Abstract
The ulcerogenic potential of dopamine antagonists and l-NAME in rats provides unresolved issues of anti-emetic neuroleptic application in both patients and experimental studies. Therefore, in a 1-week study, we examined the pressures within the lower oesophageal and the pyloric sphincters in rats [assessed manometrically (cm H2O)] after dopamine neuroleptics/prokinetics, l-NAME, l-arginine and stable gastric pentadecapeptide BPC 157 were administered alone and/or in combination. Medication (/kg) was given once daily intraperitoneally throughout the 7 days, with the last dose at 24 h before pressure assessment. Given as individual agents to healthy rats, all dopamine antagonists (central [haloperidol (6.25 mg, 16 mg, 25 mg), fluphenazine (5 mg), levomepromazine (50 mg), chlorpromazine (10 mg), quetiapine (10 mg), olanzapine (5 mg), clozapine (100 mg), sulpiride (160 mg), metoclopramide (25 mg)) and peripheral(domperidone (10 mg)], l-NAME (5 mg) and l-arginine (100 mg) decreased the pressure within both sphincters. As a common effect, this decreased pressure was rescued, dose-dependently, by BPC 157 (10 µg, 10 ng) (also note that l-arginine and l-NAME given together antagonized each other’s responses). With haloperidol, l-NAME worsened both the lower oesophageal and the pyloric sphincter pressure, while l-arginine ameliorated lower oesophageal sphincter but not pyloric sphincter pressure, and antagonized l-NAME effect. With domperidone, l-arginine originally had no effect, while l-NAME worsened pyloric sphincter pressure. This effect was opposed by l-arginine. All these effects were further reversed towards a stronger beneficial effect, close to normal pressure values, by the addition of BPC 157. In addition, NO level was determined in plasma, sphincters and brain tissue. Thiobarbituric acid reactive substances (TBARS) were also assessed. Haloperidol increased NO levels (in both sphincters, the plasma and brain), consistently producing increased TBARS levels in the plasma, sphincters and brain tissues. These effects were all counteracted by BPC 157 administration. In conclusion, we revealed that BPC 157 counteracts the anti-emetic neuroleptic class side effect of decreased pressure in sphincters and the dopamine/NO-system/BPC 157 relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine antagonists (Bilic et al. 2001; Jelovac et al. 1999; Sikiric et al. 1985, 1986, 1987, 2000; Szabo and Neumeyer 1983) and nitric oxide synthase (NOS) blockade have ulcerogenic effects (Cesarec et al. 2013; Skorjanec et al. 2015). Thus, we challenged the anti-emetic issue of neuroleptics (Murray-Brown and Dorman 2015; Perkins and Dorman 2009). In rats, we studied how dopamine neuroleptics/prokinetics interact with the lower oesophageal and pyloric sphincters, along with NO-agents, NOS-blocker, N(G)-nitro-l-arginine methyl ester (l-NAME), NOS-substrate, l-arginine and stable gastric pentadecapeptide BPC 157 (Sikiric et al. 2010, 2011, 2014, 2016, 2017).
This dopamine/NO-system blockade combination might be important because there is currently incomplete evidence from published randomized controlled trials to determine the effectiveness of haloperidol for nausea and vomiting in palliative care, which contrasts with haloperidol being commonly prescribed to relieve these symptoms (Murray-Brown and Dorman 2015; Perkins and Dorman 2009). Likewise, some studies have failed to demonstrate the effectiveness of metoclopramide and domperidone in GERD therapy (Grande et al. 1992) or their effects on sphincter pressure (Meng et al. 2016).
However, all these agents, in addition to dopamine receptors, may also affect various other receptors (e.g. metoclopramide is also a mixed 5-HT3 receptor antagonist/5-HT4 receptor agonist, and olanzapine has a higher affinity for 5-HT2A serotonin receptors than for D2 dopamine receptors).
Furthermore, in terms of the generally recognized significance of dopamine and the NO-system in the gastrointestinal tract (Paré and Glavin 1986; Sikiric et al. 2014; Szabo et al. 1987), the inhibition of either leads to stomach and/or duodenal ulcer induction or aggravation in rats (Bilic et al. 2001; Jelovac et al. 1999; Sikiric et al. 1985, 1986, 1987, 2000; Szabo and Neumeyer 1983; Cesarec et al. 2013; Skorjanec et al. 2015). However, the likely consequence of lower oesophageal and pyloric sphincter disability was not assumed and thereby not investigated or demonstrated. This dopamine/NO-system blockade combination should also be considered with regard to the higher doses commonly used in rat studies (Bilic et al. 2001; Jelovac et al. 1999; Sikiric et al. 1985, 1986, 1987, 2000; Szabo and Neumeyer 1983).
Indicative of the dopamine/NO relationship, NOS inhibitors induce catalepsy in mice, and an additive cataleptic effect occurs with haloperidol (Del Bel et al. 2005). Dopamine induced an increase in NOS activity in the renal medulla and cortex, whereas the hypotensive effect of l-arginine and hypertension induced by l-NAME were not modified by haloperidol (Costa et al. 2006). However, regarding this sphincter issue, a possible neuroleptics–prokinetics/NO-system relationship in vivo has not been studied.
To solve this dilemma, it may be helpful that pentadecapeptide BPC 157 interacts peripherally and centrally with both dopamine and the NO-system in various models and species, and also maintains gastrointestinal mucosa integrity and reinstates sphincter function (Sikiric et al. 2010, 2011, 2014, 2016, 2017). Interestingly, BPC 157 is an original anti-ulcer peptide in inflammatory bowel disease. In multiple sclerosis trials, LD-1 was not achieved and no side effects have been found in clinical trials (Klicek et al. 2013; Sikiric et al. 2010, 2011, 2014, 2016, 2017). In addition, BPC 157 is known to interact with several molecular pathways (Chang et al. 2011, 2014; Hsieh et al. 2017; Huang et al. 2015; Tkalcevic et al. 2007).
