Abstract
Background/Aims
We examined the contributions of gastric emptying and duodenogastric bile reflux in the formation of gastric antral ulcers induced by NSAIDs in mice.
Methods
We used the murine re-fed indomethacin (IND) experimental ulcer model. Outcome measures included the appearance of gastric lesions 24 h after IND treatment and the assessment of gastric contents and the concentration of bile acids 1.5 h after re-feeding. The effects of atropine, dopamine, SR57227 (5-HT3 receptor agonist), apomorphine, ondansetron, haloperidol, and dietary taurocholate and cholestyramine were also examined.
Results
IND (10 mg/kg, s.c.) induced severe lesions only in the gastric antrum in the re-fed model. The antral lesion index and the amount of food intake during the 2-h refeeding period were positively correlated. Atropine and dopamine delayed gastric emptying, increased bile reflux, and worsened IND-induced antral lesions. SR57227 and apomorphine worsened antral lesions with increased bile reflux. These effects were prevented by the anti-emetic drugs ondansetron and haloperidol, respectively. The anti-emetic drugs markedly decreased the severity of antral lesions and the increase of bile reflux induced by atropine or dopamine without affecting delayed gastric emptying. Antral lesions induced by IND were increased by dietary taurocholate but decreased by the addition of the bile acid sequestrant cholestyramine.
Conclusions
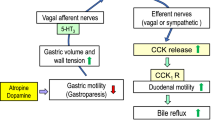
These results suggest that gastroparesis induced by atropine or dopamine worsens NSAID-induced gastric antral ulcers by increasing duodenogastric bile reflux via activation of 5-HT3 and dopamine D2 receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the importance of peptic ulcer disease has diminished since the 1990s due to the advent of potent antisecretory medications and the ability to eradicate H. pylori, peptic ulcer disease remains a major health problem and cause of morbidities such as hemorrhage and perforation, in particular in populations with a high prevalence of H. pylori and frequent use of nonsteroidal anti-inflammatory drugs (NSAIDs), which often cause gastric ulcers in the pre-pyloric (antral) area in humans [1,2,3,4] for reasons incompletely understood. In experimental models, NSAIDs induce gastric ulcers selectively in the antral mucosa when administered after 2 h of re-feeding following a 22-h fast, but induce lesions in the corpus under continuously fasted conditions [5], suggesting that the location of gastric ulcers is closely related to the timing of taking NSAIDs relative to meals, similar to the situation in humans, where NSAIDs are frequently taken after a meal. Furthermore, clinical studies show that the healing of NSAIDs-induced gastric antral ulcers is resistant to histamine H2-receptor antagonists (H2-RAs; famotidine or ranitidine), whereas proton pump inhibitors (PPIs) such as lansoprazole or omeprazole are effective [6,7,8]. In the gastric antral ulcer model in re-fed mice, the formation of antral ulcers induced by indomethacin (IND) is also resistant to H2-RAs, but responsive to PPIs [9]. In contrast, PPIs prevent the formation of antral ulcers via activation of capsaicin-sensitive afferent nerves [9] that explains, at least in part, the differential effects of H2-RAs and PPIs on NSAID-induced antral ulcers in humans.
Since the antimotility cholinergic antagonist atropine inhibits the formation of gastric corpus lesions induced by NSAIDs under fasted conditions in rats, gastric motility is likely to contribute to the formation of gastric lesions by NSAIDs [10, 11]. Interestingly, in our preliminary studies, atropine did not inhibit the formation of antral lesions, but markedly worsened antral lesions induced by IND under re-fed conditions, suggesting that gastroparesis due to atropine exacerbates the severity of antral lesions induced by IND in the re-fed model.
Bile reflux from the duodenum to the stomach is another important factor in the genesis of gastric antral ulcers induced by atropine. Atropine increases reflux of duodenal contents including bile to the stomach in humans [12] and dogs [13]. Bile acids disrupt the gastric mucosal barrier due to their detergent properties [14,15,16]. Thus, we also hypothesize that atropine may affect the formation of NSAID-induced antral ulcers by increasing bile reflux.
In the present study, we examined the effects of several pharmacologic agents that affect gastric motility or bile reflux [17,18,19,20,21,22,23,24,25], on IND-induced antral lesions in order to test the hypothesis that luminal bile acids worsen NSAID-induced antral ulcers in the re-fed mouse model.
Materials and Methods
Animals
Seven-week-old male ddy mice (Shimizu Laboratory Supplies, Shizuoka, Japan) weighing 35–40 g were used. Animals were housed in our animal facility and fed a standard pellet diet and water ad libitum until performance of the experiments as described below.
We performed two series of the experiments, gastric injury and gastric content studies since the time courses of injury development (at 24 h) and gastric content accumulation (at 1.5 h) are different. The animals were fed a regular pellet diet for the injury study, while a powder diet was given for the gastric content study since exact amounts of food intake should be measured to calculate gastric emptying. The details were described below.
Drugs and Diets
The following drugs and chemicals were used: cholestyramine (COL) (Questran®, 44.4% powder, Sanofi, Tokyo), mosapride citrate (MOS) (Nipro, Osaka, Japan), ondansetron hydrochloride (OND) (TCI, Tokyo, Japan), apomorphine hydrochloride (APO), atropine sulfate monohydrate (ATR), carboxymethylcellulose, dopamine hydrochloride (DOP), sodium diclofenac (DIC), haloperidol hydrochloride (HAL), indomethacin (IND), neostigmine bromide (NEO), SR 57227 hydrochloride (SR), and sodium taurocholate (TCA) (Fuji-Wako, Osaka, Japan). Drugs for subcutaneous (s.c.) administration were suspended in saline (SAL) containing 1% carboxymethylcellulose, and drugs for intraperitoneal (i.p.) administration were dissolved in SAL. The drugs were prepared immediately before the experiments and administered in a volume of 0.05 ml/10 g body weight.
Regular chow pellets (CE-2 pellets; Clea Japan, Osaka, Japan) or regular powder diet (PD) (CE-2 powder; Clea Japan) were used. In some experiments, PD with taurocholate (TCA) or cholestyramine (COL) was given to mice during the 2-h re-feeding period. To block the binding capability of COL for bile acids, 1 g of COL was suspended in 50 ml of 0.1 M TCA solution and stirred for 2 h at 150 rpm at 37 °C, after which the suspension was centrifuged (800×g, 10 min). The precipitate was then washed several times with de-ionized water. The precipitate was then dried at 37 °C for 24 h, and subsequently used as COL − TCA, which was added to PD (PD + COL − TCA).
Induction and Measurement of Gastric Lesions
NSAIDs (IND 10 mg/kg or DIC 30 mg/kg) were administered s.c. after a 24-h fast or after 2 h of re-feeding of diet following a 21–22-h fast (Fig. 1A–C). A submaximal dose of IND at 10 mg/kg [5] was used, since enhancement or inhibition of gastric lesions was assessed in the present study.
