Abstract
Purpose
The interferon regulatory factor 2 (IRF-2) acted as a tumor suppressor. We inspected IRF-2 as a predictor of prognosis in gastric cancer (GC) patients and tried to find out the potential molecular mechanism.
Methods
In this study, the association between IRF-2 expression and clinical or prognosis significance was investigated in 86 pairs of tumor and the adjacent normal gastric tissues from GC patients. After establishing the stable cell lines, the Transwell assays were deduced to evaluate the malignancy of tumor. Then, microarray assay was carried out and the GO/KEGG pathway analyses were conducted to identify IRF-2’s target gene. The relationship between IRF-2 and matrix metalloproteinases 1 (MMP-1) was also investigated by the immunohistochemistry in 15 pairs of tumor and adjacent normal gastric tissues.
Results
We found that IRF-2 expression level in GC was significantly correlated with the prognosis of the patients. Transwell assays suggested an impaired ability of invasion and migration in IRF-2-overexpressed GC cells and a progressive malignant phenotype in IRF-2-knockdown GC cells. Ninety differentially expressed genes were found between IRF-2-overexpressed GC cells and its normal control sets by microarray. We demonstrated that MMP-1 was canonical in the network of differentially expressed genes by GO and KEGG pathway analysis and its expression level was markedly decreased in IRF-2-overexpressed cells of MKN-45 and increased in IRF-2-knockdown cells of SGC-7901. The expression of MMP-1 was inversely correlated with IRF-2 in GAC TMA specimens.
Conclusion
IRF-2 may inhibit GC progression by down-regulating MMP-1 level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is ranked as the fourth most common malignancy worldwide, with 80% mortality in more than 70% countries. Asia countries comprise above 75% of newly diagnosed GC cases, and Chinese morbidity reached 405 thousand in 2010 [1]. Since great advances in recognizing the molecular pathogenesis in the initiation, progression, and metastasis of GC, genetic alterations such as tumor biomarkers are central to predict the prognosis, guide the individualized treatment and provide new directions for future targeted therapy in GC.
The mammalian interferon regulatory factor (IRF) family is a kind of transcription factors which can regulate the gene expression by binding to DNA [2]. The amino-terminal of IRF contains a 115-amino-acid DNA-binding domain (DBD), which is similar to the DBD of c-myc oncogenic protein and can regulate the transcription of DNA. The carboxyl-terminal of IRF is a variable region, which gives many kinds of biological functions to IRFs [3]. Recently, a large amount of studies has indicated that IRFs play a critical role in immune regulation, cell differentiation, cell apoptosis, and oncogenesis. IRF-2 is an important member of the IRFs family. It locates on chromosome 4q34.1-q35.1, and no expression of tissue specificity has been found so far. Zucman-Rossi et al. [4] performed high-resolution copy-number analysis on 125 hepatocellular carcinoma (HCC) and whole-exome sequencing on 24 HCC and found recurrent alterations of IRF-2 gene in HCC. Further studies found that IRF-2 served as a tumor suppressor and its inactivation led to impaired P53 function [4,5,6].
Matrix metalloproteinase-1 is a kind of endopeptidase that needs Ca2+, Zn2+ and other metal ions as cofactors. By decomposition of extracellular matrix, the proteinase is involved in physiology process, including embryo development, reproduction, and tissue remodeling [8]. However, in pathological setting such as tumors, the expression level of MMP-1 is markedly increased by regulation of transcription factor [9].
We have already found that miR-18a modulates P53 expression by directly targeting IRF-2 in GC [7]. In this study, we inspected the relationship between IRF-2 and clinicopathological characteristics by analyzing paired sample of tumor and its adjacent normal tissues from GC patients. Then,we inferred the IRF-2 expression in GC cells,observed the malignancy behavior, and investigated the differential genes expression by a series of biological assays as well as bioinformatics analyses.
Materials and Methods
Patients and Specimens
Tumor samples used in this research were obtained from 101 patients with gastric adenocarcinoma (GAC) who underwent curative surgical treatment at the Department of General Surgery, Zhongshan Hospital, Fudan University, from 2007 to 2008. The inclusion and exclusion criteria are as follows: (a) having curative gastric surgical treatment, defined as a complete resection of all the cancer nodules and histological examination showing free of tumor on the cut surface and no cancerous thrombus in the vein, (b) having no distant metastasis, (c) having a definite pathological diagnosis of GAC, (d) having no anticancer treatment before the surgery, (e) having a complete follow-up data until August 2013, and (f) having suitable formalin-fixed and paraffin-embedded tissues. The diagnosis of GAC was based on WHO criteria, and tumor differentiation was defined according to the seventh edition of tumor–node–metastasis (TNM) classification of Union International Contra Cancrum (UICC).