Thus, we hypothesized that a decrease in pressure within the lower oesophageal and pyloric sphincters is a class side effect in living rats administered with dopamine antagonists and/or NOS-blocker l-NAME, not reported heretofore. The regimens include those used as neuroleptics, typical and atypical, and those used as anti-emetics, acting centrally (haloperidol, fluphenazine, levomepromazine, chlorpromazine, quetiapine, olanzapine, sulpiride, clozapine, metoclopramide) and/or peripherally (domperidone) (Abi-Dargham 2014; Acosta and Camilleri 2015; Sikiric et al. 2014). Against this large background, we explored the potential of the stable gastric pentadecapeptide BPC 157 (Sikiric et al. 2010, 2011, 2014, 2016, 2017) and the NOS-substrate l-arginine. In particular, BPC 157 reinstates sphincter function that has failed due to acute and chronic oesophagitis, acute pancreatitis, oesophagocutaneous and duodenocutaneous fistulas or severe hyperkalaemia, and counteracts failed functioning of urethral and sphincter stress urinary incontinence (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Jandric et al. 2013; Petrovic et al. 2006, 2011; Sikiric et al. 2010, 2011, 2014, 2016, 2017; Skorjanec et al. 2015) as well as pupil sphincter and counteracts atropine-mydriasis (Kokot et al. 2016), where its interaction with dopamine and NO-system may be a convincing background (Sikiric et al. 2011, 2014, 2016, 2017).
A basic extension of the previous findings on mucosal damaging effects of both dopamine and NO-system blockades (Bilic et al. 2001; Jelovac et al. 1999; Sikiric et al. 1985, 1986, 1987, 2000; Szabo and Neumeyer 1983; Cesarec et al. 2013; Skorjanec et al. 2015) on sphincter failure (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Jandric et al. 2013; Petrovic et al. 2006, 2011; Sikiric et al. 2010, 2011, 2014, 2016, 2017) would prevail over previous studies that were not related to injurious conditions (Palheta et al. 2014; Braverman et al. 2011;Niedringhaus et al. 2008). In these studies, NO-lower oesophageal sphincter relaxation is based on the effect obtained in intact dogs (Palheta et al. 2014), rats (Braverman et al. 2011) and muscle strips (Niedringhaus et al. 2008). More specifically, our approach (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Jandric et al. 2013; Petrovic et al. 2006, 2011; Sikiric et al. 2010, 2011, 2014, 2016, 2017) favours NO-related lower oesophageal and pyloric sphincter disability in addition to the neighbouring tissue damage; i.e. defects, oesophagitis and even a systemic disturbance such as hyperkalaemia or arrhythmias (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Jandric et al. 2013; Petrovic et al. 2006, 2011). As a novel combination, we would clearly suggest a possible NO-sphincter disability along with a neuroleptic/prokinetic effect. Of note, nitrergic inhibitory neurons of the myenteric plexus mediate inhibition in different parts of the stomach, including the pyloric sphincter (Ramkumar and Schulze 2005), while basal pyloric hypotension with normal nitrergic inhibition predisposes interstitial cells of Cajal (ICC)-deficient mice to duodeno-gastric bile reflux (Sivarao et al. 2008).
Thus, using rat lower oesophageal and pyloric sphincter failure assessments, as previously described (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Petrovic et al. 2006, 2011; Skorjanec et al. 2015), we used a 1-week protocol to investigate whether dopamine antagonists acting centrally/peripherally and/or NOS-blocker l-NAME would induce a pressure decrease within sphincters and whether BPC 157 and/or l-arginine administration would benefit from the decreased pressure within the lower oesophageal and pyloric sphincters in rats. The experimental groups included (i) dopamine blockade (central and/or peripheral); (ii) NOS-blockade; and (iii) blockades combined, dopamine blockade and NOS-blockade (dopamine central blockade + NOS-blockade; dopamine peripheral blockade + NOS-blockade). Dopamine antagonists were given in the regimen known to produce gastric ulcer and/or central disturbances (Bilic et al. 2001; Jelovac et al. 1999; Sikiric et al. 1985, 1986, 1987, 2000). l-NAME was administered in the regimen known to aggravate gastric and oesophageal lesions and/or disabled sphincter functioning (Cesarec et al. 2013; Skorjanec et al. 2015). In addition, NO level was determined in plasma, sphincters and brain tissue. Accordingly, thiobarbituric acid reactive substance (TBARS)-oxidative stress was also assessed (Ohkawa et al. 1979).
Materials and methods
Animals
Wistar Albino male rats (200 g b.w.) were randomly assigned to the experiments (at least 10 animals per experimental group). The study was approved by the Local Ethics Committee at the School of Medicine (University of Zagreb, Zagreb, Croatia). Furthermore, all experiments were carried out under a blind protocol, and the effect was assessed by examiners who were completely unaware of the given protocol.
Drugs
Pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, M.W.1419), (Diagen, Ljubljana, Slovenia) (10 µg/kg or 10 ng/kg) dissolved in saline was used in all experiments. The peptide, BPC 157, is part of the sequence of the human gastric juice protein BPC and is freely soluble in water at pH 7.0 and saline. It was prepared as previously described (Seiwerth et al. 2014; Sikiric et al. 2014; Tkalcevic et al. 2007), with 99% high-pressure liquid chromatography (HPLC) purity, expressing 1-des-Glypeptideasan impurity. Haloperidol, fluphenazine, levomepromazine, chlorpromazine, quetiapine, olanzapine, clozapine, sulpiride, metoclopramide, domperidone, l-NAME and l-arginine (Sigma, USA) were used accordingly (Sikiric et al. 2010, 2011, 2014, 2016, 2017).
Experimental protocol
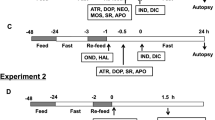
All agents (/kg) were administered intraperitoneally once daily for 7 days, with the last application occurring 24 h before pressure assessment. Neuroleptics (typical, atypical) and prokinetics acting centrally [haloperidol (6.25, 16, 25 mg), fluphenazine (5 mg), levomepromazine (50 mg), chlorpromazine (10 mg), quetiapine (10 mg), olanzapine (5 mg), clozapine (100 mg), sulpiride (160 mg), metoclopramide (25 mg)] or peripherally [domperidone (10 mg)] were administered alone or together with BPC 157 (10 µg or 10 ng). l-NAME (5 mg) and l-arginine (100 mg) were administered alone and/or together, as well as with haloperidol (25 mg), domperidone (10 mg) or BPC 157 (10 µg). To ascertain the procedure assessment and the pressure values for lower oesophageal sphincter and for pyloric sphincter which were considered to be normal as determined previously, healthy rats received an equal volume (5 ml/kg) of saline intraperitoneally once daily for 7 days, with the last application at 24 h before pressure assessment.