Experimental schedule. Experiment 1. Effects of drugs on gastric antral ulcers induced by indomethacin (IND) or diclofenac (DIC): A IND or DIC was administered subcutaneously (s.c.) after 2-h re-feeding of diet (chow pellets) after a 22-h fast. B Atropine (ATR), dopamine (DOP), neostigmine (NEO), mosapride (MOS), SR 57227 (SR) or apomorphine (APO) was administered just after 2-h re-feeding, and 0.5 h later IND or DIC was administered. C Ondansetron (OND) or haloperidol (HAL) was administered just after re-feeding, and 0.5 h later ATR, DOP, SR, or APO was administered. IND or DIC was administered 0.5 h later, and gastric lesions were examined 24 h after IND or DIC treatment. Experiment 2. Effects of drugs on gastric contents and concentration of bile acids: D ATR, DOP, NEO, MOS, SR, or APO was administered just after 2-h re-feeding of powder diet, and 1.5 h later gastric contents were collected. E OND or HAL was administered just after the re-feeding of diet, and 0.5 h later ATR, DOP, SR, or APO was administered. Gastric contents were collected 1.5 h after pretreatment with OND or HAL
Animals were sacrificed by cervical dislocation under isoflurane (Escain®, Pfizer, Tokyo, Japan) anesthesia at 24 h after NSAID treatment. The abdominal wall was incised through a midline incision, and changes in the abdominal organs and presence of ascites were observed macroscopically. The stomach was removed and filled with 1.5 ml of 0.4% formalin solution and then immersed in the same formalin solution for 15 min. The stomach was opened along the greater curvature; the length (in mm, of the corpus) or area (mm2, of the antrum) of individual lesions was measured using a dissecting microscope with a 1 mm square grid eyepiece (× 10). The sum of the lengths or areas of all the lesions separately in the corpus, or antrum was expressed as the lesion index.
In the food intake study, restricted amounts of the chow pellet diet (0, 0.2, 0.4, 0.6, 0.8, or 1 g) or free-access amounts (2 g) were given during 2-h re-feeding period, followed by IND (30 mg/kg) treatment in order to induce the maximal injuries [5]. The correlation between the measured food consumption as food intake (g/2 h) and lesion index (mm for corpus lesions and mm2 for antral lesions) was analyzed.
Measurements of Amounts of Gastric Contents and Concentration of Bile Acids in the Gastric Juice
Measurement of Gastric Contents
Animals were sacrificed by cervical dislocation under isoflurane anesthesia at 1.5 h after 2 h of re-feeding of diet (PD) following a 22-h fast (Fig. 1D, E). After an abdominal midline incision, the pyloric ring was immediately closed using mosquito forceps, and the stomach was removed with the lower esophagus, after which the gastric contents were collected carefully in a 5 ml plastic tube after cutting the greater curvature of the stomach. The wet weight of the contents was measured, after which 500 μl of de-ionized water was added to the contents. The contents were suspended and centrifuged for 10 min at 20,000×g, and 400 μl of the supernatant was collected and frozen at − 30 °C until the bile acids were assayed. The pellet from the gastric contents was dried for 24 h at 70 °C, after which the dry weight of the pellet was measured. The volume of gastric juice was calculated from the difference between the wet weight and dry weight. Gastric contents (%) were calculated as follows; (dry weight of gastric contents)/(amount of food intake during the 2 h re-feeding period) × 100. Increased gastric contents represent the delayed gastric emptying.
Measurement of Gastric Bile Acid Concentrations
After the pH of a 400 μl aliquot of the supernatant solution was adjusted to 6.5–7.5 by addition of 0.1 N NaOH, the concentration of bile acids was measured using a kit for total bile acids (Fuji-Wako) with a method using 3α-hydroxysteroid dehydrogenase [26]. The color of the resulting generated diformazan was measured at 490 nm to evaluate the concentration of bile acids using a microplate reader (iMark®, Bio-Rad, Tokyo). The concentration of total bile acids was obtained by correction of the dilution effect of the samples, i.e., the addition of 500 μl of de-ionized water to each sample of gastric contents. Increased bile acid concentrations in the gastric juice reflect duodenogastric bile reflux.
Statistics
All data are expressed as mean ± SEM. Differences between groups were analyzed using Student’s t-test for two group comparisons, one way analysis of variance (Dunnett’s multiple range test or Tukey’s multiple comparison test) if more than two variables were considered, or χ2 test (Fisher’s exact probability test), using GraphPad™ Prism 10 (San Diego, CA, USA) with the significance level set at P < 0.05. Correlations were assessed by the Pearson correlation method using GraphPad.
Results
Effect of Food Intake on the Formation of Gastric Lesions Induced by IND in Fasted or in Re-fed Mice
IND (10 mg/kg) was administered subcutaneously (s.c.) after a 24-h fast, or just after 2-h re-feeding of diet (chow pellets) following a 22-h fast (Fig. 1A). Gastric lesions were examined 24 h after IND treatment. IND treatment after a 24-h fast induced many linear lesions in the gastric corpus, but no lesions in the antrum (Fig. 2A, Fasted). In contrast, IND treatment after 2-h refeeding induced large ulcers only in the antrum (Fig. 2B, Re-fed). The lesions in the antrum were often observed in the anterior and posterior walls, and occasionally accompanied by perforations (Fig. 2B).
Effects of food intake and atropine on indomethacin-induced gastric lesions. The images depict gastric lesions 24 h after IND treatment (10 mg/kg, s.c.) administered after a 24 h fasted (A, Fasted) or just after 2-h re-feeding with chow pellets (B, Re-fed) following a 22-h fast. Under fasted conditions (A) many linear lesions were observed in the corpus, but no lesions in the antrum. Under re-fed conditions (B) many large ulcers were observed in the antral mucosa accompanied by perforations, but no lesions in the corpus. Yellow arrows in A and B show the lesions, and a blue grid on each photo shows a 1-cm scale. Effect of food intake on IND-induced gastric lesions: The mice were given restricted amounts (0, 0.2, 0.4, 0.6, 0.8, or 1 g) or 2 g of chow pellets for 2 h after a 22-h fast, and the amount of food intake (g) was measured for each individual mouse. IND (30 mg/kg, s.c.) was administered just after the refeeding of diet. Gastric lesions were examined 24 h after IND treatment. Each point shows the lesion index in the corpus (C) and antrum (D) against the amounts of food intake in each animal (n = 40). Closed circles in the antrum (D) show cases of perforation. Statistical correlations were assessed using the Pearson correlation method. Effect of atropine on IND-induced gastric lesions: Atropine (3–30 mg/kg, s.c.) was administered after a 24-h fast (E) or after 2 h of re-feeding (F), and 0.5 h later IND (10 mg/kg, s.c.) was administered. Gastric lesions were examined 24 h after IND treatment. Each point represents data from an individual animal. Data are expressed as mean ± SEM (n = 8). b and c: P < 0.01, and 0.001 vs. vehicle (VEH) (Dunnett’s test). Ratio in parentheses indicates the number of mice with antral perforation vs. the total number of mice. ##P < 0.01 vs. VEH (χ2 test)
To further study the mechanism of antral ulcer formation, we examined the effects of the amounts of remaining chow in the re-fed mice that calculated food intake on IND-induced gastric lesions at maximal dose (30 mg/kg). Mice were fed chow pellets (0 – 1 g or ad libitum) during the 2-h re-feeding period in order to generate a range of food intake. The lesion index in the corpus under fasted conditions was decreased with increasing food intake (Fig. 2C). In contrast, the lesion index in the antrum was increased with increasing food intake, with some perforations evident (shown as closed circles) (Fig. 2D). The antral lesion index was near maximal at 1–1.5 g of food intake. The lesion index in the corpus inversely correlated with the amounts of food intake (R2 0.5696, p = 0.0001), whereas the antral lesions were positively correlated with food intake (R2 0.7486, p < 0.0001). These results implicate the amount of food consumption and presumably the rate of gastric emptying in the formation of IND-induced antral lesions.
