Abstract
Objective
The role of gastritis in dyspepsia remains controversial. We aimed to examine the efficacy of rebamipide, a gastric mucosal protective agent, in both organic and functional dyspepsia.
Design
A systematic review and meta-analysis was performed. The following databases were searched using the keywords (“rebamipide” OR “gastroprotective agent*” OR “mucosta”) AND (“dyspepsia” OR “indigestion” OR “gastrointestinal symptoms”): PubMed, Wed of Science, Embase, CINAHL, Cochrane Clinical Trials Register. The primary outcome was dyspepsia or upper GI symptom score improvement. Pooled analysis of the main outcome data were presented as risk ratio (RR) for dichotomous data and standardized mean difference (SMD) for continuous data.
Results
From an initial 248 records, 17 randomised controlled trial (RCT) publications involving 2170 subjects (1224 rebamipide, 946 placebo/control) were included in the final analysis. Twelve RCTs were conducted in subjects with organic dyspepsia (peptic ulcer disease, reflux esophagitis or NSAID-induced gastropathy) and five RCTs were conducted in patients with functional dyspepsia (FD). Overall, dyspepsia symptom improvement was significantly better with rebamipide compared to placebo/control drug (RR 0.77, 95% CI = 0.64–0.93; SMD −0.46, 95% CI = −0.83 to −0.09). Significant symptom improvement was observed both in pooled RR and SMD in subjects with organic dyspepsia (RR 0.72, 95% CI = 0.61–0.86; SMD −0.23, 95% CI = −0.4 to −0.07), while symptom improvement in FD was observed in pooled SMD but not RR (SMD −0.62, 95% CI = −1.16 to −0.08; RR 1.01, 95% CI = 0.71–1.45).
Conclusion
Rebamipide is effective in organic dyspepsia and may improve symptoms in functional dyspepsia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyspepsia refers to a collection of upper gastrointestinal (UGI) symptoms including abdominal pain/discomfort, nausea, and early satiety, which are chronic in nature. It is a common condition with a prevalence rate of 21% in the global population [1, 2] and is broadly categorised as either organic (due to structural diseases such as peptic ulcer disease, reflux esophagitis, gastro-esophageal malignancy) or functional (absence of structural lesions in the UGI tract) dyspepsia, usually following endoscopic investigation. Although organic causes of dyspepsia are infrequent in both the West [3] and the East [4], differences in the epidemiology and clinical characteristics of organic dyspepsia between populations have been observed [5]. Similarly, clinical and epidemiological differences in functional dyspepsia (FD) have been reported between Western and Eastern populations [6], suggesting that certain pathophysiological mechanisms of FD may vary between populations.
Gastric mucosal inflammation or gastritis has been shown to relate to several pathophysiological aspects of FD, particularly in relation to altered gastro-duodenal motility. In a previous Japanese study of 198 patients with FD, the severity of histological gastritis was shown to correlate with a reduction in gastric motility [7]. Previous studies have demonstrated that specific patterns of gastritis appeared to correlate with dyspepsia sub-types, regardless of H. Pylori infection status, in patients with FD, [8,9,10]. Chronic gastritis might also affect a variety of endocrine functions of the stomach, such as the production of the GI hormones and neurotransmitters somatostatin, gastrin, and ghrelin, which influence the severity and frequency of symptoms in FD [11]. A recent review article highlighted that symptom improvement with H. pylori eradication appears to have a larger magnitude in Eastern compared to Western patients with FD [6]. A greater degree of gastric inflammation (gastritis) in Eastern patients with FD compared to their Western counterparts [12] may be one explanation for greater symptom resolution with H. pylori eradication in the former.
Rebamipide, a mucosal protective agent, is widely used in East Asia for the relief of various UGI disorders [13]. Preclinical and animal studies have demonstrated that rebamipide enhances mucosal protection by increasing gastric mucosal prostaglandin and mucus secretion, whilst additionally reducing mucosal inflammation in the stomach by inhibiting inflammatory cytokines such as IL-8, impeding neutrophil activation and adhesion of vascular endothelium [14,15,16,17]. The efficacy of rebamipide in improving symptoms of dyspepsia has not been comprehensively and systematically evaluated. A few studies in FD, largely from the West, have produced negative results [18, 19]. However, several recent un-controlled studies in Asian patients with chronic gastritis [20] and type 2 diabetes mellitus [21] have suggested a benefit for UGI symptoms using rebamipide. This systematic review and meta-analysis, pooling data from both the East and the West, aims to summarize the efficacy of rebamipide in both organic and functional dyspepsia and to explore differences in efficacy between the two types of dyspepsia.