The clinicopathological characteristics of all the participants are listed in Supplementary Table S1. Ethical approval of human subjects was obtained from the research ethics committee of Zhongshan Hospital. Informed consent was obtained from each participant.
Follow-Up and Treatment for Cancer Recurrences
Patients received lifelong regular follow-up and physical examinations in our hospital. The latest follow-up time in this article was on August 2013. The median follow-up was 39 months, ranging from 0 to 75 months. Patients were monitored by tumor markers such as CEA, CA724, CA199, endoscopy, and typical imaging tests such as enhanced CT and PET-CT every 6–12 months, according to the postoperative time. Patients who were confirmed recurrence received further treatments such as chemotherapy, radiotherapy, or palliation therapy according to their physical conditions.
Immunohistochemistry (IHC) and Its Variables Evaluation
Formalin-fixed and paraffin-embedded tissues were made into tissue microarrays (TMAs) after histopathology and HE (hematoxylin and eosin) staining guided location. Five-micron-thick sections of TMA were deparaffinized and rehydrated, followed by high-temperature antigen retrieval via microwave in 0.1 M citrate solution (pH 6.0) for 15 min. After being blocked for 30 min in 5% normal goat serum at room temperature, the sections were incubated at 4 °C with the rabbit anti-IRF-2 antibody (Abcam, Cambridge, UK) and rabbit anti-MMP1 antibody (Abcam, Cambridge, UK) overnight. Then, they were incubated at room temperature for 30 min with biotinylated secondary antibody and finally immunostained by the avidin–biotin complex (ABC) technique using 3,3′-diaminobenzidine (DAB). Hematoxylin was used as a counterstain.
The immunohistochemical staining was evaluated by two pathologists without knowing the characteristics of the patients. Any discrepancy was settled in the consensus review. The interpretation of immunoreactivity was performed in a semiquantitative manner which was mentioned previously [8]. In brief, the interpretation score was calculated by analyzing the extent and intensity of staining positivity of cells: 0, ≤ 5% cell positivity or negative staining; + 1, 6–20% cell positivity or mild staining; and + 2, 21–50% cell positivity or intense staining. Total scores were the product of the two. The final scores were the differentials of the tumor total score and the adjacent normal tissues total score; the scores greater than − 3.0 were considered high expression; otherwise, they were identified as low expression.
Cell Lines and Transwell Assay
Gastric cancer cells (AGS, MKN-4, SGC-7901, and MKN-38) were cultured. IRF-2 was overexpressed by plasmid and knocked down by specific shRNAs. The influence of IRF-2 on cell invasion and migration was investigated by Transwell assay.
Overexpression and Knockdown Experiments
For the experiments utilizing overexpression, the IRF-2 full-length sequence was synthesized and subcloned into a pcDNA3.1 vector (GeneChem, China). AGS and MKN45 cells were transfected with pcDNA3.1-IRF-2 using Lipofectamine 3000 (Invitrogen, USA) according to manufacturer’s instructions. For the knockdown experiments, short hairpin RNA for IRF-2 (shIRF-2) was generated by GeneChem (China) and inserted into the pHY-LV-KD1.4 lentiviral shRNA vector (Hanyinbt, China). SGC-7901 and MKN-28 cells were transfected with lentiviral shIRF-2 and subjected to selection with puromycin to establish a stable cell line. The stable monoclonal cell lines with up-regulated and down-regulated IRF-2 were screened. The efficacy of overexpression and knockdown of IRF-2 was verified by real-time PCR and Western blotting.
Migration and Invasion Assays
Cell migration and invasion were assayed using the transwell chamber (Millipore, USA) with and without Matrigel (BD, Franklin Lakes, USA). For the invasion assay, the transwell insert was coated with 30 μl Matrigel for 1 h at 37 °C. Cells, 48 h after transfection, were trypsinized and seeded into the upper chamber of the insert at the density of 6 × 104 cells per well and cultured in serum-free media, while media containing 10% FBS were added to the lower chamber. After 48 h of incubation, migrated or invaded cells were fixed with 4% paraformaldehyde, followed by staining with Giemsa. Cell images were obtained under a phase-contrast microscope (Olympus, Tokyo, Japan).
