3031, a biosimilar pegylated G-CSF, compared with daily filgrastim in patients receiving TAC chemotherapy for early-stage breast cancer. Investig New Drugs 31:1300–1306. https://doi.org/10.1007/s10637-013-9973-4
von Minckwitz G, Kummel S, du Bois A et al (2008) Pegfilgrastim ± ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 19:292–298. https://doi.org/10.1093/annonc/mdm438
Weycker D, Hackett J, Edelsberg JS, Oster GGlass AG (2006) Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother 40:402–407. https://doi.org/10.1345/aph.1G516
Scott SD, Chrischilles EA, Link BK, Delgado DJ, Fridman MStolshek BS (2003) Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin's lymphoma treated with chemotherapy. J Manag Care Pharm 9(2 Suppl):15–21. https://doi.org/10.18553/jmcp.2003.9.s2.15
Koumakis G, Vassilomanolakis M, Barbounis V, Hatzichristou E, Demiri S, Plataniotis G, Pamouktsoglou FEfremidis AP (1999) Optimal timing (preemptive versus supportive) of granulocyte colony-stimulating factor administration following high-dose cyclophosphamide. Oncology 56:28–35. https://doi.org/10.1159/000011926
Martin M, Lluch A, Segui MA et al (2006) Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol 17:1205–1212. https://doi.org/10.1093/annonc/mdl135
Papaldo P, Lopez M, Marolla P et al (2005) Impact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamide. J Clin Oncol 23:6908–6918. https://doi.org/10.1200/jco.2005.03.099
Morrison VA, Wong M, Hershman D, Campos LT, Ding BMalin J (2007) Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3-4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm 13:337–348. https://doi.org/10.18553/jmcp.2007.13.4.337
Ozer H, Mirtsching B, Rader M, Luedke S, Noga SJ, Ding BDreiling L (2007) Neutropenic events in community practices reduced by first and subsequent cycle pegfilgrastim use. Oncologist 12:484–494. https://doi.org/10.1634/theoncologist.12-4-484
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/jco.2015.62.3488
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32. https://doi.org/10.1016/j.ejca.2010.10.013
National Comprehensive Cancer Network. (2019) Hematopoietic growth factors: NCCN clinical practice guidelines in oncology (NCCN Guidelines®) version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed 21 August 2019
Bonadonna G, Moliterni A, Zambetti M, Daidone MG, Pilotti S, Gianni LValagussa P (2005) 30 years' follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. Bmj 330:217. https://doi.org/10.1136/bmj.38314.622095.8F
Rader M (2006) Granulocyte colony-stimulating factor use in patients with chemotherapy-induced neutropenia: clinical and economic benefits. Oncology (Williston Park) 20(5 Suppl 4):16–21
Nabholtz JM, Mackey JR, Smylie M et al (2001) Phase II study of docetaxel, doxorubicin, and cyclophosphamide as first-line chemotherapy for metastatic breast cancer. J Clin Oncol 19:314–321. https://doi.org/10.1200/jco.2001.19.2.314
Smith RE, Anderson SJ, Brown A, Scholnik AP, Desai AM, Kardinal CG, Lembersky BC, Mamounas EP (2002) Phase II trial of doxorubicin/docetaxel/cyclophosphamide for locally advanced and metastatic breast cancer: results from NSABP trial BP-58. Clin Breast Cancer 3:333–340. https://doi.org/10.3816/CBC.2002.n.036
Martin M, Pienkowski T, Mackey J et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313. https://doi.org/10.1056/NEJMoa043681
Holmes FA, Jones SE, O'Shaughnessy J et al (2002) Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol 13:903–909. https://doi.org/10.1093/annonc/mdf130
Meza LA, Charu V, Campos L, Davis DXie F (2005) Pegfilgrastim and filgrastim patterns of use in community oncology practices: results of the ACCEPT study. J Clin Oncol 23(16 suppl):6113–6113. https://doi.org/10.1200/jco.2005.23.16_suppl.6113
von Minckwitz G, Raab G, Caputo A et al (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German breast group. J Clin Oncol 23:2676–2685. https://doi.org/10.1200/jco.2005.05.078
Nabholtz JM, Cantin J, Chang J et al (2002) Phase III trial comparing granulocyte colony-stimulating factor to leridistim in the prevention of neutropenic complications in breast cancer patients treated with docetaxel/doxorubicin/cyclophosphamide: results of the BCIRG 004 trial. Clin Breast Cancer 3:268–275. https://doi.org/10.3816/CBC.2002.n.030
Funding
Funding for this study came from Kyowa Hakko Kirin, Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there exist no conflicts of interest in this study.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sohn, B.S., Jeong, J.H., Ahn, JH. et al. A pilot study on intermittent every other days of 5-dose Filgrastim compared with single Pegfilgrastim in breast Cancer patients receiving adjuvant Docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy. Invest New Drugs 38, 866–873 (2020). https://doi.org/10.1007/s10637-019-00863-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00863-8