Effect of Atropine on the Formation of Gastric Lesions Induced by IND in Fasted or in Re-fed Mice
To test the hypothesis that gastroparesis and excess luminal bile acids exacerbate NSAID-induced antral ulcers in the re-fed model, we first examined the effects of the anticholinergic drug atropine (ATR) that inhibits gastric motility. ATR was administered according to the protocol in Fig. 1B. Gastric lesions were examined 24 h after IND treatment at submaximal dose (10 mg/kg).
In fasted mice, IND (10 mg/kg, s.c.) induced many linear lesions only in the corpus (Fig. 2A), with the lesion index in the vehicle control group was 23.3 ± 2.9 mm (n = 8) (Fig. 2E). ATR (3–30 mg/kg, s.c.) dose-dependently and significantly inhibited the formation of corpus lesions (Fig. 2E), suggesting that enhanced gastric motility by IND is involved in the formation of corpus lesions under fasted conditions, as previously reported in rats [10].
In re-fed mice, IND (10 mg/kg, s.c.) induced lesions only in the antrum (Fig. 2B); the vehicle group lesion index was 4.5 ± 1.3 mm2 (n = 8) (Fig. 2F). In contrast to the effects on corpus lesions, ATR (3–30 mg/kg, s.c.) dose-dependently and significantly worsened the antral lesions (Fig. 2F). ATR also significantly increased the perforation rate from 3/8 mice in the vehicle group to 8/8 mice in the ATR groups (Fig. 2F). On the other hand, ATR (30 mg/kg, s.c.) alone without IND treatment, given just after 2 h of re-feeding did not cause any visible lesions in the antrum at 24 h later (lesion index was 0.0 ± 0.0 mm2; n = 6).
Effect of ATR on Gastric Emptying and Bile Reflux
We hypothesized that the worsening effects of ATR on IND-induced antral lesions were implicated in the changes in gastric emptying and bile reflux. First, we confirmed that IND treatment alone had no effect on the amounts of gastric contents or concentration of bile acids in the stomach, compared with vehicle treatment. Gastric contents were 21.3 ± 1.1% (n = 12) in vehicle group, and 22.6 ± 1.3% (n = 12) in IND group (p = 0.455). The concentration of bile acid was 4.7 ± 1.8 μM (n = 12) in the vehicle group and 3.9 ± 2.1 μM (n = 12) in the IND group (p = 0.607). This result suggests that IND treatment has no effect on the rate of gastric emptying or on duodenogastric reflux. Therefore, we examined the effects of drugs on the gastric contents and the concentration of bile acids without IND treatment as outlined in Fig. 1D, E.
ATR was administered after 2 h of re-feeding of powder diet (PD) as in Fig. 1D. We measured the amounts of gastric contents (shown as % of the amounts of food intake during the 2 h of re-feeding) and the concentration of bile acids in the stomach. The amounts of gastric contents in the group given vehicle was 21.1 ± 2.0% (n = 10) at 1.5 h after re-feeding. The content was dose-dependently and significantly increased by ATR (3–30 mg/kg, s.c.) (Fig. 3A), confirming that ATR slows the rate of gastric emptying. The concentration of bile acids in the group given vehicle at 1.5 h after refeeding was 4.1 ± 0.7 μM (n = 10). ATR (3–30 mg/kg, s.c.) dose-dependently and significantly increased the concentration of bile acids (Fig. 3B), suggesting that ATR increases duodenogastric bile reflux. These results support that the worsening effects of ATR on IND-induced antral lesions are associated with delayed gastric emptying, accompanied by increased bile reflux, suggesting that increased luminal bile acids increase the severity of NSAID-induced antral ulcers.
Effects of atropine on the amounts of gastric contents and the concentration of bile acids in the stomach. The mice were given powder diet (PD) for 2 h after a 22-h fast. Atropine (ATR, 3–30 mg/kg) was administered s.c. just after the re-feeding period, and 1.5 h later the gastric contents were collected. The amounts of gastric contents (A) and the concentration of bile acids (BA, B) were measured as described in the Materials and Methods. Each point represents data from an individual animal. Data are expressed as mean ± SEM (n = 10). b and c: P < 0.01, and 0.001 vs. vehicle (VEH) (Dunnett’s test)
Effects of Dopamine, Neostigmine and Mosapride on Antral Lesions, Gastric Emptying, and Bile Reflux
To further test our hypothesis, we studied the effect of a variety of pharmacologic agents with known effects on gastric emptying, dopamine (DOP), another inhibitor of gastric emptying [17, 18], the prokinetic acetylcholine esterase inhibitor neostigmine (NEO) [23], and the selective 5-HT4 receptor agonist mosapride (MOS) [24, 25] on antral lesions, gastric emptying, and bile reflux. DOP, NEO, or MOS was administered according to the protocol shown in Fig. 1B. The results were shown in comparison with that of ATR in Fig. 4A. DOP (1–30 mg/kg, i.p.) dose-dependently and significantly worsened the antral lesions at 10 and 30 mg/kg (Fig. 4A). On the other hand, NEO (0.003–0.03 mg/kg, s.c.) and MOS (0.3–3 mg/kg, s.c.) dose-dependently and significantly decreased antral lesions at 0.03 mg/kg of NEO and at 1 and 3 mg/kg of MOS (Fig. 4A).