Western Blotting
Western blotting was performed according to standard procedure by antibodies against IRF-2, p-Erk 1/2, total-Erk 1/2, p-p38, p-JNK, total-JNK, active-MMP1. GAPDH was selected as a loading control.
Microarray Assay and Data Analyses
We used Whole Human Genome Oligo Microarray (provided by Aksomics Inc., Shanghai, China) for microarray analysis to survey the gene expression from samples of overexpressed IRF-2 and its normal control cells. RNA quantity and quality were measured by NanoDrop ND-1000. RNA integrity was assessed by standard denaturing agarose gel electrophoresis. Sample labeling and array hybridization were performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technology). Agilent Feature Extraction software (version 11.0.1.1) was used to analyze acquired array images. Quantile normalization and subsequent data processing were performed with using the GeneSpring GX v12.1 software package (Agilent Technologies). Hierarchical clustering was performed using the R scripts. GO analysis and pathway analysis were performed in the standard enrichment computation method.
Statistical Analyses
Statistical analyses were done by SPSS 19.0 (SPSS Inc., IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc., CA, USA). The Chi-square test, Fisher’s exact probability, Student’s t test, and Mann–Whitney test were used for comparison between groups. Cumulative survival time was calculated by Kaplan–Meier method and analyzed by log-rank test. Univariate and multivariate analyses were used to measure the prognostic value of IRF-2 based on the Cox proportional hazard regression model. The linear regression was used to explore the casual relationship between the expression levels of IRF-2 and MMP-1. P < 0.05 was regarded as statistically significant.
Results
IRF-2 Expression Is Down-Regulated in Most GC Tissues
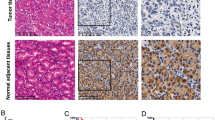
To determine the expression level of IRF-2 in GC, we performed the immunohistochemical analyses in patients’ GC tissues and found IRF-2 mostly located in the cytoplasm. By using the Chi-square test, we revealed it was down-regulated in human GC tissues compared with that in the normal adjacent tissues (P < 0.001; Fig. 1). Sixty-eight (68/86, 79.07%) pairs showed a lower expression of IRF-2 in cancer tissues and nine (9/86, 10.47%) pairs showed almost the same expression of IRF-2 in both the GC tissues and the normal adjacent tissues, while another nine (9/86, 10.47%) pairs performed the opposite results with the overall tendency.
Association of IRF-2 Expression with Clinicopathological Characteristics
To find out the correlation between the expression level of IRF-2 and various clinicopathological characteristics of the GC patients, we collected the data which are summarized in Table 1. However, IRF-2 expression was correlated with none of the clinical parameters including sex, age, tumor differentiation, invasive depth, lymph nodes metastasis, position, size, and TNM stage (all P > 0.05).
Association of IRF-2 Expression with Survival Outcomes
To make sure the IRF-2’s influence on survival, we evaluated the relationship between expression and survival data by Kaplan–Meier analysis and log-rank test. It was found that IRF-2 expression level in GC was significantly correlated with patients’ overall survival time (OS) (P = 0.013, Fig. 2a); i.e., the higher level of IRF-2 expression was correlated with longer OS. The cumulative 3-year and 5-year OS was 62.3% and 42.6% in the high IRF-2 expression group, respectively, whereas it was only 28.0% and 20.0% in the low IRF-2 expression group.
Prognostic significance assessed by using Kaplan–Meier survival estimates and log-rank tests. The overall survival periods of patients with high IRF-2 expression were significantly longer than those of patients with low IRF-2 expression in general patients cohort (a), male patients subgroup (b), young subgroup (c), gastric antrum site subgroup (d), big size subgroup (e), and TNM stage III subgroup (f)
We also evaluate the prognostic value of IRF-2 expression level in selective patient subgroups stratified according to sex, age, tumor differentiation, primary tumor site, size, and TNM stage. The statistical results showed that IRF-2 had more important prognostic value in male patients who were younger than 65 years of age, with GC primarily located in the gastric antrum and bigger than 5 cm in size, and had a higher TNM stage (Table 2, Fig. 2b–f). Particularly in the gastric antrum subgroup and bigger size (> 5 cm) subgroup, the 3-year OS rates of the patients who showed high IRF-2 expression were 73.3% and 53.3%, respectively, much higher than IRF-2 lower expression patients (18.2% and 0.0%, both P < 0.001); similar results were also found in the 5-year OS rates, and the high IRF-2 expression patients were 53.3% and 33.3%, while the low IRF-2 expression patients were only 9.1% and 0.0%, respectively (both P < 0.001).