Lower oesophageal sphincter pressure and pyloric sphincter pressure assessment
As described previously (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Petrovic et al. 2006, 2011; Skorjanec et al. 2015), manometric evaluation (cm H2O) was performed in all rats, with a water manometer connected to the drainage port of the Foley catheter as described. The values of 68–76 cm H2O for lower oesophageal sphincter and 68–74 cm H2O for pyloric sphincter which were considered to be normal as determined previously were ascertained by saline (5 ml/kg intraperitoneally) given alone once daily for 7 days. The proximal side of the oesophageal incision, or the distal side of the duodenal incision, was ligated to prevent regurgitation (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Petrovic et al. 2006, 2011; Skorjanec et al. 2015).
Nitric oxide determination in plasma, lower oesophageal and pyloric sphincter and brain tissue
We determined nitric oxide (NO) in plasma and tissue samples using the Griess reaction (Griess Reagent System, Promega, USA). Sulphanilamide was added to the homogenized tissue and incubated, after which N-1-naphthylethylenediamine dihydrochloride was added. The Griess reaction is based on the diazotization reaction in which acidified nitrite reacts with diazonium ions and, in a further step, is coupled to N-1-naphthylethylenediamine dihydrochloride, forming chromophoric azo derivate. Absorbance was measured at 540 nm, using sodium nitrite solution as standard. NO levels are reported in µmol/mg protein. Proteins are determined using a commercial kit (BioRad Protein DR Assay Reagent Kit, USA).
TBARS determination in plasma, lower oesophageal and pyloric sphincter and brain tissue
Measurement of TBARS concentration with thiobarbituric acid following the formation of TBARS was performed on tissue samples (Ohkawa et al. 1979). Briefly, tissue homogenate was mixed with a solution containing trichloroacetic acid and incubated to precipitate the proteins. Following centrifugation, thiobarbituric acid was added. The mixture was heated, and the absorbance of the supernatant fraction was determined (532 nm). A standard curve was prepared with serial dilutions of standard 1,1,3,3-tetramethoxypropane. The results are expressed as µmol/mg of protein (determined with bovine serum albumin as standard using BioRad Protein Reagent Kit).
Statistical analysis
Statistical analysis was performed using a parametric two-way mixed model ANOVA (one factor is repeated measures) and Student–Newman–Keuls test to compare the differences between groups. Additionally, a non-parametric Kruskal–Wallis and a post hoc Mann–Whitney U test were used. Fisher’s exact probability test was used to assess the differences in frequencies between groups. P values of 0.05 or less were considered to be statistically significant.
Results
Regarding this sphincter issue and the evidence that a possible neuroleptics–prokinetics/NO-system relationship in vivo has not been studied, we used an additional procedure for ascertaining. The ascertaining protocol (saline intraperitoneally once daily for 7 days in healthy rats) revealed that the values within the values for lower oesophageal sphincter and pyloric sphincter which were considered to be normal as determined before.
Effects of haloperidol, fluphenazine, levomepromazine, chlorpromazine, quetiapine, olanzapine, sulpiride, clozapine, metoclopramide and domperidone
Considering the previously determined normal values in normal rats in the lower oesophageal and pyloric sphincters and, specifically, the values obtained with saline protocol given intraperitoneally in healthy rats once daily for 7 days, all the applied agents, neuroleptics (typical and atypical) and prokinetics (acting centrally and/or peripherally) induced a decrease in pressure within both the lower oesophageal and the pyloric sphincters (Figs. 1, 2, 3, 4, 5), an effect which was not dose related, at least with haloperidol. The pylorus sphincter seemed to be more affected.
Pressure within sphincters after administration of haloperidol alone or with BPC 157. Pressure within lower oesophageal sphincter (LES) (empty bars) and pyloric sphincter (PS) (black bars). Pressure outcome after administration of haloperidol (6.25 mg/kg, 16 mg/kg, 25 mg/kg) and saline (5 ml/kg) or BPC 157 (10 µg/kg, 10 ng/kg) intraperitoneally, once daily through 7 days, with the last application 24 h before pressure assessment. Saline (5 ml/kg) administered intraperitoneally once daily for 7 days in healthy rats, with the last application 24 h before pressure assessment, revealed the values (cm H2O) of 72 ± 2 for lower esophageal sphincter and 70 ± 3 for the pyloric sphincter. The values were within the values which were considered to be normal as determined before. *P < 0.05 at least, vs. haloperidol + saline; ΔP < 0.05 at least, vs. saline in healthy
Pressure within sphincters after administration of chlorpromazine, levomepromazine and fluphenazine alone or with BPC 157. Pressure within the lower oesophageal sphincter (LES) (empty bars) and pyloric sphincter (PS) (black bars). Pressure outcome after administration of dopamine antagonist (DA-ANTAG), chlorpromazine (10 mg/kg), levomepromazine(50 mg/kg), fluphenazine (5 mg/kg) and saline (5 ml/kg) or BPC 157 (10 µg/kg, 10 ng/kg) intraperitoneally, once daily through 7 days, with the last application 24 h before pressure assessment. Saline (5 ml/kg) administered intraperitoneally once daily for 7 days in healthy rats, with the last application 24 h before pressure assessment, revealed the values (cm H2O) of 72 ± 2 for lower oesophageal sphincter and 70 ± 3 for pyloric sphincter. The values were within the values which were considered to be normal as determined before. *P < 0.05 at least, vs. dopamine antagonist + saline; ΔP < 0.05 at least, vs. saline in healthy
Pressure within sphincters after administration of clozapine, olanzapine, quetiapine alone or with BPC 157. Pressure within lower oesophageal sphincter (LES) (empty bars) and pyloric sphincter (PS) (black bars). Pressure outcome after administration of dopamine antagonist (DA-ANTAG), clozapine (100 mg/kg), olanzapine (5 mg/kg), quetiapine (10 mg/kg) and saline (5 ml/kg) or BPC 157 (10 µg/kg, 10 ng/kg) intraperitoneally, once daily through 7 days, with the last application 24 h before pressure assessment. Saline administered (5 ml/kg) intraperitoneally once daily for 7 days in healthy rats, with the last application 24 h before pressure assessment, revealed the values (cm H2O) of 72 ± 2 for lower oesophageal sphincter and 70 ± 3 for pyloric sphincter. The values were within the values which were considered to be normal as determined before. *P < 0.05 at least, vs. dopamine antagonist + saline; ΔP < 0.05 at least, vs. saline in healthy