Effects of prokinetic and antimotility drugs on indomethacin-induced antral lesions, gastric emptying, and bile reflux. Effects on antral lesions: A Atropine (ATR), dopamine (DOP), neostigmine (NEO), mosapride (MOS), SR 57227 (SR) or apomorphine (APO) was administered subcutaneously (s.c.) or intraperitoneally (i.p. for DOP) just after 2-h re-feeding with chow pellets after a 22-h fast. IND (10 mg/kg, s.c.) was administered 0.5 h later, and 24 h later the antral lesions were examined. Data are expressed as mean % changes of lesion index (n = 8) at doses of each drug compared with that given vehicle. a, b and c: P < 0.05, 0.01, and 0.001 vs. vehicle (VEH) (Dunnett’s test). NEO was administered s.c. at doses of 0.003–0.03 mg/kg. Effects on gastric emptying B and bile reflux C: The mice were given powder diet (PD) for 2 h after a 22-h fast. Gastrokinetic drugs were administered s.c. or i.p. just after the re-feeding period, and 1.5 h later the gastric contents were collected. The amounts of gastric contents and the concentration of bile acids (BA) were measured. Data are expressed as mean % changes (n = 8–12) of the amounts of gastric contents (B) and the concentration of bile acids (C) at doses of each drug compared with those given vehicle (VEH). a, b, and c: P < 0.05, 0.01, and 0.001 vs. VEH (Dunnett’s test)
DOP, NEO, or MOS was administered according to the protocol in Fig. 1D. The results were shown in comparison with those of ATR in Fig. 4B, C. DOP (1–30 mg/kg, i.p.) dose-dependently and significantly increased the amounts of gastric contents at 30 mg/kg (Fig. 4B). On the other hand, NEO (0.003–0.03 mg/kg, s.c.) and MOS (0.3–3 mg/kg, s.c.) dose-dependently and significantly decreased the amounts of gastric contents at 0.03 mg/kg of NEO, and at 1 and 3 mg/kg of MOS (Fig. 4B). These results indicate that gastric emptying was inhibited by DOP, but accelerated by NEO and MOS. DOP (1–30 mg/kg, i.p.) increased the concentration of bile acids dose-dependently and significantly at 10 and 30 mg/kg (Fig. 4C). On the other hand, NEO (0.003–0.03 mg/kg, s.c.) and MOS (0.3–3 mg/kg, s.c.) dose-dependently and significantly reduced the bile acid concentrations at 0.03 mg/kg of NEO and 3 mg/kg of MOS (Fig. 4C). These results indicated that bile reflux was increased by DOP, but reduced by NEO and MOS.
These results suggest that delayed gastric emptying with increased bile reflux by ATR or DOP worsens IND-induced antral lesions, whereas the gastroprokinetic drugs NEO and MOS reduce antral lesions accompanied by accelerated gastric emptying with reduced bile reflux.
Effects of Atropine and Mosapride on the Formation of Gastric Antral Lesions Induced by Diclofenac
To confirm whether the induction of antral lesions was specific for IND, we also examined the effects of the NSAID diclofenac (DIC) on the formation of antral lesions. Using the protocol depicted in Fig. 1B, ATR (30 mg/kg) or MOS (3 mg/kg) and DIC (100 mg/kg) was administered s.c. DIC induced lesions only in the antrum. The lesion index in the group given vehicle was 9.3 ± 1.7 mm2 (n = 8). As shown in Table 1, the lesion index was significantly increased by ATR and decreased by MOS, similar to the results of IND-induced antral lesions.
Effects of SR 57227 and Apomorphine on Antral Lesions, Gastric Emptying, and Bile Reflux
To further test our hypothesis, we examined the effects of emetogenic drugs on antral lesions, gastric emptying, and bile reflux using the protocol depicted in Fig. 1B, D: the selective 5-HT3 receptor agonist SR 57227 [27] (SR, 0.03–1 mg/kg, s.c.), and the central dopamine D2 receptor agonist apomorphine [28] (APO, 0.1–3 mg/kg, s.c.). SR and APO dose-dependently and significantly worsened the antral lesions at 0.3 and 1 mg/kg SR, and at 1 and 3 mg/kg APO (Fig. 4A). SR dose-dependently and significantly decreased the amounts of gastric contents at 0.3 and 1 mg/kg, whereas APO did not affect the gastric contents (Fig. 4B). SR and APO dose-dependently and significantly increased the concentration of bile acids at 1 mg/kg of SR and at 0.3 and 1 mg/kg of APO (Fig. 4C). These results suggest that emetogenic drugs worsen IND-induced antral lesions by increasing the amount of bile reflux, although SR accelerated gastric emptying.
Effects of Ondansetron and Haloperidol on Aggravation of Antral Lesions and Increase of Bile Reflux Caused by SR or APO
Since emetogenic drugs worsened antral lesions with increased bile reflux, we next examined the effects of the selective 5-HT3 receptor antagonist ondansetron [29], (OND, 0.3–3 mg/kg, s.c.) and the selective dopamine D2 receptor antagonist haloperidol [30] (HAL, 0.3–3 mg/kg, s.c.) on these parameters. OND or HAL was administered according to the protocol depicted in Fig. 1C. The lesion index in the group given vehicle was 3.0 ± 0.4 mm2 (n = 10), and perforations were observed in 2/10 mice in the group given vehicle (Fig. 5A). SR (1 mg/kg, s.c.) significantly increased the lesion index and the number of mice with perforations (Fig. 5A). OND dose-dependently and significantly prevented the increases of lesion index and the perforation ratio induced by SR (Fig. 5A). OND (3 mg/kg, s.c.) itself did not affect the formation of antral lesions induced by IND (10 mg/kg, s.c.) in the group given vehicle (data not shown). APO (1 mg/kg, s.c.) significantly increased the lesion index and the number of mice with perforations, compared with the group given vehicle (Fig. 5B). HAL dose-dependently and significantly prevented the increases of lesion index and the perforation ratio induced by APO (Fig. 5B). HAL (3 mg/kg, s.c.) itself did not affect the formation of antral lesions induced by IND (10 mg/kg, s.c.) in the group given vehicle (data not shown). For bile acid concentration measurements, OND or HAL was administered according to the protocol depicted in Fig. 1E. As shown in Fig. 5C and D, SR (1 mg/kg, s.c.) and APO (1 mg/kg, s.c.) significantly increased the concentration of bile acids in the stomach. This increase was significantly prevented by pretreatment with OND (3 mg/kg, s.c.) or HAL (3 mg/kg, s.c.), respectively (Fig. 5C, D). These results support the hypothesis that the selective activation of 5-HT3 or of dopamine D2 receptor worsens IND-induced antral lesions by increasing bile reflux.
Effects of ondansetron and haloperidol both on aggravation of antral lesions and increase of bile reflux induced by SR 57227 and apomorphine. Effects on antral lesions: Ondansetron (OND, 0.3–3 mg/kg) (A) or haloperidol (HAL, 0.3–3 mg/kg) (B) was administered s.c. just after 2 h of re-feeding of chow pellets, and 0.5 h later SR 57227 (SR, 1 mg/kg, s.c.) or apomorphine (APO, 1 mg/kg, s.c.) was administered. IND (10 mg/kg, s.c.) was administered 0.5 h after pretreatment with SR or APO, and 24 h later the antral lesions were examined. Effects on bile reflux: OND (3 mg/kg) (C) or HAL (3 mg/kg) (D) was administered s.c. just after 2 h of re-feeding of powder diet, and 0.5 h later SR (1 mg/kg, s.c.) or APO (1 mg/kg, s.c.) was administered. Gastric contents were collected 1.5 h after dosing with OND or HAL, and concentration of bile acids (BA) was measured. Each point represents data from an individual animal. Data are expressed as mean ± SEM (n = 8–10). b and c: P < 0.01 and 0.001 vs. vehicle (VEH) + VEH, (b) and (c): P < 0.01 and 0.001 vs. VEH + SR or APO (Tukey’s test). Ratio in parenthesis indicates the number of mice with antral perforation vs. the total number of mice. # and ##P < 0.05 and 0.01 vs. VEH + VEH, †, and ††P < 0.05 and 0.01 vs. VEH + SR or APO (χ2 test)
Effects of Ondansetron and Haloperidol on Aggravation of Antral Lesions by Atropine and Dopamine
Next, we examined the contribution of 5-HT3 and dopamine D2 receptors towards the worsening effects of ATR and DOP on IND-induced antral lesions, according to the protocol depicted in Fig. 1C. ATR and DOP markedly increased the formation of antral lesions and the number of mice with perforation, compared with the corresponding vehicle groups (Fig. 6A, B). These effects of ATR and DOP were dose-dependently and significantly prevented by OND (0.3–3 mg/kg, s.c.) (Fig. 6A, B). HAL (1 and 3 mg/kg, s.c.) also dose-dependently and significantly prevented the worsening effects of ATR and DOP on the formation of antral lesions and number of mice with perforation (Fig. 6C, D).
