Abstract
Introduction
Pelvic schwannomas are rare, mostly benign tumors. They are usually asymptomatic until their massive growth compresses adjacent organs. We describe the case of a 53-year-old man with a pelvic schwannoma who initially complained of constipation and urinary retention.
Areas Covered
We analyzed the clinical presentation, histopathology, diagnostic imaging tools, and the treatment options for pelvic schwannomas, compared with the few other cases reported in the literature.
Expert Commentary
Pelvic schwannomas are masses that can grow to considerable size, producing symptoms over time. Due to their size and localization, surgery, although difficult, is the only available treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Case Presentation
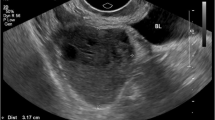
A 53-year-old man complained of mostly nocturnal constipation and urinary retention. The family doctor attributed the former to probable IBS-C, for which he was told to take laxatives when needed. For the latter, BPH was suspected, but not confirmed by rectal exploration. Since these symptoms worsened over the ensuing months, he obtained an abdominal US at the local hospital that showed the bladder displaced by a large, non-homogeneous mass with many hypoechoic areas and a few calcifications. A CT scan of the abdomen (Fig. 1A) with contrast confirmed the presence of an ovoid mass in the pelvic area of considerable size (11.3 cm × 12.2 cm × 13.6 cm) anterior to the sacrum that extended to the lesser pelvic cavity and the obturator fossa. The descending colon was dilated up to 8 cm, and the bladder was displaced cranially. The parenchyma was homogeneous, with a few calcifications noted. The mass was firmly adhered to the anterior sacral surface, compressing the rectosigmoid junction and the bladder, likely explaining the patient’s constipation and urinary retention. The CT characteristics of the mass were thought to be consistent with a peripheral schwannoma, probably originating from the root of the sacral plexus. A CT-guided biopsy (Fig. 1B) was performed in order to confirm this diagnosis.
Histologically, the tumor was composed of fascicles of spindle-shaped cells with elongated tapering nuclei and eosinophilic cytoplasm with indistinct cell borders. The tumor showed variable cellularity (Fig. 2A), with cellular areas (Fig. 2B) and hyalinized thick-walled vessels characteristic of schwannomas. Focal degenerative nuclear atypia and mitotic activity were present. By immunohistochemistry, tumor cells expressed S-100 protein with a strong and diffuse nuclear and cytoplasmic staining (Fig. 2C). The cytomorphologic features combined with a strong expression of S-100 protein and the absence of human melanoma black monoclonal (HMB45) and murine double minute type 2 (MDM2) positivity confirmed the diagnosis of cellular schwannoma as distinguished from spindle cell melanoma and dedifferentiated liposarcoma. The tumor was also KIT negative, excluding the diagnosis of gastrointestinal stromal tumor (GIST). The proliferation marker Kiel (ki)-67 stained 8% of cells.
a–c Tumor histology. a Low power view of the tumor, displaying irregular fascicles of spindle cells (red arrow) with palisading (Antoni type A) and hypocellular myxoid areas (green arrow) (Antoni type B), stained by HE (× 4). b Higher power showing spindle-shaped cells stained by HE (× 20). c Immunochemical examination. The tumor cell showed a diffuse staining for S100 protein (× 10)
A PET scan confirmed the presence of the pelvic mass with increased FDG uptake, consistent with its heteroplastic nature. The MRI clarified the relationship of the mass with other structures—it was limited superiorly by the sacral margin, caudally by the coccyx, posteriorly by the anterior sacral surface, anteriorly by the pubic symphysis (compressing the rectosigmoid junction and bladder), and more dislocated on the right to its probable origin. The mass was iso-intense or modestly hypointense in T1 and had large areas of hypersignal in T2.
The patient was admitted in the hospital for surgical tumor treatment. The excision of the mass was challenging due to its large size and its location in a very narrow male pelvis. After the radical removal of the tumor attached to the pre-sacral fascia (Fig. 3), ongoing bleeding of the sacral vessels was observed, requiring packing of the pelvis. The patient was transfused with two units of blood intraoperatively and an additional seven in the ICU. Two days later, the patient was brought back to the operating room and the packing was removed with completed hemostasis. Afterward, due to the appearance of pulmonary consolidation, the patient was treated successfully by antibiotic therapy. The patient was discharged on the 14th postoperative day in good clinical condition.