Pressure within sphincters after administration of domperidone, metoclopramide and sulpiride alone or with BPC 157. Pressure within lower oesophageal sphincter (LES) (empty bars) and pyloric sphincter (PS) (black bars). Pressure outcome after administration of dopamine antagonist (DA-ANTAG), domperidone (10 mg/kg), metoclopramide (25 mg/kg) and sulpiride (160 mg/kg) and saline (5 ml/kg) or BPC 157 (10 µg/kg, 10 ng/kg) intraperitoneally, once daily through 7 days, with the last application 24 h before pressure assessment. Saline (5 ml/kg) administered intraperitoneally once daily for 7 days in healthy rats, with the last application 24 h before pressure assessment, revealed the values (cm H2O) of 72 ± 2 for lower oesophageal sphincter and 70 ± 3 for pyloric sphincter. The values were within the values which were considered to be normal as determined before. *P < 0.05 at least, vs. dopamine antagonist + saline; ΔP < 0.05 at least, vs. saline in healthy
Pressure within sphincters after administration of haloperidol, domperidone, l-NAME, l-arginine and BPC 157. Pressure within lower oesophageal sphincter (LES) (empty bars) and pyloric sphincter (PS) (black bars). a Pressure outcome after administration of l-NAME (LN), l-arginine (LA) and/or BPC 157 (b), #P < 0.05 at least, vs. l-NAME; xP < 0.05 at least, vs. l-arginine. b Pressure outcome after administration of dopamine antagonist [haloperidol (H)] acting mostly centrally with NOS-blockade (l-NAME), NOS-substrate (l-arginine) and BPC 157. *P < 0.05 at least, vs. haloperidol, + P < 0.05 at least, vs. haloperidol + l-NAME, &P < 0.05 at least, vs. haloperidol + l-arginine. c Pressure outcome after administration of dopamine antagonist [domperidone (D)] acting mostly peripherally with NOS-blockade (l-NAME), NOS-substrate (l-arginine) and BPC 157. Saline (5 ml/kg) administered intraperitoneally once daily for 7 days in healthy rats, with the last application 24 h before pressure assessment, revealed the values (cm H2O) of 72 ± 2 for lower oesophageal sphincter and 70 ± 3 for pyloric sphincter. The values were within the values which were considered to be normal as determined before. *P < 0.05 at least, vs. domperidone, +P < 0.05 at least, vs. domperidone +l-NAME, &P < 0.05 at least, vs. domperidone +l-arginine; ΔP < 0.05 at least, vs. saline in healthy
Effect of BPC 157 on the effect of haloperidol, fluphenazine, levomepromazine, chlorpromazine, quetiapine, olanzapine, sulpiride, clozapine, metoclopramide and domperidone
The counteracting effect of BPC 157 on pressure decreases in the sphincters rescued the decreased pressure induced by all neuroleptics, metoclopramide and domperidone in both the lower oesophageal and the pyloric sphincters. This may be seen with µg regimens (Fig. s1, 2, 3, 4, 5). The BPC 157 effect included all the increasing doses of haloperidol (Fig. 1).
Effect of l-NAME and l-arginine
Considering the previously determined normal values in normal rats in the lower oesophageal and the pyloric sphincters, given as individual agents, both l-arginine (l-arginine-rats) and l-NAME (l-NAME-rats) produced a decrease in both lower oesophageal and pyloric sphincter pressures, which were probably distinct and NO-specific effects (l-arginine produced a small decrease, while l-NAME induced a more prominent decrease) because they attenuated each other’s responses when combined (l-NAME + l-arginine-rats) (Fig. 5).
Effect of BPC 157 on the effect of l-NAME and on the effect of l-arginine
Decreased values in rats administered with l-arginine (l-arginine-rats) and more greatly decreased values in rats administered with l-NAME (l-NAME-rats) were increased with the addition of BPC 157, but not over normal values (l-arginine + BPC 157-rats; l-NAME + BPC 157-rats; l-NAME + l-arginine + BPC 157-rats) (Fig. 5).
Haloperidol and l-NAME and l-arginine
In rats administered haloperidol, l-NAME led to an additional decrease in pressure for both the lower oesophageal and the pyloric sphincters. l-arginine rescued only the lower oesophageal sphincter. In rats administered haloperidol and l-NAME (haloperidol + l-NAME-rats), l-arginine rescued both the lower oesophageal and pyloric sphincters (haloperidol + l-NAME + l-arginine-rats) (Fig. 5).
BPC 157
A consistent increase in both lower oesophageal and pyloric sphincter pressure resulted from the addition of BPC 157 to rats administered haloperidol and l-NAME (haloperidol + l-NAME-rats) or l-arginine (haloperidol + l-arginine-rats) and l-NAME and l-arginine (haloperidol + l-NAME + l-arginine-rats). This BPC 157 treatment brought the pressure values in those rats close to normal values (haloperidol + l-NAME + BPC 157-rats; haloperidol + l-arginine + BPC 157 rats haloperidol + l-NAME + l-arginine + BPC 157-rats) (Fig. 5).
Domperidone and l-NAME and l-arginine
In rats administered domperidone presenting with already decreased pressure values in both sphincters, l-NAME additionally decreased values in the pyloric sphincter, and l-arginine showed no rescuing effects on its own. However, in rats administered with domperidone and l-NAME, l-arginine administration reversed the pylorus pressure (domperidone + l-NAME + l-arginine-rats) to the values found in rats administered only domperidone (Fig. 5).
BPC 157
The addition of BPC 157 was consistently effective in increasing the pressure values. Rats administered domperidone and l-NAME (domperidone + l-NAME-rats) or l-arginine (domperidone + l-arginine-rats) and l-NAME and l-arginine (domperidone + l-NAME + l-arginine-rats) exhibited a consistent increase in both lower oesophageal and pyloric sphincter pressure with the addition of BPC 157. The pressure values in those rats were close to normal values (haloperidol + l-NAME + BPC 157-rats; haloperidol + l-arginine + BPC 157-rats haloperidol + l-NAME + l-arginine + BPC 157-rats) (Fig. 5).
In summary, we noted decreases in lower oesophageal and pyloric sphincter pressure as a common effect of all neuroleptics, metoclopramide, domperidone and rescue, which was dose dependent with BPC 157. Interestingly, while a decrease in pressure appeared in the lower oesophageal and pyloric sphincter pressure with l-arginine and l-NAME, both were rescued with BPC 157.
However, l-NAME augmented the effects of haloperidol on the lower oesophageal and the pyloric sphincters, as well as the effect of domperidone on the pyloric sphincter. l-arginine rescued the lower oesophageal sphincter when haloperidol was administered, but not domperidone, and it interfered with the effect of l-NAME in both haloperidol and domperidone rats. All of these rats exhibited a consistent increase towards normal values in both lower oesophageal and pyloric sphincter pressures with the addition of BPC 157.