Effects of ondansetron and haloperidol on the aggravation of antral lesions induced by atropine or dopamine. OND (0.3–3 mg/kg) (A, B) or HAL (1 and 3 mg/kg) (C, D) was administered s.c. just after 2 h of re-feeding, and 0.5 h later ATR (30 mg/kg, s.c.) or DOP (10 mg/kg, i.p.) was administered. IND (10 mg/kg, s.c.) was administered 0.5 h after ATR or DOP treatment, and 24 h later the antral lesions were examined. Each point represents data from an individual animal. Data are expressed as mean ± SEM (n = 8). a and c: P < 0.05 and 0.001 vs. vehicle (VEH) + VEH or SAL, (a), (b) and (c): P < 0.05, 0.01 and 0.001 vs. VEH + ATR or DOP (Tukey’s test). Ratio in parenthesis indicates the number of mice with antral perforation vs. the total number of mice. #P < 0.05 vs. VEH + VEH or SAL, †, and ††P < 0.05 and 0.01 vs. VEH + ATR or DOP (χ2 test)
Effect of Ondansetron on Aggravation of DIC-Induced Antral Lesions by Atropine
We also confirmed the protective effects of OND on DIC-induced antral lesions. DIC induced lesions only in the antrum (lesion index in the group given vehicle was 8.4 ± 1.4 mm2; n = 8). ATR markedly worsened lesion formation, with a lesion index of 15.5 ± 2.0 mm2 (n = 8, P < 0.05 vs. vehicle) (Table 2). The increase in lesion index induced by ATR was significantly prevented by pretreatment with OND with observed change in perforation rate (Table 2).
Effects of Ondansetron and Haloperidol on Inhibition of Gastric Emptying and Increase of Bile Reflux Induced by Atropine or Dopamine
OND (3 mg/kg, s.c.) or HAL (3 mg/kg, s.c.) was administered according to the protocol depicted in Fig. 1E. The gastric contents in the group given vehicle (control of those given ATR or DOP) were 19.3 ± 1.2% (n = 12) and 21.9 ± 1.1% (n = 10). ATR and DOP significantly increased the amounts of gastric contents, unaffected by OND (Fig. 7A, B). The concentrations of bile acids in the group given vehicle (control of those given ATR or DOP) were 3.4 ± 0.5 μM (n = 12) and 4.1 ± 1.2 μM (n = 10). ATR and DOP significantly increased the concentration of bile acids (Fig. 7C, D). OND (3 mg/kg, s.c.) significantly inhibited the increase in bile reflux by ATR or DOP (Fig. 7C, D).
Effects of ondansetron on inhibition of gastric emptying and increase of bile reflux induced by atropine or dopamine. OND (3 mg/kg) was administered s.c. just after 2 h of re-feeding, and 0.5 h later ATR (30 mg/kg, s.c.) or DOP (10 mg/kg, i.p.) was administered. Gastric contents were collected 1.5 h after pretreatment with OND. The amounts of gastric contents (A, B) and concentration of bile acids (BA) (C, D) were measured. Each point represents data from an individual animal. Data are expressed as mean ± SEM (n = 10–12). a, and b: P < 0.05, and 0.01 vs. vehicle (VEH) + VEH or SAL, ns: not significant, (b): P < 0.01 vs. VEH + ATR or DOP (Tukey’s test)
The gastric contents in the group given vehicle (control of those given ATR or DOP) were 17.3 ± 1.2% (n = 10) and 17.5 ± 0.9% (n = 10). ATR and DOP markedly increased the amounts of gastric contents, though HAL (3 mg/kg, s.c.) had no effect on the increase in gastric contents by ATR or DOP (Fig. 8A, B). The concentrations of bile acids in the group given vehicle (control of those given ATR or DOP) were 7.3 ± 1.9 μM (n = 10) and 6.1 ± 1.4 μM (n = 10). ATR and DOP significantly increased the concentration of bile acids (Fig. 8C, D). HAL (3 mg/kg, s.c.) significantly inhibited the increase of bile reflux by ATR or DOP (Fig. 8C, D).
Effects of haloperidol on inhibition of gastric emptying and increase of bile reflux induced by atropine or dopamine. HAL (3 mg/kg) was administered s.c. just after 2 h of re-feeding, and 0.5 h later ATR (30 mg/kg, s.c.), or DOP (10 mg/kg, i.p.) was administered. Gastric contents were collected 1.5 h after dosing with HAL. Both the amounts of gastric contents (A, B) and concentration of bile acids (BA) (C, D) were examined. Each point represents data from an individual animal. Data are expressed as mean ± SEM (n = 9 or 10). a, and b: P < 0.05, and 0.01 vs. vehicle (VEH) + VEH or SAL, ns: not significant, (a), and (c): P < 0.05 and 0.001 vs. VEH + ATR or DOP (Tukey’s test)
These results suggest that anti-emetic drugs OND and HAL prevent the aggravation of IND-induced antral lesions induced by ATR or DOP through the inhibition of bile reflux increased by ATR or DOP with no effect on delayed gastric emptying.
Effects of Sodium Taurocholate and Cholestyramine on Gastric Antral Lesions Induced by IND
Since our data suggest that bile reflux is likely an important contributor to increasing the severity of antral lesions induced by ATR and DOP, we further examined the effects of exogenous bile acids or bile acid sequestrants in the stomach on IND-induced antral lesions.
The antral lesion index induced by IND in control mice given powder diet (PD) for 2 h was 3.4 ± 0.6 mm2 (n = 8); antral perforation was observed in 2/8 mice. Sodium taurocholate (TCA, 0.01–0.3%) added to the PD increased the lesions in a concentration-dependent manner. The effects at 0.1 and 0.3% TCA were significant (P < 0.05 and 0.01 vs. PD alone), and the number of mice with perforation at 0.3% TCA was also significantly increased to 7/8 mice (P < 0.05 vs. PD alone) (Fig. 9A). TCA (0.3%) alone added to the PD without IND did not cause any visible lesions in the antral mucosa at 24 h in re-fed mice (lesion index: 0.0 ± 0.0 mm2, n = 6).