Methods
Search Strategy
Relevant peer-reviewed articles were identified by searching the following databases until 9 November 2016: PubMed, Web of Science, EMBASE, CINAHL, and the Cochrane Library. Where possible, medical subject heading (MESH) terms were employed. Articles with titles, abstracts, keywords, or text words containing the keywords [“rebamipide” OR “gastroprotective agent*” OR “mucosta (trade name of rebamipide)”] AND (“dyspepsia” OR “indigestion” OR “gastrointestinal symptoms”) were selected for title search. We also hand-searched reference lists of relevant studies, electronic theses, review articles, and abstracts published in international conferences on this topic in both English and non-English languages. Articles were excluded at this stage if they did not fulfill the title search criteria as above. This process was completed by three of the authors (SZS, MPT, and SM). Full-text articles were then retrieved for further screening and data extraction by the three authors. Disagreements were resolved by consensus.
Study Selection
This study was conducted according to the PRISMA statement for the reporting of systematic reviews and meta-analysis [22]. We included all RCTs comparing rebamipide with placebo or other treatments, involving human adults aged 18 years or over. All articles were required to contain rebamipide as their intervention while the control arms could employ placebo or standard treatment. The articles were required to report symptom change or the presence of symptom improvement, over any duration of treatment.
Definition of Organic and Functional Dyspepsia
Organic dyspepsia was defined as dyspepsia due to recognized structural diseases in the upper GI tract—i.e. peptic ulcer disease, reflux esophagitis, or nonsteroidal anti-inflammatory drug (NSAID)-induced gastropathy [4]. Functional dyspepsia was defined as the absence of any such structural lesions upon endoscopy, which included “chronic gastritis” not associated with NSAIDs. The latter was included in the “Functional Dyspepsia” category due to a lack of evidence for symptom association with chronic gastritis [23–25].
Data Extraction and Risk of Bias
Three authors (SZS, MPT, and SM) independently extracted data from the selected articles including the baseline demographics, sample size, duration of rebamipide treatment, dose of rebamipide, and key outcome data. Outcome data were recorded as either: (1) proportion of subjects with symptom improvement; or (2) changes in GI symptom scores. The Cochrane Collaboration’s tool for assessing risk of biases was used to assess the methodological quality of each study [26].
Statistical Analysis
The primary outcome for this review was UGI symptom improvement. A cumulative score of various UGI symptoms or specific UGI symptom scores was used in most studies. The association between rebamipide therapy and UGI symptom improvement was estimated using pooled risk ratio (RR) with 95% confidence intervals (CIs) for dichotomous data and standardized mean difference (SMD) with 95% CIs for continuous data. The pooled estimate was computed by weighting each estimate by the inverse variance method using a random effects model. We used the forest plot to illustrate pooled estimates and Cochran Q and I 2 statistics to evaluate statistical heterogeneity between studies and by type of lesion, age, duration, and study quality. Publication bias was assessed using a funnel plot. We attempted to obtain missing data from authors and assumed missing data to be completely at random when the outcomes were not available. The meta-analyses were conducted using RevMan 5.3.
Results
Literature Search
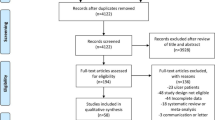
Our search strategy resulted in 248 articles. A total of 17 articles remained for this systematic review and meta-analysis after excluding duplicate and irrelevant articles (Fig. 1). Sixteen articles were published in the English language and one article was published in Korean [27]. All articles were full-text papers, but one study was only published as an abstract. The studies were carried out in Japan [14, 28–33], Korea [27, 34, 35], China [36, 37], Thailand [38], Brazil [39], and USA [18]. Pertinent details of the 17 studies have been highlighted in Table 1.
