Abstract
Pelvic schwannomas are rare tumors that may occur either sporadically or in the context of schwannomatosis. We retrospectively reviewed the charts of patients harboring a pelvic schwannoma under conservative management or operated at our reference center between 2016 and 2023. All patients were operated by a multidisciplinary team, combining a vascular surgeon and a neurosurgeon. Twenty-four patients harboring 33 pelvic tumors were included in the cohort, including 12 patients with sporadic lesions, 2 patients with NF2-related schwannomatosis, and 10 patients with NF2-independent schwannomatosis. Multi-nodular tumors were more frequent in schwannomatosis compared to sporadic cases (p = 0.005). The mean age at diagnosis was 41 years old. Schwannomas were located on branches of the sciatic nerve (23/33, 70%), the femoral nerve (6/33, 18%), and the obturator nerve (4/33, 12%). Over the course of the study, 16 patients were operated, including 11 sporadic cases. The indication for surgery was pain (12/16, 75%) or tumor growth (4/16, 25%). Complete resection was achieved in 14 of 16 patients (87%). The mean post-operative follow-up was 37 months (range: 2–168 months). At last-follow-up, complete pain relief was achieved in all 12 patients with pre-operative pain. Post-operative morbidity included 3 long-term localized numbness and one MRC class 4 motor deficit in a multi-nodular tumor in a schwannomatosis patient. Despite its limited size, our series suggests that nerve-sparing resection of pelvic schwannomas offers satisfying rates of functional outcome both in sporadic and schwannomatosis cases, except for multi-nodular tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic schwannomas are rare tumors that may occur either sporadically or in the context of Schwannomatosis [18]. They represent only 0.3–3.2% of all schwannomas and 0.4–15% of all retrorectal tumors [16]. Despite their rarity, pelvic schwannomas drew the attention of neurosurgeons and general surgeons alike with the publication of several cohorts of patients [3, 5,6,7, 13, 16, 21, 22]. While most series published by general surgeons have focused on the extent of resection and disease-free survival, fewer series have highlighted the functional results of this surgery on pain and sensory symptoms. As pelvic schwannomas are indeed treated by surgeons from a variety of disciplines including visceral surgery, oncologic surgery, and neurosurgery, these studies have also highlighted two different strategies in the surgical management of pelvic schwannomas: either aggressive en bloc resection advocated by general surgeons [22] in the context of a possibly more aggressive tumor or nerve-sparing tumor removal advocated by neurosurgeons [6]. As these two strategies have led to variable reported rates of post-operative recurrence and surgical complications, mostly based on small surgical series, the ideal management of pelvic schwannomas remains to be defined.
As a tertiary care reference center for Schwannomatosis, we wanted to evaluate the results of surgery in this specific clinical setting, as concerns have raised on the morbidity of surgery in peripheral nerve lesions of patients harboring genetic tumor-predisposing syndromes [1, 10]. Pelvic schwannomas have been reported in schwannomatosis, either as single case reports [2, 8] or in surgical series [13, 22], but without a separate analysis describing the specific clinical and radiological presentations found in this disease. As previous studies have led to the description of different radiological presentations, summarized in the classification proposed by Klimo et al. [11], influencing the choice of surgical approach and the rate of post-operative complications [16], we also wanted to determine which radiological presentation was more prominent in patients with schwannomatosis.
We therefore decided to conduct a retrospective study on pelvic schwannoma cases operated at our institution, both in sporadic setting and in tumor predisposing syndromes in order to evaluate the functional results of nerve-sparing schwannoma resection in both groups.
Material and methods
Study cohort
We retrospectively reviewed the charts of patients operated for a pelvic schwannoma in the department of neurosurgery of a single center between 2016 and 2023 and the charts of patients harboring a pelvic schwannoma under conservative management. Patients with pelvic neurofibromas and patients harboring a presumed pelvic peripheral nerve sheath tumor without histological diagnosis were excluded from the study. Clinical records of the 272 NF2-related and 46 NF2-independent Schwannomatosis patients monitored at our national NF2 and Schwannomatosis reference center were also reviewed searching for pelvic schwannoma cases. All schwannomatosis diagnoses were based upon clinical criteria with additional genetic confirmation when available. As all patients had regular spinal MRIs, pelvic tumors were diagnosed either on sagittal sections of the lumbar spine in the presacral space or on dedicated pelvic MRIs due to associated symptoms. Considering the rarity of other tumor types (perineuriomas, neurofibromas), all non-operated pelvic tumors from schwannomatosis patients included in the series were presumed to be schwannomas. Tumor volume was measured using the dedicated tools on Carestream PACS (AGFA Healthcare©). Pelvic schwannomas were classified according to the previously published classification proposed by Klimo et al. [11] (Fig. 1A). For sporadic cases, only confirmed schwannomas based on biopsy or final histological findings of a resected specimen were included. For patients with syndromic tumors, all tumors identified in the pelvis were deemed to be schwannomas. Data were obtained retrospectively from clinical records. All patients provided informed written consent and the local institutional review committee approved the retrospective chart review.