Effects of sodium taurocholate and cholestyramine on gastric antral lesions induced by indomethacin. Powder diets (PD) alone, PD with taurocholate (TCA, A), cholestyramine (COL, B), or COL pretreated with TCA (COL-TCA, C) were given to mice during a 2-h re-feeding period. IND (10 mg/kg, s.c.) was administered just after the re-feeding period (A–C). Gastric lesions were examined 24 h after IND treatment. Each point represents data from an individual animal. Data are expressed as mean ± SEM (n = 8). A, B: a, b, and c: P < 0.05, 0.01, and 0.001 vs. PD (Dunnett’s test). C: c: P < 0.001 vs. PD, (b): P < 0.01 vs. PD + COL (Tukey’s test). Ratio in parenthesis indicates the number of mice with antral perforation vs. the total number of mice. #P < 0.05 vs. PD (χ2 test)
The lesion index of antral lesions induced by IND (10 mg/kg, s.c.) in control mice given PD for 2 h was 5.4 ± 0.6 mm2 (n = 8) with antral perforation observed in 5/8 mice. Cholestyramine (COL; a bile acid sequestrant, 0.3–10.0%) added to PD decreased the severity of antral lesions in a concentration-dependent manner; the maximal effect was observed at 3% COL (Fig. 9B). The effects at doses of 1% and more were significant (P < 0.05–0.001 vs. PD alone). The number of mice with perforation also decreased to 1/8 or 3/8 mice (Fig. 9B).
To elucidate whether the effect of COL is dependent on the binding activity of COL to bile acids, we examined the effect of pretreatment of COL with TCA on the formation of IND-induced antral lesions. As shown in Fig. 9C, the inhibitory effect of COL (1%) on antral lesions was significantly eliminated by pretreatment of COL with 0.1 M TCA (COL-TCA), suggesting that the binding of bile acids by COL prevents the formation of antral lesions.
Effect of Cholestyramine on Gastric Antral Lesions Induced by Diclofenac
DIC (100 mg/kg, s.c.) was administered just after 2 h of re-feeding with PD alone or PD supplemented with COL (0.3 and 1%), and the lesions in the antrum were examined 24 h thereafter. The lesion index of antral lesions in the control mice given PD alone for 2 h was 10.9 ± 1.7 mm2 (n = 8); antral perforation was observed in 8/8 mice (Table 3). The lesion index and the number of mice with perforation were decreased by the addition of COL to PD in concentration-dependent manner. The effect of COL at 1% were significant (P < 0.05 vs. PD alone) (Table 3).
Correlations Between Lesion Index and Gastric Contents or Bile Acid Concentrations
To elucidate the contributions of gastric contents and bile reflux to IND-induced antral ulceration, we analyzed the correlation between lesion index and gastric contents or bile acid concentrations. Since the lesion index and measurements of gastric contents and bile acid concentrations were analyzed in the different animals, we plotted the mean ± SEM of the corresponding treatment groups as depicted in Figs. 2, 3, 4, 5, 6, 7. Note the significant correlation between the gastric contents and the lesion index (Fig. 10A), and bile acid concentrations and the lesion index (Fig. 10B). These results strongly support our hypothesis that delayed gastric emptying and increased bile reflux worsen IND-induced antral ulcers.
Discussion
The results of the present study strongly suggest that ATR and DOP increase the severity of NSAID-induced gastric antral ulcers in re-fed mice by increasing duodenogastric bile reflux via activation of 5-HT3 and dopamine D2 receptors.
NSAIDs often cause gastric ulcers in the pre-pyloric antral area in humans [1,2,3,4]. In the present mouse study, NSAIDs induced lesions only in the antral mucosa in the re-fed model. The formation of antral lesions correlated with the amount of food intake during the 2-h re-feeding period, suggesting that the amount of residual gastric content contributes to the formation of NSAIDs-induced gastric antral ulcers. We first examined the effects of ATR on the formation of gastric corpus and antral lesions induced by IND, finding that the formation of gastric corpus lesions in fasted mice was markedly inhibited by ATR pretreatment. In re-fed mice, ATR unexpectedly increased the severity of antral lesions in a dose-dependent manner. Furthermore, DOP, another antimotility drug [17, 18], also dose-dependently worsened the antral lesions. On the other hand, the gastroprokinetic drugs NEO (acetylcholine esterase inhibitor) [23] and MOS (5-HT4 receptor agonist) [24, 25] dose-dependently decreased the severity of antral lesions. These effects of ATR and MOS on the formation of antral lesions were also observed when DIC, another NSAID, was used.
Then, we examined the effects of the motility-altering drugs on the amounts of gastric contents. The gastric contents were increased by ATR and DOP, but decreased by NEO and MOS, suggesting that the alteration of the rate of gastric emptying affects the formation of antral lesions, since the amounts of food intake (i.e., gastric contents) are closely related to the lesion index. This result is also implicated in the utility of gastroprokinetic drugs in the treatment of functional dyspepsia (FD) associated with gastroparesis [31]. These results suggest that gastroparesis worsens NSAID-induced antral lesions in the re-fed model, supported by the correlation analysis (Fig. 10A).
Duodenogastric bile reflux is another factor affecting the formation of gastric antral ulcers, supported by the finding that the concentration of bile acids in the stomach is higher in patients with gastric antral ulcers than in healthy volunteers [32, 33], and that excessive bile reflux is often observed in patients with active antral ulcers [34, 35]. Furthermore, bile acids disrupt the gastric mucosal barrier due to their detergent properties [14,15,16]. Moreover, gastric antral ulcers occur spontaneously in W/Wv mutant mice that have delayed gastric emptying and increased bile reflux [36, 37]. Likewise, ATR increased the amount of duodenogastric reflux and delayed gastric emptying in humans [12] and in dogs [13]. These findings suggest that ATR may alter the formation of gastric antral ulcers by increasing the amount of bile reflux. In the present study in re-fed mice, ATR and DOP increased bile reflux associated with delayed gastric emptying, whereas NEO and MOS reduced bile reflux while accelerating gastric emptying. Furthermore, direct exposure of bile acids to the antral mucosa may facilitate NSAIDs induced antral mucosal injury in humans. These results suggest that duodenogastric bile reflux worsens NSAID-induced antral lesions in re-fed model, also supported by the correlation analysis (Fig. 10B).
To further elucidate the contribution of bile reflux in the formation of gastric antral ulcers, we further examined the effects of TCA and COL on the formation of antral lesions induced by IND or DIC in re-fed mice. The addition of TCA to diet worsened IND-induced antral lesions whereas the sequestration of bile acids with COL reduced antral lesions. These results support our hypothesis that bile reflux is an important cause of the antral lesions.