Discussion
Schwannomas are neoplastic lesions composed of Schwann cells, benign in 97–98% of reported cases [1, 2]. Malignant tumors are usually associated with neurofibromatosis (Von Recklinghausen’s disease) [3]. Due to their benignity, they can reach considerable size prior to detection, which usually occurs after they are large enough to compress adjacent organs, producing a variety of symptoms. A recent Australian case study [4] describes a patient who was followed for 5 years, reporting very slow growth, without malignant transformation.
Pelvic schwannomas are considered to be rare tumors (1/40,000 hospitalizations) [5, 6]. The majority are located in the thorax, abdomen, and head [7]. Rarely, they occur in the pelvis, with an estimated prevalence of 0.3%–3.2% among all schwannoma locations and 0.4%–15% among all retrorectal tumors [6]. Usually, they are masses of small size (< 5–6 cm). Giant schwannomas (> 100 cm3) have been reported rarely [2, 3, 8,9,10,11,12,13,14]. Table 1 describes the main findings obtained from case reports and case series identified by a PubMed™ search spanning the last 25 years (the “CT scan era”) in the English language [15,16,17,18,19,20,21,22,23,24,25,26,27,28].
According to Klimo et al. [29], sacral schwannomas can be subdivided into three categories: Type I tumors are limited to the sacrum; type II originate from the sacrum but also expand to pre-sacral or subcutaneous space; and type III are located in the pelvis or retroperitoneum. Other masses can, however, also arise in the pelvic cavity, distant from the sacrum. Our case report describes a giant pelvic mass that, since it was completely pre-sacral, can be classified as Klimo III. Considering that our patient started to experience constipation and urinary retention one year before his admission to our department, our case confirms the slow growth of these tumors. Unlike the majority of cases of giant pelvic schwannomas [6], our patient had neither neurological symptoms nor pain radiating to the thigh, likely due to the lack of obvious compression of the sacral nerves.
In our case, the initial detection of the pelvic mass was obtained by ultrasound that showed a hypoechoic lesion with a few calcifications and without cystic areas. Although ultrasound is generally the first imaging technique performed, it cannot be used to specifically diagnose schwannomas. CT scan can give more information—in this case the mass was large, solitary with an ovoid or round that did not infiltrate the nearby organs [10]. In 66% of cases, CT scan can detect cysts [1] although our patient did not have any detectable cysts, but rather calcifications, which are also common in schwannomas [30].
MRI is the optimal imaging tool for these kinds of tumors, since it is able also to show its possible origin, vascularization, and invasiveness [31]. Schwannomas are usually characterized by the association of a T1 iso-signal in the adjacent skeletal muscle, with a T2 hypersignal which is more intense where there are few cells. It is also possible in this manner to differentiate Antoni type A from type B (a histopathological classification described later), since, by definition, the latter has more matrix, thus showing a more intense signal [11]. MRI is also useful to define the relationship with other pelvic organs (sacrum, rectum, and bladder), which is important for surgical procedural planning.
An uncomplicated CT-guided biopsy was performed in our patient to definitively diagnose the mass. A recent meta-analysis conducted among 358 biopsies [32] shows that this procedure is associated with very low early and late complication rates (3.1% and 0.5%, respectively). This study did not support the assertion that such biopsies are complicated by hemorrhage, tumoral seeding, or infections or are the subject to misdiagnosis due to tumor pleomorphism. Needle tract seeding was only reported in one case. Sarcomas were differentiated from benign entities in 98% of cases, high-grade from low-grade lesions in 86% of cases, and an accurate tumor subtype was identified in 86% of cases. This information, when available prior to surgery, can inform surgical strategy. In summary, due to the absence of pathognomonic features, the diagnosis of schwannoma rests on the use of multiple imaging modalities and histological markers, as was used in this case [10].
Histopathologically, schwannomas present two types of cellular organization, called Antoni type A and Antoni type B. Type A is characterized by highly organized patterns, with elongated compact cells, whereas B types are more disorganized with sparse cells [8]. Immunohistochemically schwannomas are intensely stained with S-100 protein, vimentin, and neuron specific enolase, but not by smooth muscle actin (SMA) or CD117 [33]. The biopsy report of our patient showed these findings, confirming the diagnosis of mixed-type (Antoni type A and B) schwannoma. Moreover, SOX10 was positive as is characteristic of tumors derived from cells differentiated from the neural crest such as Schwann cells [34].