It was found that invasive depth, lymph nodes metastasis, TNM stage, and tumor size were unfavorable predictors for OS of GC, while IRF-2 was the favorable factor for OS. On the other hand, sex, age, tumor differentiation, and position had no prognostic value for OS by univariate analysis (Table 3). We also performed the multivariate survival analysis to evaluate the independent prognostic values of TNM stage, tumor size, and IRF-2. Considering the invasive depth and lymph nodes metastasis were included in the TNM stage, we did not bring these two factors into the multivariate analysis. We found that IRF-2 was an independent prognosticator for OS in this analysis (P = 0.004), even better than the TNM stage (P = 0.012) (Table 3).
IRF-2 Modulates the Invasion and Migration of GC Cells
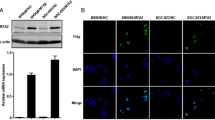
To discover IRF-2’s function of inhibiting tumor, we overexpressed the IRF-2 in AGS, MKN-45 gastric cancer cells by plasmid and constructed IRF-2-knockdown cells of SGC-7901, MKN-38 using two IRF-2-specific shRNAs (Fig. 3a). The Transwell assays suggested that overexpression of IRF-2 significantly decreased the AGS, MKN-45 invasion and migration, while knockdown of IRF-2 increased the SGC-7901, MKN-28 invasion and migration (Fig. 3b, c).
IRF-2 inhibits cell migration and invasion in vitro. a IRF-2 was overexpressed in AGS and MKN-45 cells and knocked down by two IRF-2-specific shRNAs in SGC-7901 and MKN-28 cells. It was found that the overexpression of IRF-2 significantly decreased the migration and invasion abilities in AGS and MKN-45 gastric cancer cells, while knockdown of IRF-2 increased these abilities in SGC-7901 and MKN-28 gastric cancer cells (b, c the magnification was 200). Columns represented the average of four random fields; bars, SD
IRF-2 Inhibits Tumorigeneses and Progression of Gastric Cancer by Suppressing MMP-1
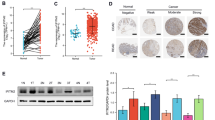
To identify IRF-2’s target molecule, we applied microarray assays for IRF-2-overexpressed GC cells versus its normal control sets and revealed 90 differentially expressed genes (fold change > 1.5, P < 0.05), of which 47 were up-regulated and 43 were down-regulated (Supplemental Table 1). There were four pathways, including extracellular matrix (ECM)-related pathway, adhesive plaque-related pathway, blood small plate activation-related pathway, and PI3K-AKT signal pathway enriched in IRF-2-overexpressed GC cells by KEGG pathway analysis (Fig. 4a). We demonstrated that matrix metalloproteinase 1 (MMP-1) was canonical in the network of differentially expressed genes by GO pathway analysis (Fig. 4b) and its expression level was markedly decreased in IRF-2-overexpressed cells of AGS and increased in IRF-2-knockdown cells of SGC-7901 (Fig. 4c).
IRF-2 can downregulate the protein expression of MMP-1 and its associated signaling pathways. Agilent gene array obtained the different gene expressions in overexpressed IRF-2 and control cells. The KEGG pathway enrichment analysis was carried out as well as the functional analysis (a). The GO and pathway enrichment analyses were performed and found that MMP-1 was canonical in the network of differentially expressed genes (b). Western blot showed that the protein expression of MMP-1 and its associated signaling pathways was related to the expression of IRF-2 (c). The expression of MMP-1 inversely correlated with IRF-2 in GAC TMA specimens (d)
The Expression of MMP-1 was Inversely Correlated with IRF-2 in GAC TMA Specimens
To further evaluate the relationship between IRF-2 and MMP-1 in GAC patients, we examine the expression levels of both IRF-2 and MMP-1 by immunohistochemical assay using anti-IRF-2 antibody and anti-MMP-1 antibody in the same TMA specimens which contain 15 pairs of tumor and adjacent tissues. In tumor tissues, IRF-2 expression was lower (P < 0.001), whereas MMP-1 expression was higher (P < 0.001) than that in the adjacent non-tumor tissues (Fig. 4d). A significant inverse correlation was also observed between the expressions of MMP-1 and IRF-2 in the same TMA specimens (r = − 0.5066, P = 0.0043; Fig. 4d). These data further support the hypothesis that IRF2 reduces the expression of MMP-1.