The next question is whether bile acid composition contributes to the pathogenesis of the antral lesions. In mice, the primary bile acids include cholic acid (CA), chenodeoxycholic acid (CDCA) and muricholic acids (α, β, ω), and their conjugated forms, such as taurocholic acid (TCA) and glycocholic acid (GCA), TCDCA and GCDCA. Deoxycholic acid (DCA) and lithocholic acid (LCA), and their conjugated forms TDCA and TLCA comprise the major secondary bile acids. Although previous studies showed that mucosal application of bile acids injure the bullfrog gastric mucosa at ~ mM concentrations with DCA > CA > TCA [38], in humans, gastric bile acid concentrations were higher in the gastric juice of gastric ulcer patients than in controls, while gastric bile acid compositions (including conjugated CA, CDCA, and DCA) had no difference between controls and gastric ulcer patients [39]. In contrast, bile acid reflux and secondary bile acids such as DCA increase gastric inflammation and carcinogenesis in humans [40, 41] and in mice under certain conditions [42]. These data suggest that though gastric bile acid compositions are implicated in carcinogenesis, bile acid concentration, without reference to specific bile acids, is related to gastric ulcer pathogenesis in certain instances.
In the present study, limited amounts of bile acids were often observed in the gastric contents even in the mice given vehicle, supported by clinical [12] and canine data [13] that duodenogastric reflux is a common post-prandial phenomenon of uncertain significance. Interestingly, IND treatment itself had no effect on the amounts of gastric contents and the concentration of bile acids, whereas IND induced antral lesions after re-feeding, suggesting that pre-existing bile acids in the stomach during re-feeding are implicated in the induction of antral lesions after IND treatment. This hypothesis is supported by the results of COL feeding, which prevented IND-induced antral lesions further implicating bile acids in the pathogenesis of antral ulcers.
Another question is the mechanism by which ATR and DOP accelerate bile reflux with delayed gastric emptying. Possibilities include bile reflux due to decreased gastric motility associated with reduced gastric tone or intra-gastric pressure, an increase in duodenal motility with opening of the pyloric ring, or by both. Since APO stimulates retrograde motility of upper small intestine with resultant nausea and vomiting in dogs [43], it is possible that gastroparesis caused by ATR and DOP may accelerate bile reflux through the stimulation of duodenal motility. Increased bile reflux with delayed gastric emptying or reduced bile reflux with accelerated gastric emptying were observed in the ATR and DOP, or NEO and MOS groups, respectively. In contrast, the anti-emetic drugs OND and HAL reduced bile reflux without affecting delayed gastric emptying by ATR or DOP. These discrepancies may be explained by the balance between bile reflux due to retrograde duodenal peristalsis and gastric emptying, since bile reflux may be enhanced by gastroparesis due to reduced clearance and/or enhanced retrograde duodenal motility. Although no data are available regarding the balance between gastric emptying and retrograde propulsion of the duodenal content in terms of gastric bile acid concentrations, we speculate that OND and HAL may predominantly inhibit duodenal motility with consequent reduced bile reflux into the relaxed stomach by ATR or DOP.
Distension of the gastric antrum (e.g., by over-eating) stimulates 5-HT3 receptor localized in vagal afferent nerves in the stomach, then induces nausea and vomiting [3, 19, 20]. In the present study, gastric contents were significantly increased by ATR and DOP, suggesting that these drugs may stimulate vagal afferent nerves by distension of the antrum. It is possible that stimulation of vagal afferent nerves may stimulate dopamine D2 receptors localized in the chemoreceptor trigger zone (CTZ) and/or central vomiting center [44, 45], then stimulate duodenal motility through vago-vagal or vago-sympathetic nerve reflexes. This reflex may include “adaptive relaxation” of the stomach [46], and nausea and vomiting induced by anesthetics [47] and by cytotoxic drugs for chemotherapy [48]. Therefore, we speculate that ATR and DOP may accelerate bile reflux by increasing duodenal motility induced by vagal reflex via an activation of 5-HT3 receptors on the vagal afferent nerves and dopamine D2 receptors localized in the CTZ and/or vomiting center.
NSAID-induced corpus gastric injury in fasted rats and small intestinal injury in fed rats are caused by gastric or small intestinal hypermotility, respectively, since ATR abolishes these lesions by inhibiting hypermotility [10, 49]. In contrast, the duodenal mucosal injury caused by 5-HT release with IND treatment in fed rats is inhibited by OND, while ATR has no effect, although ATR reduces jejunal and ileal injury [50]. These results suggest that duodenal motility is regulated, at least in part, by ATR-resistant, 5-HT3 receptor-mediated mechanisms. The precise mechanisms of accelerated bile reflux induced by ATR and DOP under re-fed conditions remain to be elucidated.
In conclusion, the results of the present study suggest that gastric emptying and bile reflux are important factors in the formation of gastric antral ulcers induced by NSAIDs. We speculate that gastroparesis may worsen gastric antral ulcers by increasing the volume of gastric contents and bile reflux by a mechanism in which 5-HT3 and dopamine D2 receptors localized in vagal afferent nerves, the chemoreceptor trigger zone (CTZ) and/or central vomiting center, are involved. The results further suggest that cholestyramine and gastroprokinetic agents such as mosapride are predicted to reduce the formation of gastric antral ulcers induced by NSAIDs in patients with FD or gastroparesis.
References
Taylor RT, Huskisson EC, Whitehouse GH, Hart FD, Trapnell DH. Gastric ulceration occurring during indomethacin therapy. Br Med J 1968;4:734–737.
Roth SH, Bennett RE. Nonsteroidal anti-inflammatory drug gastropathy. Recognition and response. . Arch Intern Med 1987;147:2093–2100.
Talley NJ, Locke GR 3rd, Lahr BD et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut 2006;55:933–939.
McCarthy DM. Nonsteroidal antiinflammatory drug-induced ulcers: management by traditional therapies. Gastroenterology 1989;96:662–674.
Satoh H, Urushidani T. Soluble dietary fiber can protect the gastrointestinal mucosa against nonsteroidal anti-inflammatory drugs in mice. Dig Dis Sci 2016;61:1903–1914.
Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. NSAID-associated gastric ulcer study group. Arch Intern Med 2000;160:1455–1461.
Campbell DR, Haber MM, Sheldon E et al. Effect of H. pylori status on gastric ulcer healing in patients continuing nonsteroidal anti-inflammatory therapy and receiving treatment with lansoprazole or ranitidine. Am J Gastroenterol 2002;97:2208–2214.
Yeomans ND, Svedberg LE, Naesdal J. Is ranitidine therapy sufficient for healing peptic ulcers associated with non-steroidal anti-inflammatory drug use? Int J Clin Pract 2006;60:1401–1407.
Satoh H, Akiba Y, Urushidani T. Proton pump inhibitors prevent gastric antral ulcers induced by NSAIDs via activation of capsaicin-sensitive afferent nerves in mice. Dig Dis Sci 2020;65:2580–2594.
Takeuchi K, Ueki S, Okabe S. Importance of gastric motility in the pathogenesis of indomethacin-induced gastric lesions in rats. Dig Dis Sci 1986;31:1114–1122.
Ueki S, Takeuchi K, Okabe S. Gastric motility is an important factor in the pathogenesis of indomethacin-induced gastric mucosal lesions in rats. Dig Dis Sci 1988;33:209–216.
Müller-Lissner SA, Fimmel CJ, Sonnenberg A et al. Novel approach to quantify duodenogastric reflux in healthy volunteers and in patients with type I gastric ulcer. Gut 1983;24:510–518.