A Classification of pelvic schwannomas in 3 different subtypes depending on the level of sacral invasion (adapted from [11]). B Anatomical distribution of operated pelvic schwannomas (adapted from the Netter Atlas of Human Anatomy): S1 and S2 schwannomas originating from branches of the sciatic nerve; F1, F2, and F3 schwannomas originating from branches of the femoral nerve; O1 schwannomas originating from branches of the obturator nerve
Surgical technique
In our department, surgery of pelvic schwannomas is performed by a multidisciplinary team composed of a vascular surgeon and a neurosurgeon specialized in peripheral nerve surgery, as already reported by others [6]. Patients are operated supine, via retroperitoneal approach. An angio-CT was performed in all patients during pre-operative work-up to evaluate the vessels adjacent to the tumor. Nerve monitoring was either performed by an electrophysiologist (I.B) using EMG or by direct nerve stimulation (Isis Inomed©) to allow the identification of motor nerve fibers at the surface of the tumor. The vascular surgeon chose and performed the surgical approach, identified, and mobilized vital structures such as the ureter. Vascular dissection of iliac vessels was then performed whenever needed to expose the tumor pseudo-capsule in its largest diameter. The neurosurgeon then performed tumor removal under microscope by incision of the pseudo-capsule, incidentally held up by sutures, then dissection in the plane between pseudo- and true capsule and en-bloc tumor enucleation as previously reported [20] in order to preserve the normal nerve fibers and vessels embedded in the pseudo-capsule. While the neurosurgeon remained the same in all operated cases but one (MP), the vascular surgeon differed from surgery to surgery.
Statistical analysis
Statistical analyses were performed using Microsoft Excel© and GraphPad Prism 9©. The chi-square test was used for categorical variables and the unpaired t-test for continuous unpaired variables. Statistical significance was presumed if p < 0.05.
Results
General characteristics of the cohort
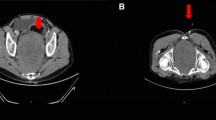
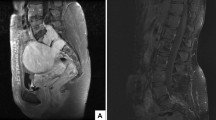
From 2016 to march 2023, 24 patients presented to our department of neurosurgery with pelvic schwannomas. Twelve patients had sporadic lesions, while 12 patients had a tumor-predisposing syndrome. There were 10 men and 14 women in the cohort. The mean age at diagnosis was 41 years old (median, 41; range, 18–75 years old) and did not differ between sporadic and syndrome cases (p > 0.05, unpaired t-test). In six patients (25%), the schwannomas were identified incidentally during investigations for unrelated symptoms (one case), the staging of other malignancies (one case), or the workup of their tumor-predisposing syndrome (4 cases). In all other cases, pelvic schwannomas were revealed by associated symptoms including local or irradiated pain (14 cases), paresthesias (12 cases), hypoesthesia (2 cases), and/or motor deficit (2 cases). There was a total of 33 pelvic schwannomas in the cohort. Most tumors originated from the branches of the sciatic nerve, either from L5 or S1 (Fig. 1B, S1, and S2 and Fig. 2A–C) (23 cases, 70%), while 6 tumors arose from branches of the femoral nerve, from its initial extra-foraminal portion behind the psoas muscle (Fig. 1B, F1 and Fig. 2D) to its terminal part above the inguinal ligament (Fig. 1B, F2–F3 and Fig. 2E–F) (18%) and 4 from the obturator nerve (Fig. 1B, O1) (12%). All patients harbored Klimo type III lesions, with purely presacral development (Fig. 1), except one patient with schwannomatosis harboring both a Klimo type II and a Klimo type III tumor.