Study Characteristics
Of the 17 RCT studies on subjects with dyspepsia, 8 studies compared rebamipide to placebo [18, 19, 28, 30, 33, 34, 37, 39], 5 studies compared rebamipide to controlled/comparator medication [32, 35, 36, 40, 41], 1 study compared rebamipide to no treatment [29], and 3 studies compared rebamipide + anti-secretory therapy versus placebo + anti-secretory therapy [31, 38, 42]. The efficacy of rebamipide in subjects with organic dyspepsia (NSAID-induced gastropathy/ulcers, reflux esophagitis, and H. pylori-associated peptic ulcers) was examined in 12 RCTs [27–35, 39–43], whilst the effect of rebamipide in subjects with functional dyspepsia was evaluated in 5 RCTs [18, 19, 36–38]. Rebamipide was administered at a dose of mostly 300 mg daily, for a median period of 7 weeks (range 1–48 weeks). Symptoms of dyspepsia were scored using (1) Likert scales of individual UGI symptoms in 12 studies, (2) Gastrointestinal Symptom Rating Scale (GSRS) in 3 studies, and (3) global UGI symptoms in 2 studies (Table 1). A total of 2170 subjects were included, of whom 1224 received rebamipide (57 with rebamipide and anti-secretory therapy), and 946 received placebo/control drugs.
Meta-Analysis
The relative risk of dyspepsia improvement was pooled initially among studies which reported dichotomous outcomes of symptom improvement. Rebamipide therapy was associated with a 23% improvement in dyspepsia (RR = 0.77; 95% CI = 0.64–0.93; P < 0.001; I 2 = 21%) compared to placebo/control medication (Fig. 2). Rebamipide therapy significantly improved dyspepsia in those with organic dyspepsia (RR = 0.72; 95% CI 0.61–0.86; P < 0.001; I 2 = 8%), but not in those with functional dyspepsia (RR = 1.01; 95% CI 0.71–1.45; P = 0.94; I 2 = 0%). There was suggestive evidence that the efficacy of rebamipide varied between the two types of dyspepsia (between-group P = 0.09). From the funnel plot, there was slight asymmetry in studies with smaller-size samples (Fig. 3). Sensitivity analyses (not shown) excluding these smaller-sized studies did not significantly change our findings.
For studies which reported outcomes as continuous data, the SMDs of UGI symptom scores in studies were pooled for meta-analysis (Fig. 4). Rebamipide therapy was associated with a 0.46 standard deviation (SD) reduction in UGI symptom scores (SMD = −0.46; 95% CI −0.83 to −0.09; P = 0.02; I 2 = 86%). The mean reduction in UGI symptom scores associated with rebamipide therapy for those with organic dyspepsia (SMD = −0.23; 95% CI −0.40 to −0.07; P = 0.005; I 2 = 0%) and functional dyspepsia (SMD = −0.62; 95% CI −1.16 to −0.08; P = 0.03; I 2 = 87%) was not significantly different (between-group P = 0.18). The funnel plots for the published studies was symmetrical (Fig. 3).
Due to the heterogeneity of RCT studies included in the meta-analysis, we performed additional sensitivity analyses as follows:
-
(i)
Duration of rebamipide therapy
Pooled data for symptom improvement was analysed based on treatment duration of ≤ 4 weeks versus > 4 weeks. A greater improvement of dyspepsia symptoms was observed in studies which had > 4 weeks of therapy (SMD = −0.65; 95% CI −0.12 to −0.18; P = 0.007; I 2 = 90%), in contrast to studies which had ≤ 4 weeks of rebamipide therapy (Supplementary Fig. 6).
-
(ii)
Studies using different UGI symptom scales
Pooled data for symptom improvement was analysed based on studies which used the GSRS (n = 3), an individual UGI symptom Likert scale (n = 15), and a global/overall scale (n = 2). Pooled analysis revealed that improvement of dyspepsia was observed in studies which used individual and global UGI symptoms, but not in those which used the GSRS (Supplementary Fig. 7).
-
(iii)
Studies with placebo versus active drug as controls
Pooled data for symptom improvement was analysed for studies which had placebo compared with active drug (e.g. ranitidine or misoprostol) as controls. No significant difference in symptom improvement was observed in studies which reported either placebo or an active drug as controls (Supplementary Fig. 8).