Sonnenberg A, Müller-Lissner SA, Schattenmann G, Siewert JR, Blum AL. Duodenogastric reflux in the dog. Am J Physiol 1982;242:G603–G607.
Davenport HW. Destruction of the gastric mucosal barrier by detergents and urea. Gastroenterology 1968;54:175–181.
Duane WC, Wiegand DM. Mechanism by which bile salt disrupts the gastric mucosal barrier in the dog. J Clin Invest 1980;66:1044–1049.
Armstrong D, Rytina ER, Murphy GM, Dowling RH. Gastric mucosal toxicity of duodenal juice constituents in the rat. Acute studies using ex vivo rat gastric chamber model. Dig Dis Sci 1994;39:327–339.
Nagahata Y, Azumi Y, Kawakita N, Wada T, Saitoh Y. Inhibitory effect of dopamine on gastric motility in rats. Scand J Gastroenterol 1995;30:880–885.
Levein NG, Thörn SE, Wattwil M. Dopamine delays gastric emptying and prolongs orocaecal transit time in volunteers. Eur J Anaesthesiol 1999;16:246–250.
Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut 2006;55:1685–1691.
Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol 2018;43:111–117.
Feinle C, Read NW. Ondansetron reduces nausea induced by gastroduodenal stimulation without changing gastric motility. Am J Physiol 1996;271:G591–G597.
Sanger GJ, Andrews PLR. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front Pharmacol 2018;9:913.
Parthasarathy G, Ravi K, Camilleri M et al. Effect of neostigmine on gastroduodenal motility in patients with suspected gastrointestinal motility disorders. Neurogastroenterol Motil 2015;27:1736–1746.
Uchida M, Yamato S, Shimizu K, Amano T, Ariga H. Dual role of mosapride citrate hydrate on the gastric emptying evaluated by the breath test in conscious rats. J Pharmacol Sci 2013;121:282–287.
Seto Y, Yoshida N, Kaneko H. Effects of mosapride citrate, a 5-HT4-receptor agonist, on gastric distension-induced visceromotor response in conscious rats. J Pharmacol Sci 2011;116:47–53.
Mashige F, Tanaka N, Maki A, Kamei S, Yamanaka M. Direct spectrophotometry of total bile acids in serum. Clin Chem 1981;27:1352–1356.
Bachy A, Héaulme M, Giudice A et al. SR 57227A: a potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eur J Pharmacol 1993;237:299–309.
Depoortère R, Barret-Grévoz C, Bardin L, Newman-Tancredi A. Apomorphine-induced emesis in dogs: differential sensitivity to established and novel dopamine D2/5-HT1A antipsychotic compounds. Eur J Pharmacol 2008;597:34–38.
Markham A, Sorkin EM. An update of its therapeutic use in chemotherapy-induced and postoperative nausea and vomiting. Drugs 1993;45:931–952.
Schwalbe T, Kaindl J, Hübner H, Gmeiner P. Potent haloperidol derivatives covalently binding to the dopamine D2 receptor. Bioorg Med Chem 2017;25:5084–5094.
Acosta A, Camilleri M. Prokinetics in gastroparesis. Gastroenterol Clin North Am 2015;44:97–111.
Gotthard R, Bodemar G, Tjädermo M, Tobiasson P, Walan A. High gastric bile acid concentration in prepyloric ulcer patients. Scand J Gastroenterol 1985;20:439–446.
Rydning A, Berstad A. Intragastric bile acid concentrations in healthy subjects and in patients with gastric and duodenal ulcer and the influence of fiber-enriched wheat bran in patients with gastric ulcer. Scand J Gastroenterol 1985;20:801–804.
Duplessis DJ. Pathogenesis of gastric ulceration. Lancet 1965;1:974–978.
Rhodes J, Barnardo DE, Phillips SF, Rovelstad RA, Hofmann AF. Increased reflux of bile into the stomach in patients with gastric ulcer. Gastroenterology 1969;57:241–252.
Yokoyama M, Tatsuta M, Baba M, Kitamura Y. Bile reflux: a possible cause of stomach ulcer in nontreated mutant mice of W/Wv genotype. Gastroenterology 1982;82:857–863.
Azuma T, Dojyo M, Ito S et al. Bile reflux due to disturbed gastric movement is a cause of spontaneous gastric ulcer in W/Wv mice. Dig Dis Sci 1999;44:1177–1183.
Silen W, Forte JG. Effects of bile salts on amphibian gastric mucosa. Am J Physiol 1975;228:637–644.
Schindlbeck NE, Heinrich C, Stellaard F, Paumgartner G, Müller-Lissner SA. Healthy controls have as much bile reflux as gastric ulcer patients. Gut 1987;28:1577–1583.
Li D, Zhang J, Yao WZ et al. The relationship between gastric cancer, its precancerous lesions and bile reflux: a retrospective study. J Dig Dis 2020;21:222–229.
Režen T, Rozman D, Kovács T et al. The role of bile acids in carcinogenesis. Cell Mol Life Sci 2022;79:243.
Noto JM, Piazuelo MB, Shah SC et al. Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis. J Clin Invest. 2022. https://doi.org/10.1172/JCI147822.
Lang IM. Physiology of the digestive tract correlates of vomiting. J Neurogastroenterol Motil. 2023;29:20–30.
Mitchelson F. Pharmacological agents affecting emesis. A review (Part I). Drugs 1992;43:295–315.
Belkacemi L, Darmani NA. Dopamine receptors in emesis: molecular mechanisms and potential therapeutic function. Pharmacol Res 2020;161:105–124.
Currò D, Ipavec V, Preziosi P. Neurotransmitters of the non-adrenergic non-cholinergic relaxation of proximal stomach. Eur Rev Med Pharmacol Sci 2008;12:53–62.
Glatzle J, Sternini C, Robin C et al. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 2002;123:217–226.
Minami M, Endo T, Hirafuji M et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther 2003;99:149–165.
Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion 2002;66:30–41.
Akiba Y, Maruta K, Narimatsu K et al. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am J Physiol Gastrointest Liver Physiol 2017;313:G117–G128.
Acknowledgments
The authors are greatly indebted to Ms. Ai Sato, Ms. Chihiro Takagi, Ms. Miki Horikawa and Ms. Fumika Kotera, students in our Department, Doshisha Women’s College of Liberal Arts, for their technical assistance, and Drs. Y. Amagase and Y. Mizukawa for valuable discussions and suggestions.
Funding
Doshisha Women’s College of Liberal Arts (TU), VA Merit Review I01BX001245 (JDK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
Experimental protocols were approved by the Animal Research Committees at Doshisha Women’s College of Liberal Arts, Kodo, Kyotanabe, Kyoto, Japan (Approval Number Y16-031).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satoh, H., Akiba, Y., Urushidani, T. et al. Gastroparesis Worsens Indomethacin-Induced Gastric Antral Ulcers by Bile Reflux via Activation of 5-HT3 and Dopamine D2 Receptors in Mice. Dig Dis Sci 68, 3886–3901 (2023). https://doi.org/10.1007/s10620-023-08086-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08086-x