Surgical series
Sixteen patients were operated over the course of the study (Table 1). There was a majority of sporadic cases and among syndromic cases, a majority of patients with non-NF2-schwannomatosis. The indication for surgery was pain in most cases (12/16, 75%) or tumor growth (4/16, 25%). There was no case of pre-operative sexual dysfunction or bowel/bladder incontinence in our series. Tumors were right-sided in 56% of cases and left-sided in 44% of cases. Schwannomas were all discrete, especially in sporadic cases (Fig. 1A–F), but also in most syndromic cases (Fig. 2A, C) except one case of multi-nodular schwannoma of the obturator nerve (Fig. 2B) in a schwannomatosis patient for which 6 nodules were resected. In this case, we did not perform en-bloc resection but rather staged resection of each of the six nodules inside the same obturator nerve. This strategy leads to a satisfying pain relief while limiting post-operative deficits.
During surgery, vascular dissection of iliac vessels for extended access to the tumor capsule was performed in 10 cases (62%). In one case, the primary iliac vein was injured during the incision of the tumor pseudo-capsule and was sewed by the vascular surgeon. Intra-operative nerve monitoring was performed in all cases and lead to the anatomic localization of motor nerve fibers in 9 patients (56%), mostly found at the medial and lower side of the tumor, thus allowing for a safe opening of the tumor pseudo-capsule at its ventral surface. Mean operative blood loss was 226 ml (median, 100 ml; range, 100–1100 ml). The mean duration of surgery was 137 min (median, 120 min; range, 60–300 min). The duration of surgery was influenced by tumor volume and the existence of adhesions between the capsular and the pseudo-capsular plane. Complete resection was achieved in 14 of 16 patients (87%); 2 patients with pelvic schwannomas underwent a subtotal resection. A tumor remnant was left in place in the nerve foramen in one case of sporadic schwannoma and in the obturator canal in the case of multi-nodular schwannoma. Tumor size of resected schwannomas ranged from 3.4 to 247 cm3 (median, 38 cm3; mean, 65 cm3). The mean size of sporadic cases (77.1 cm3) was larger compared to syndromic cases (33.7 cm3), but this difference did not reach statistical significance (p = 0.7, unpaired t-test). There was no post-operative mortality and no general surgical morbidity (no infection or post-operative hematoma). Histological analysis confirmed the diagnosis of cellular schwannoma in all cases. The mean duration of hospital stay was 5 days and did not differ between syndromic and non-syndromic cases.
The mean post-operative follow-up was 37 months (range: 2–168 months). At last-follow-up, complete pain relief was achieved in all 12 patients with pre-operative pain. There were 3 cases of long-term localized hypoesthesia on the front of the thigh (including one patient with similar pre-operative symptoms) and one case of post-operative motor deficit (adductor muscles MRC class 4) in the patient with a multi-nodular schwannoma. The global rate of neurological post-operative morbidity was 12%. There was no statistical difference in terms of pain and sensory symptoms relief between syndromic and sporadic patients. In the 2 tumors with subtotal resection, no progression of residual disease was observed on follow-up imaging 15 and 44 months after resection.
Pelvic schwannomas in schwannomatosis
The clinical characteristics of patients with schwannomatosis included in the study are detailed in Table 2. Considering all schwannomatosis patients managed in our reference center, the prevalence of symptomatic pelvic schwannomas was 3.7% but was higher in NF2-independent (19.5%) compared to NF2-related (0.7%) cases. The majority of patients (66%) with schwannomatosis did not present with spinal schwannomas. All syndromic patients but one (patient 4) had other peripheral nerve tumors, ranging from 1 to over 40 peripheral nerve schwannomas. Multi-nodular schwannomas occurred only in NF2-independent schwannomatosis cases and represented 43% of tumors, significantly differing from sporadic cases (p = 0.005, chi-square test). As in previous reports on multi-nodular schwannomas [9], we observed that multi-nodular tumors might correspond to multiple discontinuous nodules in the course of a single nerve, forming a “string of beads” (Fig. 3B), or involve multiple adjacent fascicles and form a “profiteroles cake” (Fig. 3C, D, and E), the former being more amenable to surgery than the latter. Consequently, most patients with multi-nodular schwannomas were managed conservatively while presenting with symptomatic tumors due to the inoperable nature of such cases (Fig. 3D–F). Despite the high frequency of pelvic schwannomas encountered in schwannomatosis patients, all patients but one harbored Klimo [11] type III lesions with only one reported case of trans-sacral (type II) tumor in our cohort.