Assessment of Bias Due to Methods
The methodological quality and risk of bias of the 17 RCT studies are presented in Fig. 2. Overall, all 17 RCTs were judged to have lower risk of attrition and reporting bias, 3 studies were at risk of bias because blinding was not employed, and the risk of selection, performance, and detection biases for the remaining 14 studies was unclear (Fig. 5). Computer-generated number sequences and sealed envelopes were used for eight and three studies, respectively.
Discussion
In the current meta-analysis, treatment with rebamipide in 17 RCTs has demonstrated a 23% improvement of dyspepsia symptoms compared to placebo or a control drug (either anti-secretory or misoprostol). Fifteen of the 17 studies were conducted in East Asia (Korea, Japan, and China), whilst 2 studies were conducted in Western patients [18, 39]. The improvement in symptoms was present for both categorical and continuous symptom outcomes in organic dyspepsia, naturally due to a resolution of structural lesions in the upper GI tract. Interestingly, the pooled analysis demonstrated benefit in FD studies which reported continuous symptom outcomes but not in FD studies which reported categorical outcomes. The latter was heavily weighted by a single large study in Western patients [18], which may have influenced the pooled analysis.
Previous studies conducted among mainly Caucasian populations with dyspepsia have shown a poor correlation between chronic gastritis and dyspepsia symptoms [23–25]. However, recent reports have indicated that the clinical significance of chronic gastritis in Asians may be different to that of Western patients [6]. In a study comparing age and symptom-matched adults from Japan and the UK, Naylor et al. were able to demonstrate a greater severity of histological gastritis in Japanese adults with dyspepsia compared to their British counterparts [12]. Furthermore, a meta-analysis of RCTs in China of H. pylori eradication in adults with FD (which had only been published in the Chinese literature) demonstrated a threefold (OR 3.61) chance of symptom improvement following H. pylori eradication [44], which is a significantly greater magnitude of symptom improvement than that reported in Western FD patients with H. pylori eradication [45].
A previous nationwide, endoscopic survey of 8892 adults with a label of FD in China reported that chronic gastritis was the commonest endoscopic finding. However, when compared to histology, endoscopy had a lower diagnostic validity [47]. This suggests that many patients labelled as FD may have underlying chronic gastritis. This review suggests that rebamipide therapy may improve symptoms in Asians with FD, as many of them may have chronic gastritis as well.
This systematic review and meta-analysis has several limitations. First, the subjects included in the meta-analysis had a variety of UGI diseases and were therefore quite heterogeneous. However, as the primary outcome measure of symptom improvement is not known to differ between various causes of dyspepsia [47], the inclusion of these studies is valid in this meta-analysis. Second, the methods of assessing dyspepsia symptoms were not identical in all studies but were based on similar domains—i.e. individual components of dyspepsia. The improvement of symptoms were pooled using risk ratios (dichotomous outcome) and/or SMD (continuous outcomes) to account for the different assessment methods. Third, three studies included anti-secretory acid suppressants (either proton pump inhibitor or H2-receptor antagonist) with rebamipide in the treatment arm—two studies with organic dyspepsia [27, 31] and one study with FD [38]. Although anti-secretory acid suppressants are a recognised proven therapy in organic dyspepsia [48], their efficacy in FD is less established [49]. Furthermore, the combination of rebamipide and anti-secretory acid suppressants was found to be superior to anti-secretory acid suppressants alone in these three studies, demonstrating the added benefit of rebamipide in these cases. Fourth, the duration of rebamipide therapy varied between studies considerably. Nevertheless, we have performed additional sensitivity analyses for these factors accounting for heterogeneity and found no significant difference apart from duration of therapy—i.e. a longer duration of therapy was associated with better symptom improvement. However, more studies are needed to explore whether the efficacy of rebamipide in the different types of dyspepsia is associated with duration of therapy. Last, the studies from which we have based this systematic review were of moderate quality. However, the studies were carried out in various populations across three continents, which suggests wide representation of the data.
In summary, rebamipide is associated with improvement of symptoms in organic dyspepsia. The evidence for its efficacy is less consistent in FD. The mechanism of symptom improvement is probably related to a resolution of chronic gastritis, which is not easily diagnosed in routine clinical practice with endoscopy. With a growing concern over the safety of proton pump inhibitors lately [50], rebamipide therapy may offer an alternative treatment option for patients with FD or those at risk of recurrent NSAID-induced peptic ulcers. Further large, multi-center, clinical trials in such patients are warranted to confirm the findings from this meta-analysis.