Discussion
Despite the improvements in surveillance and clinical treatment strategies, the prognosis of GC still remains dismal. It is important to classify those patients who have the high probability of recurrent and short survival period to initiate a timely intervention. Current clinical factors such as TNM stage cannot accurately predict the outcome of GC patients. Therefore, studies of tumor biomarkers are helpful in predicting the prognosis of patients with GC, guiding the individualized treatment, revealing the possible molecular mechanism of oncogenesis, and providing new directions for future targeted therapy.
In the clinicopathological analysis, we found that IRF-2 had more important prognostic value in male patients who were younger than 65 years of age, with GC primarily located in the gastric antrum, bigger than 5 cm in size, and had a higher TNM stage. The possible reasons can be listed as follows: I. Some studies showed sex hormones might be partly involved in GC development and progression [9,10,11], although the effect of sex hormones on the oncogenesis is still unclear. The survival time of the female patients is affected by the level of their hormones, so the prognostic value of IRF-2 is weakened in such kind of patients. In addition, the role of sex hormones in regulation of IRF-2 should also be further studied. II. To those patients older than 65 years of age, poor physical quality, bad surgical tolerance, slow postoperative recovery, and low immunity will affect the stability of survival. Therefore, IRF-2 is more valuable for the prognosis of the relatively young patients. III. The reason why IRF-2 had higher prognostic value in patients with GC primarily located in the gastric antrum, bigger than 5 cm in size, and had a higher TNM stage may be the survival time of these kinds of patients is more relevant to the characteristics of the tumor. Further studies will be conducted to determine why IRF-2 expression is more associated with prognosis in these specific populations.
The IRF family comprise nine members and was first characterized as transcriptional regulators of type I interferon (IFN) and IFN-inducible genes. But recent studies have found that this family plays a crucial role in regulation of cell growth, apoptosis, and oncogenesis. IRF-2 is an important member of the IRF family. In addition to its role as an IFN attenuator, IRF-2 manifests a tumor-related activity. Initial studies showed IRF-2 served as an oncogenic protein through its transcriptional interference of other IRF family members that bind to the same IFN-stimulated response element [12, 13] or its acetylation make it preferentially bind to H4 promoter in proliferating cells [14,15,16]. And the expression level of IRF-2 was also found up-regulated in human esophageal cancers [17] and pancreatic cancers [18, 19]. But recently, Zucman-Rossi and his colleagues performed high-resolution copy-number analysis on 125 HCC and whole-exome sequencing on 24 HCC and found recurrent alterations of IRF-2 gene in HCC [4]. Further studies found that IRF-2 served as a tumor suppressor and its inactivation led to impaired P53 function [4,5,6]. All the results indicated that IRF-2 plays a complex role in the development of tumor.
In this study, we found IRF-2 mostly located in the cytoplasm and its level decreased in human GC tissues compared with that in the normal adjacent tissues, since it was previously believed that IRF-2 expressed in the nucleus as a transcriptional regulator [20]. During oncogenesis, the expression of IRF-2 neither increased nor decreased significantly in the nucleus, while it was down-regulated in the cytoplasm (P < 0.001). As transcriptional regulation and binding H4 are required to be carried out in the nucleus, while the regulation of ubiquitination of P53 can be performed in the cytoplasm, we believe that IRF-2 is more likely to inhibit the oncogenesis by regulating the P53 pathway in the cytoplasm. But all these speculations depend on further investigation.
MMP-1 contributed to the degradation of histological barrier of tumor cell and provided conditions for tumor invasion and metastasis by destroying extracellular matrix [9]. Studies reported that increased MMP-1 expression could be found in about 73% of gastric cancer patients [20]. It has previously been shown that the ERK and p38 mitogen-activated protein kinase (MAPK) pathways are the major regulators for MMP-1 expression [21, 22]. It has been shown that EGFR-mediated p38 MAKP signaling pathway augments MMP-1 expression and then leads to promote cancer tumorigenesis and angiogenesis [22, 23]. In this study, we revealed that the expression of IRF-2 was related ERK and p38 MAKP signaling pathway and also the expression of MMP-1, which involved in the tumorigenesis of gastric cancer as well as the invasion and metastasis of the gastric cancer.
The relationship between the expression level of IRF-2 and the prognosis of GC patient was first found in this paper. All the participants had a long follow-up time. But there are still some limitations in this study; for instance, the number of samples is limited and the level of hormone receptors in GC tissues or the hormone levels of the patients were not detected. In addition, further studies are also required to find out the molecular mechanism of IRF-2 regulating MMP-1.