Radiological presentation of schwannomatosis-associated pelvic schwannomas. A Discrete schwannoma in NF2-related Schwannomatosis. B Operated case of multi-nodular schwannoma presenting with a “string of beads” appearance (black arrowheads). C–F Cases of multi-nodular schwannomas with conservative management, presenting with simultaneous involvement of several nerves (D), and contiguous nodules in the same nerve, with a “profiteroles cake” appearance (C). Note the possible simultaneous involvement of terminal branches of the sciatic nerve (C, white arrowheads)
Discussion
This study on the surgical results of nerve-sparing removal of pelvic schwannomas in sporadic and schwannomatosis patients confirms the excellent rate of pain relief (100%) in both clinical settings, with a similar frequency of post-operative neurological morbidity (12%) compared to previous reports. In the literature, the rate of surgical complications varied from 11 [12] to 27% [7, 22]. The rates of pain relief varied between 72 [6] and 100% [12] in monocentric series but was not informed in most studies [5, 7, 13], especially when reported by non-neurosurgical teams.
Most series reported by general surgeons have indeed focused on the quality of tumor resection and associated surgical morbidity rather than functional and neurological outcome, thus reflecting a culture inherited from sarcoma management, denoted by the use of the R classification to assess quality of tumor resection [22]. Similar to other neurosurgical teams [6], we consider that nerve-sparing tumor resection [20] minimizes surgical morbidity, by preserving nerve bundles embedded in the tumor pseudo-capsule and vessels that might be adherent to it. R0 tumor resection entails the resection of the tumor pseudo-capsule and may therefore require vascular division and/or resection, reported in 25% of cases in the Australasian group meta-analysis and statistically associated with the incidence of high-grade complications [22]. Extensive dissection of major blood vessels adherent to the tumor pseudo-capsule may also increase mean operative blood loss. In our series, correct exposure of the tumor pseudo-capsule in its longest diameter required dissection of major blood vessels in 62% of cases, and we encountered one case of accidental incision of the primitive iliac vein collapsed on the tumor pseudo-capsule. In a comparable surgical series on nerve-sparing resection of pelvic schwannomas [14], careful mobilization of iliac vessels was required in 16% of cases, and 11% cases of ruptured branches of the inner iliac vein were encountered. In both series, the mean operative blood loss was less than 1000 ml (226 ml and 680 ml [6], respectively) compared to a mean operative blood loss of 1987.7 ± 609.4 ml in a recent meta-analysis [16]. Other studies have also reported massive blood loss after retroperitoneal schwannoma removal [13] and tearing of adjacent arteries [5]. At the same time, gross total resection could be achieved in 86% of cases, which is in line with other studies, which reported complete tumor removal rates ranging from 73 [13, 16] to 100% [5].
This diversity in surgical approaches might reflect the existence of knowledge silos between neurosurgeons and general surgeons on the topic of pelvic schwannomas, as already reported in vestibular schwannomas with otologists [19]. In the biggest series reported to date by general surgeons [22], the citations included only one reference (8%) from a neurosurgical team. On the other hand, the latest meta-analysis published by neurosurgeons [14] included 45 references, with 38% of citations from non-neurosurgical teams.
Consequently, it is difficult to compare the different surgical series due to the small sample size of most series and the diversity of tumors. Pelvic schwannomas may indeed be of various sizes, and some authors have only focused on giant schwannomas, defined as tumors with a maximum diameter > 5 cm. In their meta-analysis, Pennington et al. have included tumors with a mean diameter of 445.5 cm3, far greater than in the present series (71 cm3), while the recent cohort study of the Transatlantic Australasian Retroperitoneal Sarcoma Working Group reported 188 operated patients including 96 (51%) cases with tumor volume < 100 cm3. The rate of pre- and post-operative neurological morbidity may also vary depending of the type of pelvic schwannoma: Klimo type II tumors, which were prominent (76% of cases) in a recent series by Mualem et al. [14] and are more amenable to a posterior approach, presented with 37% of pre-operative bowel/bladder incontinence, and were associated with 33% of per-procedure nerve sacrifice and cases of post-operative paraplegia and incontinence, all of which were never encountered in our series of Klimo type III tumors. Finally, neurological outcome may vary depending on the affected nerve, as pelvic schwannomas may arise from small bundles of the lumbosacral plexus or involve major retroperitoneal nerves. Pelvic schwannomas involving major nerves represented only 20% [22] of operated cases in the most important series reported to date. Even in schwannomas involving major nerves, the neurological outcome was not systematically reported in all studies, especially sensory outcome, so small areas of numbness defined as post-operative morbidity in our series might have been overlooked in previous reports focused on extent of resection and rates of recurrence.