NO levels in plasma, lower oesophageal and pyloric sphincter and brain tissue
Haloperidol consistently increased NO values (lower oesophageal and pyloric sphincter, plasma and brain). All these increased values were counteracted by the addition of BPC 157 (Fig. 6).
Nitric oxide levels in different tissues. Nitric oxide levels in lower oesophageal (a) and pyloric (b) sphincter, plasma (c) and brain (d) of control, haloperidol- and haloperidol + BPC 157 (10 µg)-treated rats. Using colorimetric assay, we determined levels of NO. *P < 0.05 versus control group and &P < 0.05 versus haloperidol-treated group
Levels of TBARS in plasma, lower oesophageal and pyloric sphincter and brain tissue
Haloperidol consistently increased TBARS values (lower oesophageal and pyloric sphincter, plasma and brain). All these increased values were counteracted by the addition of BPC 157 (Fig. 7).
TBARS levels in different tissues. TBARS levels in lower oesophageal (a) and pyloric (b) sphicter, plasma (c) and brain (d) of control, haloperidol- and haloperidol + BPC 157 (10 µg)-treated rats. Using colorimetric assay, we determined the levels of TBARS as a measure of lipid peroxidation in the tissue. *P < 0.05 versus control group and &P < 0.05 versus haloperidol-treated group
Discussion
We emphasized various unresolved issues of anti-emetic, neuroleptic applications in both patients and experimental studies (Bilic et al. 2001; Jelovac et al. 1999; Murray-Brown and Dorman 2015; Perkins and Dorman 2009; Grande et al. 1992; Meng et al. 2016; Sikiric et al. 1985, 1986, 1987, 2000; Szabo and Neumeyer 1983). Controlled trials object to the common prescription of haloperidol (Murray-Brown and Dorman 2015; Perkins and Dorman 2009). The ulcerogenic potential of dopamine antagonists (Bilic et al. 2001; Jelovac et al. 1999; Sikiric et al. 1985, 1986, 1987, 2000; Szabo and Neumeyer 1983) was not related to possible sphincter failure, and relationships with the NO-system were not defined [NOS blockade also has ulcerogenic effects (Cesarec et al. 2013; Skorjanec et al. 2015)]. Thus, we studied how dopamine neuroleptics/prokinetics interact with the lower oesophageal and pyloric sphincters along with NO-agents, NOS-blocker, l-NAME, NOS-substrate, l-arginine and stable gastric pentadecapeptide BPC 157 in rats (Sikiric et al. 2010, 2011, 2014, 2016, 2017).
We found the characteristic effect of a common pressure decrease in both sphincters alongside all dopamine antagonists, a probable class effect that was counteracted by BPC 157 administration. Furthermore, the additional involvement of different receptors in the effects of these agents, as mentioned earlier (i.e. metoclopramide vs. olanzapine; haloperidol vs. clozapine vs. quetiapine, etc.), would additionally emphasize the significance of the uniform counteraction obtained by BPC 157 administration.
Most importantly for dopamine/NO-system/BPC 157 relationships in normal and disturbed conditions, NO-agents, l-NAME and l-arginine are distinctive under healthy conditions and in the presentation of dopamine central and peripheral blockade in the lower oesophageal and pyloric sphincters. Given as individual agents in healthy rats, all neuroleptics, metoclopramide, domperidone, l-arginine and l-NAME decreased the pressures within the lower oesophageal and the pyloric sphincters. As a common effect, the decreased pressure was rescued dose-dependently with BPC 157 (also note that l-arginine and l-NAME administered together antagonized each other’s responses). Administered with dopamine blockade, and with haloperidol, l-NAME worsened both lower oesophageal sphincter pressure and pyloric sphincter pressure, while l-arginine ameliorated (lower oesophageal sphincter pressure) and antagonized the effect of l-NAME (lower oesophageal sphincter and pyloric sphincter pressure).With domperidone, l-arginine originally had no effect, while l-NAME worsened pyloric sphincter pressure. This effect was opposed by l-arginine. As mentioned above, all these effects were further reversed towards a stronger beneficial effect, close to normal pressure values, with the addition of BPC 157. In principle, the effects of l-NAME/l-arginine/BPC 157 are in line with the results obtained in other sphincter failure models (Barisic et al. 2013; Cesarec et al. 2013; Skorjanec et al. 2015; Kokot et al. 2016).
This means that, as individual agents, both l-NAME and l-arginine affect sphincter pressure in particular ways, in which each of the effects is specific (i.e. NO-related) as a particular aspect of the NO-system dual (l-NAME vs. l-arginine) role (vs. combination) (for reviews see Moncada et al. 1991; Whittle et al., 1992) and are prepared in relation to the dopamine system in the particular case of the pressure within the lower oesophageal and pyloric sphincters. Namely, given together (l-NAME + l-arginine), they regularly attenuated or antagonized each other’s response. Likewise, the remaining pathology (decreased sphincter pressure) in the l-NAME + l-arginine animals means that other system(s) (i.e. dopamine, central and/or peripheral, BPC 157-system) may function along with the NO-system (previously supposed to be immobilized by the mutual actions of the combination of l-NAME and l-arginine). Thus, with central and peripheral dopamine blockade, l-NAME exhibited worsening effects, and with central dopamine blockade, l-arginine exhibited amelioration (note that, in domperidone rats, l-arginine antagonized the effect of l-NAME but not the effect of domperidone itself).
In practice, for l-NAME/l-arginine, this means two pharmacologically distinct mechanisms with opposite effects on the same signalling NO-pathway confronted with regularly unresolvable failure (dopamine central and peripheral blockade leading to sphincter failure). Additionally, in practice, this means two distinct end points that should both be addressed to achieve the presentation seen in rats administered with BPC 157. Note that, to support the regulation of either of the effects of l-NAME and l-arginine, BPC 157 instantly prevents and reverses l-NAME-induced hypertension, as well as l-arginine-induced hypotension (Sikiric et al. 1997). Likewise, owing to its counteraction of prolonged bleeding and thrombocytopaenia, after amputation and/or anticoagulant application, BPC 157 counteracts both l-NAME (thrombocytopaenia) and l-arginine (prolonged bleeding, thrombocytopaenia) side effects (Stupnisek et al. 2015). Additionally, in a pupil assay, a common l-NAME/l-arginine point was accordingly noted (miosis, l-NAME/l-arginine antagonized each other’s responses) and explained in terms of an interaction with the cholinergic system (Kokot et al. 2016), while BPC 157 counteracts both l-NAME (miosis) and l-arginine (miosis), and, interestingly, BPC 157 also counteracts atropine mydriasis (Kokot et al. 2016).