Conclusion
To our knowledge, this is the first study showing the expression of IRF-2 in GC tissues and highlighting the clinical significance of IRF-2 in GC. We found IRF-2 expression level in GC was significantly correlated with patients’ OS time and was an independent prognosticator for OS. Further, we revealed that IRF-2 was involved in the invasion and migration of gastric cancer cells and negatively regulated the MMP-1, which is represented as a key factor in the extracellular matrix pathway.
References
Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. The lancet oncology.. 2013;14:e535–e547.
Zhang R, Chen K, Peng L, Xiong H. Regulation of T helper cell differentiation by interferon regulatory factor family members. Immunologic Research. 2012;54:169–176.
Veals SA, Schindler C, Leonard D, et al. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992;12:3315–3324.
Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698.
Amaddeo G, Guichard C, Imbeaud S, Zucman-Rossi J. Next-generation sequencing identified new oncogenes and tumor suppressor genes in human hepatic tumors. Oncoimmunology. 2012;1:1612–1613.
Amaddeo G, Cao Q, Ladeiro Y, et al. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut. 2015;64:820–829.
Chen YJ, Wu H, Zhu JM, et al. MicroRNA-18a modulates P53 expression by targeting IRF2 in gastric cancer patients. J Gastroenterol Hepatol. 2015;31:155.
Chen Y, Fu D, Xi J, et al. Expression and clinical significance of UCH37 in human esophageal squamous cell carcinoma. Dig Dis Sci. 2012;57:2310–2317.
Gan L, He J, Zhang X, et al. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer. 2012;12:566.
Wang X, Huang X, Fu Z, et al. Biphasic ER-alpha36-mediated estrogen signaling regulates growth of gastric cancer cells. Int J Oncol. 2014;45:2325–2330.
Qin J, Liu M, Ding Q, et al. The direct effect of estrogen on cell viability and apoptosis in human gastric cancer cells. Mol Cell Biochem. 2014;395:99–107.
Nguyen H, Mustafa A, Hiscott J, Lin R. Transcription factor IRF-2 exerts its oncogenic phenotype through the DNA binding/transcription repression domain. Oncogene. 1995;11:537–544.
Harada H, Kitagawa M, Tanaka N, et al. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993;259:971–974.
Vaughan PS, Aziz F, van Wijnen AJ, et al. Activation of a cell-cycle-regulated histone gene by the oncogenic transcription factor IRF-2. Nature. 1995;377:362–365.
Vaughan PS, van der Meijden CM, Aziz F, et al. Cell cycle regulation of histone H4 gene transcription requires the oncogenic factor IRF-2. J Biol Chem. 1998;273:194–199.
Masumi A, Yamakawa Y, Fukazawa H, Ozato K, Komuro K. Interferon regulatory factor-2 regulates cell growth through its acetylation. J Biol Chem. 2003;278:25401–25407.
Wang Y, Liu DP, Chen PP, Koeffler HP, Tong XJ, Xie D. Involvement of IFN regulatory factor (IRF)-1 and IRF-2 in the formation and progression of human esophageal cancers. Cancer Res. 2007;67:2535–2543.
Sakai T, Mashima H, Yamada Y, et al. The roles of interferon regulatory factors 1 and 2 in the progression of human pancreatic cancer. Pancreas. 2014;43:909–916.
Cui L, Deng Y, Rong Y, et al. IRF-2 is over-expressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2012;33:247–255.
Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59:489–510.
Xia P, Zhang R, Ge G. C/EBPbeta mediates TNF-alpha-induced cancer cell migration by inducing MMP expression dependent on p38 MAPK. J Cell Biochem. 2015;116:2766–2777.
Ferguson J, Arozarena I, Ehrhardt M, Wellbrock C. Combination of MEK and SRC inhibition suppresses melanoma cell growth and invasion. Oncogene. 2013;32:86–96.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290.
Acknowledgments
The authors would like to express gratitude to the staff of Prof. Xi-Zhong Shen’s laboratory for their critical discussion and reading of the manuscript. This study was supported by Youth Foundation of Zhongshan Hospital (No. 2015ZSQN08), Shanghai Sailing Program (No. 16YF1401500), Foundation of Shanghai Institute of Liver Diseases, and National Natural Science Foundation of China (Nos. 81101540, 81101637, 81172273, 81272388).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, YJ., Liang, L., Li, J. et al. IRF-2 Inhibits Gastric Cancer Invasion and Migration by Down-Regulating MMP-1. Dig Dis Sci 65, 168–177 (2020). https://doi.org/10.1007/s10620-019-05739-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05739-8