As far as surgical technique is concerned, with respect to partial resection, total resection is significantly associated with improved survival [1, 6]. Moreover, piecemeal resection instead of en bloc removal was considered a superior approach when the lesion is extended above the S3 level, in order to reduce the risk of damaging the sacral nerves, since the rate of recurrence is statistically similar between the two methods [1, 6]. Of the surgical approaches used for Klimo type III lesions (as was used for the case described), the anterior approach is preferred [6]. Some authors reported that outcomes were similar between laparoscopic and open surgical resections, with the caveat that the laparoscopic approach should be reserved for smaller tumors [35, 36].
In conclusion, schwannomas are mostly benign and are difficult to diagnose prior to the symptomatic stage when organ compression occurs. Since a definitive diagnosis cannot be made by imaging alone, tumor biopsies should be performed. Even if malignant transformation is rare, surgery should be considered as soon as possible, before the appearance of serious organ compression symptoms.
Key Messages
-
Schwannomas are usually benign tumors. They are asymptomatic until they gradually compress surrounding structures.
-
In the presence of a large, ovoid, smooth, and noninvasive pelvic mass, a diagnosis of schwannoma should be considered.
-
Surgery should be performed as early as possible, before the tumor compresses surrounding organs.
Abbreviations
- IBS-C:
-
Irritable bowel syndrome—constipation predominant
- BPH:
-
Benign prostatic hyperplasia
- CT:
-
Computerized
- FDG:
-
Fludeoxyglucose (18F)
- PET:
-
Positron emission tomography
- OR:
-
Operating room
- US:
-
Ultrasonography
- ICU:
-
Intensive care unit
- CD:
-
Cluster of differentiation
- SOX10:
-
SRY-related HMG-box protein 10
- HMB45:
-
Human melanoma black monoclonal antibody 45
- MDM2:
-
Mouse double minute 2 homolog
- ki-67:
-
Kiel 67 protein
- GIST:
-
Gastrointestinal stromal tumors
- MRI:
-
Magnetic resonance imaging
References
Li Q, Gao C, Juzi JT, et al. Analysis of 82 cases of retroperitoneal schwannoma. ANZ J Surg. 2007;77:237–240.
Habek D, Habek JC, Sindik N. Pelvic schwannoma. Eur J Obstet Gynecol Reprod Biol. 2004;116:248–249.
Gullo R, Zoccali M, Frim DM, et al. A large pelvic mass in a 39-year-old man. Updates Surg. 2011;63:293–296.
Tong RS, Collier N, Kaye AH. Chronic sciatica secondary to retroperitoneal pelvic schwannoma. J Clin Neurosci. 2003;10:108–111.
Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246–255.
Pennington Z, Westbroek EM, Ahmed AK, et al. Surgical management of giant presacral schwannoma: systematic review of published cases and meta-analysis. J Neurosurg Spine 2019 Jul 5 [Epub ahead of print].
Handa K, Ozawa H, Aizawa T, et al. Surgical management of Giant Sacral schwannoma: a case series and literature review. World Neurosurg. 2019;129:e216–e223.
Hide IG, Baudouin CJ, Murray SA, et al. Giant ancient schwannoma of the pelvis. Skelet Radiol. 2000;29:538–542.
Pongsthorn C, Ozawa H, Aizawa T, et al. Giant sacral schwannoma: a report of six cases. Ups J Med Sci. 2010;115:146–152.
Hoarau N, Slim K, Da Ines D. CT and MR imaging of retroperitoneal schwannoma. Diagn Interv Imag. 2013;94:1133–1139.
Zhou M, Chen K, Wu C, et al. Giant sacral schwannoma with pelvic and lumbar spine extension. Spine J. 2013;13:1154–1155.
Xu H, Sha N, Li HW, et al. A giant pelvic malignant schwannoma: a case report and literature review. Int J Clin Exp Pathol. 2015;8:15363–15368.
Togral G, Arikan M, Hasturk AE, et al. Incidentally diagnosed giant invasive sacral schwannoma. Its clinical features and surgical management without stability. Neurosciences (Riyadh). 2014;19:224–228.