In practice, for the dopamine/NO-system, this indicates two pharmacologically distinct systems and mechanisms with maintaining effects on the same signalling target (i.e. sphincter) confronted with the same regularly unresolvable, but exaggerated failure (both dopamine and NO-system central and peripheral blockade leading to sphincter failure). Namely, as demonstrated, a further decrease when combined (domperidone and l-NAME or haloperidol and l-NAME) implies that neither of the effects of peripheral or central dopamine blockade or NOS-blockade is maximal, that they could be mutually potentiated and that they are likely to collaborate in the maintenance of the normal function of both the lower oesophageal and the pyloric sphincters.
Therefore, these results should be taken into account with the consistently increased NO levels (lower oesophageal and pyloric sphincter, plasma and brain) by haloperidol. In theory, this may be seen as NO-system compensation for dopamine blockade (and, thereby, is likely to be opposed by l-NAME). As an alternative theory in haloperidol rats, this leads to NO excessive release generated by the inducible isozyme (Luetic et al. 2017). As noted recently in our cyclophosphamide studies (Luetic et al. 2017), this damages the vascular wall and other tissue cells, especially in combination with reactive oxygen intermediates [·NO is likely to rapidly react with ·O2 to form peroxynitrite, which may be directly cytotoxic (Oldham and Bowen 1998)] while failing in endothelial production and resulting in further aggravation by l-NAME, which was inhibited by l-arginine. In addition, TBARS values were also increased, indicating increased lipid peroxidation in plasma, sphincters and brain tissues. Thus, it seems to be a common peripheral and central phenomenon that is consistently produced. These effects were all counteracted by BPC 157 administration. Interestingly, as a possible antioxidant, the pentadecapeptide BPC 157 contains four carboxylic groups, which could all be active in scavenger processes. If reactivated (e.g. by glutathione or enzymatic), the overall activity could be very high. BPC 157 also counteracted other free-radical-induced lesions (Ilic et al. 2010; Luetic et al. 2017; Sikiric et al. 1993).
Illustratively, in an ability to rescue failed sphincters, BPC 157 in normal rats increases lower oesophageal sphincter pressure, but decreases pyloric sphincter pressure (Dobric et al. 2007; Petrovic et al. 2006), thus indicating an anti-reflux effect. Additionally, unlike injurious conditions, it does not affect normal pupil (Kokot et al. 2016) or urethral sphincters (Jandric et al. 2013), thus exhibiting a sphincter-specific and injury-specific effect (Sikiric et al. 2014). The particular success of BPC 157 sphincter rescue therapy includes lower oesophageal and pyloric sphincters when disabled by various procedures [i.e. stretching sphincters with temporal tube insertion (Dobric et al. 2007; Petrovic et al. 2006, 2011), potassium chloride overdose application (Barisic et al. 2013), bile duct ligation-induced pancreatitis (Petrovic et al. 2011) and oesophagocutaneous (Cesarec et al. 2013) and duodenocutaneous fistula creation (Skorjanec et al. 2015)]. Thus, sphincter failure as part of a syndrome could be rescued alongside the rescue of the complete syndrome (Barisic et al. 2013; Cesarec et al. 2013; Dobric et al. 2007; Petrovic et al. 2006, 2011; Skorjanec et al. 2015) [i.e. potassium chloride overdose application also induced hyperkalaemia, hypertension, gastric lesions, arrhythmia and short-term fatal outcomes, aggravated by l-NAME, and all were completely counteracted by BPC 157 administration (Barisic et al. 2013)]. Accordingly, in various models and species (Balenovic et al. 2009, 2012; Barisic et al. 2013; Boban-Blagaic et al. 2006; Cesarec et al. 2013; Grabarevic et al. 1997; Klicek et al. 2008; Sikiric et al. 1997, 2011, 2014; Skorjanec et al. 2015; Stupnisek et al. 2015; Zemba et al. 2015), BPC 157 counteracted l-NAME effects better than l-arginine and induced NO-release in gastric mucosa from rat stomach tissue homogenates, even in conditions in which l-arginine was not working (Sikiric et al. 1997; Turkovic et al. 2004).
In conclusion, the importance of BPC 157 and particular counteractions include attenuated gastrointestinal lesions induced by a peripherally/centrally disabled dopamine system (Bedekovic et al. 2003; Bilic et al. 2001; Petrovic et al. 2006; Sikiric et al. 1999, 2001a, b, 2011), dopamine release overstimulation (Jelovac et al. 1998; Sikiric et al. 2002), dopamine receptor blockade (Jelovac et al. 1999; Sikiric et al. 2000) and dopamine neuron destruction (Sikiric et al. 1999) alongside counteracted akinesia or catalepsy (Jelovac et al. 1999; Sikiric et al. 2000). It also implies that, when BPC 157 is given peripherally, acutely and chronically, the release of the brain’s serotonin (substantia nigra) (Tohyama et al. 2004) and resulting peptides act either centrally or indirectly by binding sites in the gut at some visceral receptive relay of the central nervous system (Aziz and Thompson 1998; Tohyama et al. 2004; Sikiric et al. 2016).
Thus, sphincter failure with dopamine antagonists and/or l-NAME could be a hallmark of ongoing injury (Bedekovic et al. 2003; Jelovac et al. 1998; Petrovic et al. 2006; Sikiric et al. 1999, 2001, 2002) alongside the injurious effects of dopamine antagonists or l-NAME itself (Balenovic et al. 2009, 2012; Barisic et al. 2013; Boban-Blagaic et al. 2006; Grabarevic et al. 1997; Klicek et al. 2008; Sikiric et al. 1997; Stupnisek et al. 2015, Zemba et al. 2015). Similarly, sphincter failure that can be reversed may further implicate the importance of BPC 157 in the corresponding therapy. A further argument is the disability of both sphincters, with a large number of agents acting both centrally (haloperidol, fluphenazine, levomepromazine, chlorpromazine, quetiapine, olanzapine, clozapine and sulpiride) and peripherally (domperidone) (Abi-Dargham 2014; Acosta and Camilleri 2015). This is in accordance with evidence that the induction or aggravation of experimental gastric (Sikiric et al. 1986) or duodenal ulcers (Gallagher et al. 1987) by dopamine antagonists or the therapeutic effects of dopamine agonists (Sikiric et al. 1991) probably involve a peripheral component. In addition to the suggested common dopamine component, the particular activities of the given agent and the distinction between the receptors involved (quetiapine less affects dopamine receptors (Mauri et al. 2014)) might indicate that each of the combinations might be responsible for prolonged sphincter failure.