In conservatively managed cases, diagnostic confirmation using CT-guided needle biopsy is still debated in pelvic schwannomas, especially when tumors present with typical diagnostic findings on imaging. In our cohort, only one patient with a sporadic tumor has a CT-guided biopsy. In a recent study on the natural history of those tumors [15], biopsy was only performed in 68% of patients managed with a “wait-and-scan” strategy.
Finally, our cohort allows the comparison of syndromic and non-syndromic pelvic schwannomas as cases from NF2-related and NF2-independent schwannomatosis patients have been analyzed separately, which was not the case in previous reports[22]. This study illustrates the good functional results of surgery and the low morbidity, in line with previous reports on the surgery of peripheral nerve schwannomas in NF2-related [1, 17] and NF2-independent-chwannomatosis [4] and apart from multi-nodular cases that are specific of NF2-independent-schwannomatosis. It also underlines the high frequency of this tumor location in schwannomatosis patients. Interestingly, despite their high frequency in schwannomatosis, pelvic schwannomas were almost exclusively Klimo type III (21/22, 95%) with no Klimo type I and only one Klimo type II (1/22, 5%) cases, while the latter groups represent 5.3% and 43.9% of pelvic schwannomas in sporadic cases [16].
Conclusion
This study suggests that nerve-sparing resection of pelvic schwannomas offers satisfying rates of functional outcome both in sporadic and syndromic cases. Review of the literature suggests the possible existence of knowledge silos that might hinder the centralized management of pelvic schwannomas by specialized neurosurgeons.
This study also underlines the high frequency of pelvic schwannomas and multi-nodular cases in schwannomatosis patients compared to sporadic cases. In our opinion, surgery should preferably be proposed for mononodular tumors in schwannomatosis patients. Additional studies are mandatory to treat efficiently multi-nodular schwannoma cases as available drugs offer only moderate pain control.
Data availability
Not applicable.
References
Bendon CL, Furniss D, Giele HP (2015) Comparison of outcomes of peripheral nerve schwannoma excision in neurofibromatosis type 2 patients and non-neurofibromatosis type 2 patients: a case control study. J Plast Reconstr Aesthetic Surg JPRAS 68:1199–1203
Braley AE, Goulart C, Chou J, Galgano M (2020) Resection of a large presacral schwannoma from an all-posterior trans-sacral approach. Surg Neurol Int 11:408
Dafford K, Kim D, Reid N, Kline D (2007) Pelvic plexus tumors. Neurosurg Focus 22:E10
Evans DG, Mostaccioli S, Pang D, Fadzil O Connor M, Pittara M, Champollion N, Wolkenstein P, Thomas N, Ferner RE, Kalamarides M, Peyre M, Papi L, Legius E, Becerra JL, King A, Duff C, Stivaros S, Blanco I (2022) ERN GENTURIS clinical practice guidelines for the diagnosis, treatment, management and surveillance of people with schwannomatosis. Eur J Hum Genet 30:812–817
Goh BKP, Tan Y-M, Chung Y-FA, Chow PKH, Ooi LLPJ, Wong W-K (2006) Retroperitoneal schwannoma. Am J Surg 192:14–18
Hajiabadi MM, Campos B, Sedlaczek O, Khajeh E, Nikdad M, von Deimling A, Mehrabi A, Unterberg A, Ahmadi R (2020) Interdisciplinary approach allows minimally invasive, nerve-sparing removal of retroperitoneal peripheral nerve sheath tumors. Langenbecks Arch Surg 405:199–205
Handa K, Ozawa H, Aizawa T, Hashimoto K, Kanno H, Tateda S, Itoi E (2019) Surgical management of giant sacral schwannoma: a case series and literature review. World Neurosurg 129:e216–e223
Harbaugh K, Smith P, Towfighi J (2007) Schwannomatosis in a patient with a pelvic mass: case report. Neurosurg Focus 22:E8