Khan UA, Ismayl G, Malik I. Giant Sacral schwannoma treated with a 360 approach: A rare case and systematic review of the literature. World Neurosurg. 2018;115:65–72.
Domínguez J, Lobato RD, Ramos A, et al. Giant intrasacral schwannomas: report of six cases. Acta Neurochir (Wien). 1997;139:954–960.
Surendrababu NR, Cherian SR, Janakiraman R, et al. Large retroperitoneal schwannoma mimicking a cystic ovarian mass in a patient with Hansen’s disease. J Clin Ultrasound. 2008;36:318–320.
Choudry HA, Nikfarjam M, Liang JJ, et al. Diagnosis and management of retroperitoneal ancient schwannomas. World J Surg Oncol. 2009;7:12.
Samarakoon L, Weerasekera A, Sanjeewa R, et al. Giant presacral schwannoma presenting with constipation: a case report. J Med Case Rep. 2012;6:285.
Park NY, Chong GO, Lee YS. Laparoscopic resection of schwannoma in the anomaly of obturator nerve. J Laparoendosc Adv Surg Tech A.. 2007;17:769–773.
Giona S, Oderda M, Garbossa D, et al. Laparoscopic management of sacral neurinoma causing hydronephrosis. Urologia. 2012;79:107–110.
De Sousa A, Frydenberg M. Giant obturator schwannoma: an unusual finding in a patient with prostate cancer. ANZ J Surg. 2012;82:853–854.
Labedz W, Kubaszewski L, Adamek J. Operative treatment of schwannoma, the primary sacral tumor— case presentation. Pol Orthop Traumatol. 2012;77:10–16.
Ningshu L, Min Y, Xieqiao Y, et al. Laparoscopic management of obturator nerve schwannomas: experiences with 6 cases and review of the literature. Surg Laparosc Endosc Percutan Tech. 2012;22:143–147.
Machairiotis N, Zarogoulidis P, Stylianaki A, et al. Pelvic schwannoma in the right parametrium. Int J Gen Med. 2013;6:123–126.
Mazzola CR, Power N, Bilsky MH, et al. Pudendal schwannoma: a case report and literature review. Can Urol Assoc J. 2014;8:e199–e203.
Fris TL, Friis-Andersen H. Benign sacral schwannomas—a case and short review of the literature. Br J Neurosurg. 2015;29:595–596.
Takahashi H, Hara M, Tsuboi K, et al. Laparoscopically resected obturator nerve schwannoma: a case report. Asian J Endosc Surg. 2016;9:307–310.
Padmanaban N, Chandrabose PS, Esakki M, et al. Gynaecological perspective of schwannoma: a rare pelvic tumour. J Clin Diagn Res. 2016;10:QD03–QD05.
Klimo P Jr, Rao G, Schmidt RH, et al. Nerve sheath tumors involving the sacrum. Case report and classification scheme. Neurosurg Focus. 2003;15:12.
Kinoshita T, Naganuma H, Ishii K, et al. CT features of retroperitoneal neurilemmoma. Eur J Radiol. 1998;27:67–71.
Goh BK, Tan YM, Chung YF, et al. Retroperitoneal schwannoma. Am J Surg. 2006;192:14–18.
Berger-Richardson D, Burtenshaw SM, Ibrahim AM, et al. Early and late complications of percutaneous core needle biopsy of retroperitoneal tumors at two tertiary sarcoma centers. Ann Surg Oncol 2019 Aug 1. [Epub ahead of print].
Strauss DC, Qureshi YA, Hayes AJ, et al. Management of benign retroperitoneal schwannomas: a single-center experience. Am J Surg. 2011;202:194–198.
Karamchandani JR, Nielsen TO, Van de Rijn M, et al. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445–450.
Di Furia M, Salvatorelli A, Della Penna A, et al. Advantage of laparoscopic resection for pelvic schwannoma: Case report and review of the literature. Int J Surg Case Rep. 2018;45:38–41.
Chopra S, Dharmaraja A, Satkunasivam R, et al. Robot-assisted laparoscopic resection of a pelvic schwannoma. Urol Case Rep. 2017;11:63–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest pertaining to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Colecchia, L., Lauro, A., Vaccari, S. et al. Giant Pelvic Schwannoma: Case Report and Review of the Literature. Dig Dis Sci 65, 1315–1320 (2020). https://doi.org/10.1007/s10620-020-06128-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06128-2