Finally, this study raised several issues that will require further studies to elucidate. For instance, it is possible that the described effects that cause sphincter failure are different from the effects that may be seen from nausea and vomiting in palliative care, which are commonly ascribed to effects on the vomiting centre, chemoreceptor trigger zone, cerebral cortex and vestibular system as the four main sites of activity (Glare et al. 2011). Similarly, the negative effects on sphincters could probably be overridden by the effects on nausea and vomiting due to effects on the vomiting centre, chemoreceptor trigger zone, cerebral cortex and vestibular system (Glare et al. 2011).
In conclusion, we revealed that BPC 157 counteracts the anti-emetic neuroleptic class side effect of decreased pressure in sphincters and the dopamine/NO-system/BPC 157 relationship.
References
Abi-Dargham A (2014) Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry 75:e31
Acosta A, Camilleri M (2015) Prokinetics in gastroparesis. Gastroenterol Clin North Am 44:97–111
Aziz Q, Thompson DG (1998) Brain-gut axis in health and disease. Gastroenterology 114:559–578
Balenovic D, Bencic ML, Udovicic M et al (2009) Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: a relation with NO-system. Regul Pept 156:83–89
Balenovic D, Barisic I, Prkacin I et al (2012) Mortal furosemide-hypokalemia-disturbances in rats NO-system related. Shorten survival by l-NAME. Therapy benefit with BPC 157 more than with l-arginine. J Clin Exp Cardiolog 3:201
Barisic I, Balenovic D, Klicek R et al (2013) Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept 181:50–66
Bedekovic V, Mise S, Anic T et al (2003) Different effect of antiulcer agents on rat cysteamine-induced duodenal ulcer after sialoadenectomy, but not gastrectomy. Eur J Pharmacol 477:73–80
Bilic I, Zoricic I, Anic T et al (2001) Haloperidol-stomach lesions attenuation by pentadecapeptide BPC 157, omeprazole, bromocriptine, but not atropine, lansoprazole, pantoprazole, ranitidine, cimetidine and misoprostol in mice. Life Sci 68(16):1905–1912
Boban-Blagaic A, Blagaic V, Romic Z et al (2006) The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. The effect of N(G)-nitro-l-arginine methyl ester and l-arginine. Med Sci Monit 12(1):36–45
Braverman AS, Vegesna AK, Miller LS et al (2011) Pharmacologic specificity of nicotinic receptor-mediated relaxation of muscarinic receptor precontracted human gastric clasp and sling muscle fibers within the gastroesophageal junction. J Pharmacol Exp Ther 338:37–46
Cesarec V, Becejac T, Misic M et al (2013) Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol 701(1–3):203–212
Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH (2011) The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol 110(3):774–780
Chang CH, Tsai WC, Hsu YH, Pang JH (2014) Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 19(11):19066–19077
Costa MA, Elesgaray R, Loria A, Balaszczuk AM, Arranz C (2006) Vascular and renal effects of dopamine during extracellular volume expansion: role of nitric oxide pathway. Life Sci 78(14):1543–1549
Del Bel EA, Guimarães FS, Bermúdez-Echeverry M et al (2005) Role of nitric oxide on motor behavior. Cell Mol Neurobiol 25(2):371–392
Dobric I, Drvis P, Petrovic I et al (2007) Prolonged esophagitis after primary dysfunction of the pyloric sphincter in the rat and therapeutic potential of the gastric pentadecapeptide BPC 157. J Pharmacol Sci 104:7–18
Gallagher G, Brown A, Szabo S (1987) Effect of dopamine-related drugs on duodenal ulcer induced by cysteamine or propionitrile: prevention and aggravation may not be mediated by gastrointestinal secretory changes in the rat. J Pharmacol Exp Ther 240(3):883–889
Glare P, Miller J, Nikolova T, Tickoo R (2011) Treating nausea and vomiting in palliative care: a review. Clin Interv Aging 6:243–259
Grabarevic Z, Tisljar M, Artukovic B et al (1997) The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J Physiol Paris 91(3–5):139–149
Grande L, Lacima G, Ros E et al (1992) Lack of effect of metoclopramide and domperidone on esophageal peristalsis and esophageal acid clearance in reflux esophagitis. Digest Dis Sci 37(4):583–588
Hsieh MJ, Liu HT, Wang CN et al (2017) Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl) 95(3):323–333
Huang T, Zhang K, Sun L et al (2015) Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther 9:2485–2499
Ilic S, Drmic D, Zarkovic K et al (2010) High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol 61:241–250
Jandric I, Vrcic H, Jandric Balen M et al (2013) Salutary effect of gastric pentadecapeptide BPC 157 in two different stress urinary incontinence models in female rats. Med Sci Monit Basic Res 19:93–102
Jelovac N, Sikiric P, Rucman R et al (1998) A novel pentadecapeptide, BPC 157, blocks the stereotypy produced acutely by amphetamine and the development of haloperidol-induced supersensitivity to amphetamine. Biol Psychiatry 43:511–519
Jelovac N, Sikiric P, Rucman R et al (1999) Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: the effect on catalepsy and gastric ulcers in mice and rats. Eur J Pharmacol 379:19–31
Klicek R, Sever M, Radic B et al (2008) Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: role of the nitric oxide-system. J Pharmacol Sci 108:7–17
Klicek R, Kolenc D, Suran J et al (2013) Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon–colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol 64:597–612
Kokot A, Zlatar M, Stupnisek M et al (2016) NO system dependence of atropine-induced mydriasis and l-NAME- and l-arginine-induced miosis: reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol 771:211–219
Luetic K, Sucic M, Vlainic J et al (2017) Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: l-NAME, l-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology 25:255–264
Mauri MC, Paletta S, Maffini M et al (2014) Clinical pharmacology of atypical antipsychotics: an update. EXCLI J 13:1163–1191
Meng LN, Chen S, Chen JD, Jin HF, Lu B (2016) Effects of Transcutaneous Electrical Acustimulation on Refractory Gastroesophageal Reflux Disease. Evid Based Complement Altern Med 2016:8246171
Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology and pharmacology. Pharmacol Rev 43:109–142
Murray-Brown F, Dorman S (2015) Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev 11:CD006271