Hébert-Blouin M-N, Amrami KK, Scheithauer BW, Spinner RJ (2010) Multinodular/plexiform (multifascicular) schwannomas of major peripheral nerves: an underrecognized part of the spectrum of schwannomas. J Neurosurg 112:372–382
Hébert-Blouin M-N, Spinner RJ (2015) Commentary on: “Comparison of outcomes of peripheral nerve schwannoma excision in neurofibromatosis type 2 patients and non-neurofibromatosis type 2 patients: a case control study.” J Plast Reconstr Aesthetic Surg JPRAS 68:1204–1205
Klimo P, Rao G, Schmidt RH, Schmidt MH (2003) Nerve sheath tumors involving the sacrum. Case report and classification scheme. Neurosurg Focus 15:E12
Leclerc A, Lebreton G, Huet A, Alves A, Emery E (2021) Management of giant presacral schwannoma. Clinical series and literature review. Clin Neurol Neurosurg 200:106409
Li Q, Gao C, Juzi JT, Hao X (2007) Analysis of 82 cases of retroperitoneal schwannoma. ANZ J Surg 77:237–240
Mualem W, Ghaith A-K, Rush D, Jarrah R, Alexander Y, Zamanian C, Atkinson JLD, Yaszemski MJ, Krauss WE, Spinner RJ, Bydon M (2022) Surgical management of sacral schwannomas: a 21-year mayo clinic experience and comparative literature analysis. J Neurooncol 159:1–14
Ogose A, Kawashima H, Hatano H, Ariizumi T, Sasaki T, Yamagishi T, Oike N, Inagawa S, Endo N (2019) The natural history of incidental retroperitoneal schwannomas. PLoS ONE 14:e0215336
Pennington Z, Westbroek EM, Ahmed AK, Cottrill E, Lubelski D, Goodwin ML, Sciubba DM (2019) Surgical management of giant presacral schwannoma: systematic review of published cases and meta-analysis. J Neurosurg Spine 5:1–12
Peyre M, Tran S, Parfait B, Bernat I, Bielle F, Kalamarides M (2023) Surgical management of peripheral nerve pathology in patients with neurofibromatosis type 2. Neurosurgery 92:317–328
Plotkin SR, Messiaen L, Legius E, Pancza P, Avery RA, Blakeley JO, Babovic-Vuksanovic D, Ferner R, Fisher MJ, Friedman JM, Giovannini M, Gutmann DH, Hanemann CO, Kalamarides M, Kehrer-Sawatzki H, Korf BR, Mautner V-F, MacCollin M, Papi L, Rauen KA, Riccardi V, Schorry E, Smith MJ, Stemmer-Rachamimov A, Stevenson DA, Ullrich NJ, Viskochil D, Wimmer K, Yohay K, International Consensus Group on Neurofibromatosis Diagnostic Criteria (I-NF-DC), Huson SM, Wolkenstein P, Evans DG (2022) Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: an international consensus recommendation. Genet Med Off J Am Coll Med Genet 24:1967–1977
Schnurman Z, Golfinos JG, Roland JT, Kondziolka D (2018) Knowledge silos: assessing knowledge sharing between specialties through the vestibular schwannoma literature. J Neurosurg 129:1278–1285
Stone JJ, Spinner RJ (2020) Go for the gold: a “plane” and simple technique for resecting benign peripheral nerve sheath tumors. Oper Neurosurg Hagerstown Md 18:60–68
Strauss DC, Qureshi YA, Hayes AJ, Thomas JM (2011) Management of benign retroperitoneal schwannomas: a single-center experience. Am J Surg 202:194–198
Transatlantic Australasian Retroperitoneal Sarcoma Working Group (2020) Intercontinental collaborative experience with abdominal, retroperitoneal and pelvic schwannomas. Br J Surg 107:452–463
Acknowledgements
The authors would like to thank Emmanuelle Feve for editorial assistance.
Funding
We have no funding to disclose related to this submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All living patients provided informed written consent, and the local institutional review committee approved the retrospective chart review, submitted under the acronym PELWAN.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peyre, M., Gaudric, J., Bernat, I. et al. Surgical management of sporadic and schwannomatosis-associated pelvic schwannomas. Neurosurg Rev 46, 275 (2023). https://doi.org/10.1007/s10143-023-02186-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02186-y