Niedringhaus M, Jackson PG, Evans SR, Verbalis JG, Gillis RA, Sahibzada N (2008) Dorsal motor nucleus of the vagus: a site for evoking simultaneous changes in crural diaphragm activity, lower esophageal sphincter pressure, and fundus tone. Am J Physiol Regul Integr Comp Physiol 294:R121–R131
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oldham KM, Bowen PE (1998) Oxidative stress in critical care: is antioxidant supplementation beneficial? J Am Diet Assoc 98:1001–1008
Palheta MS, Graça JR, Santos AA et al (2014) The participation of the nitrergic pathway in increased rate of transitory relaxation of lower esophageal sphincter induced by rectal distension in dogs. Arq Gastroenterol 51:102–106
Paré WP, Glavin GB (1986) Restraint stress in biomedical research: a review. Neurosci Biobehav Rev 10:339–370
Perkins P, Dorman S (2009) Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev 2:CD006271
Petrovic I, Dobric I, Drvis P et al (2006) An experimental model of prolonged esophagitis with sphincter failure in the rat and the therapeutic potential of gastric pentadecapeptide BPC 157. J Pharmacol Sci 102:269–277
Petrovic I, Dobric I, Drmic D et al (2011) BPC 157 therapy to detriment sphincters failure-esophagitis-pancreatitis in rat and acute pancreatitis patients low sphincters pressure. J Physiol Pharmacol 62:527–534
Ramkumar D, Schulze KS (2005) The pylorus. Neurogastroenterol Motil 17(Suppl 1):22–30
Seiwerth S, Brcic L, Batelja Vuletic L et al (2014) BPC 157 and blood vessels. Curr Pharm Des 20:1121–1125
Sikiric P, Geber J, Suchanek E et al (1985) The role of dopamine in the formation of gastric ulcers in rats. Eur J Pharmacol 112:127–128
Sikiric P, Geber J, Ivanovic D et al (1986) Dopamine antagonists induce gastric lesions in rats. Eur J Pharmacol 131:105–109
Sikiric P, Rotkvic I, Mise S et al (1987) The influence of dopamine agonists and antagonists on gastric lesions in mice. Eur J Pharmacol 144:237–239
Sikiric P, Rotkvic I, Mise S et al (1991) Dopamine agonists prevent duodenal ulcer relapse. A comparative study with famotidine and cimetidine. Dig Dis Sci 36:905–910
Sikiric P, Seiwerth S, Grabarevic Z et al (1993) Hepatoprotective effect of BPC 157, a 15-amino acid peptide, on liver lesions induced by either restraint stress or bile duct and hepatic artery ligation or CCl4 administration. A comparative study with dopamine agonists and somatostatin. Life Sci 53:PL291–PL296
Sikiric P, Seiwerth S, Grabarevic Z et al (1997) The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-l-arginine methylester and l-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol 332:23–33
Sikiric P, Marovic A, Matoz W et al (1999) A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson’s disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J Physiol Paris 93:505–512
Sikiric P, Separovic J, Buljat G et al (2000) Gastric mucosal lesions induced by complete dopamine system failure in rats. The effects of dopamine agents, ranitidine, atropine, omeprazole and pentadecapeptide BPC 157. J Physiol Paris 94:105–110
Sikiric P, Seiwerth S, Grabarevic Z et al (2001a) Cysteamine-colon and cysteamine-duodenum lesions in rats. Attenuation by gastric pentadecapeptide BPC 157, cimetidine, ranitidine, atropine, omeprazole, sulphasalazine and methylprednisolone. J Physiol Paris 95:261–270
Sikiric P, Seiwerth S, Aralica G et al (2001b) Therapy effect of antiulcer agents on new chronic cysteamine colon lesion in rat. J Physiol Paris 95:283–288
Sikiric P, Jelovac N, Jelovac-Gjeldum A et al (2002) Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances. Acta Pharmacol Sin 23:412–422
Sikiric P, Seiwerth S, Brcic L et al (2010) Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des 16:1224–1234
Sikiric P, Seiwerth S, Rucman R et al (2011) Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des 17:1612–1632
Sikiric P, Seiwerth S, Rucman R et al (2014) Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des 20:1126–1135
Sikiric P, Seiwerth S, Rucman R et al (2016) Brain-gut axis and pentadecapeptide BPC 157. Theoretical and practical implications. Curr Neuropharmacol 14(8):857–865
Sikiric P, Seiwerth S, Rucman R et al (2017) Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr Pharm Des. doi:10.2174/1381612823666170220163219
Sivarao DV, Mashimo H, Goyal RK (2008) Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology 135:1258–1266
Skorjanec S, Kokot A, Drmic D et al (2015) Duodenocutaneous fistula in rats as a model for “wound healing-therapy” in ulcer healing: the effect of pentadecapeptide BPC 157, L-nitro-arginine methyl ester and l-arginine. J Physiol Pharmacol 66:581–590
Stupnisek M, Kokot A, Drmic D et al (2015) Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin l-NAME and l-arginine. PLoS ONE 10(4):e0123454
Szabo S, Neumeyer JL (1983) Dopamine agonists and antagonists in duodenal ulcer disease. In: Kaiser C, Kebabian JW (eds) Dopamine Receptors, ACS Symposium Series 224. Am Chem Soc Publ, Washington, pp 175–196
Szabo S, Horner HC, Maull H, Schnoor J, Chiueh CC, Palkovits M (1987) Biochemical changes in tissue catecholamines and serotonin in duodenal ulceration caused by cysteamine or propionitrile in the rat. J Pharmacol Exp Ther 240:871–878
Tkalcevic VI, Cuzic S, Brajsa K et al (2007) Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol 570:212–221
Tohyama Y, Sikirić P, Diksic M (2004) Effects of pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: alpha-methyl-l-tryptophan autoradiographic measurements. Life Sci 76:345–357
Turkovic B, Sikiric P, Seiwerth S et al (2004) Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 126:287
Whittle BJR, Boughton-Smith NK, Moncada S (1992) Biosynthesis and role of the endothelium-derived vasodilator, nitric oxide in gastric mucosa. Ann NY Acad Sci 664:126–139
Zemba M, Cilic AZ, Balenovic I et al (2015) BPC 157 antagonized the general anaesthetic potency of thiopental and reduced prolongation of anaesthesia induced by l-NAME/thiopental combination. Inflammopharmacology 23:329–336
Acknowledgements
This research was supported by the Ministry of Science, Education and Sports, Republic of Croatia (Grant Number 108-1083570-3635).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belosic Halle, Z., Vlainic, J., Drmic, D. et al. Class side effects: decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, l-NAME, pentadecapeptide BPC 157 and l-arginine. Inflammopharmacol 25, 511–522 (2017). https://doi.org/10.1007/s10787-017-0358-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0358-8