References
Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–2666.
Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049–1057.
Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:830-7–837e1-2.
Mahadeva S, Goh KL. Clinically significant endoscopic findings in a multi-ethnic population with uninvestigated dyspepsia. Dig Dis Sci. 2012;57:3205–3212.
Mahadeva S, Raman MC, Ford AC, et al. Gastro-oesophageal reflux is more prevalent in Western dyspeptics: a prospective comparison of British and South-East Asian patients with dyspepsia. Aliment Pharmacol Ther. 2005;21:1483–1490.
Mahadeva S, Ford AC. Clinical and epidemiological differences in functional dyspepsia between the East and the West. Neurogastroenterol Motil. 2016;28:167–174.
Matsumoto Y, Ito M, Kamino D, et al. Relation between histologic gastritis and gastric motility in Japanese patients with functional dyspepsia: evaluation by transabdominal ultrasonography. J Gastroenterol. 2008;43:332–337.
Trespi E, Broglia F, Villani L, et al. Distinct profiles of gastritis in dyspepsia subgroups. Their different clinical responses to gastritis healing after Helicobacter pylori eradication. Scand J Gastroenterol. 1994;29:884–888.
Zaitoun AM. The prevalence of lymphoid follicles in Helicobacter pylori associated gastritis in patients with ulcers and non-ulcer dyspepsia. J Clin Pathol. 1995;48:325–329.
Kyzekove J, Arlt J, Arltova M. Is there any relationship between functional dyspepsia and chronic gastritis associated with Helicobacter pylori infection? Hepatogastroenterology. 2001;48:594–602.
Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168–174.
Naylor GM, Gotoda T, Dixon M, et al. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut. 2006;55:1545–1552.
Arakawa T, Kobayashi K, Yoshikawa T, et al. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43:5S–13S.
Sun WH, Tsuji S, Tsujii M, et al. Induction of cyclooxygenase-2 in rat gastric mucosa by rebamipide, a mucoprotective agent. J Pharmacol Exp Ther. 2000;295:447–452.
Tabata M, Tomomasa T, Itoh K, et al. Effect of 10% ethanol and sofalcone on prostaglandin E2 content, mucus gel thickness, and experimental ulcers in the stomach of developing rats. Digestion. 1996;57:47–53.
Adachi K, Suetsugu H, Moriyama N, et al. Influence of Helicobacter pylori infection and cetraxate on gastric mucosal blood flow during healing of endoscopic mucosal resection-induced ulcers. J Gastroenterol Hepatol. 2001;16:1211–1216.
Arakawa T, Higuchi K, Fujiwara Y, et al. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50:S3–S11.
Talley NJ, Riff DS, Schwartz H, et al. Double-blind placebo-controlled multicentre studies of rebamipide, a gastroprotective drug, in the treatment of functional dyspepsia with or without Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:1603–1611.
Miwa H, Osada T, Nagahara A, et al. Effect of a gastro-protective agent, rebamipide, on symptom improvement in patients with functional dyspepsia: a double-blind placebo-controlled study in Japan. J Gastroenterol Hepatol. 2006;21:1826–1831.
Syam AH, Simadibrata M, Rani A, et al. Rebamipide effect on chronic gastritis in dyspeptic patient: symptoms, endoscopic and histological evaluation. Digestion. 2012;85:156.
Park S, Park SY, Kim YJ, et al. Effects of rebamipide on gastrointestinal symptoms in patients with type 2 diabetes mellitus. Diabetes Metab J. 2016;40:240–247.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Johnsen R, Bernersen B, Straume B, et al. Prevalences of endoscopic and histological findings in subjects with and without dyspepsia. BMJ. 1991;302:749–752.
Schubert TT, Schubert AB, Ma CK. Symptoms, gastritis, and Helicobacter pylori in patients referred for endoscopy. Gastrointest Endosc. 1992;38:357–360.
Parente F, Imbesi V, Maconi G, et al. Influence of bacterial CagA status on gastritis, gastric function indices, and pattern of symptoms in H. pylori-positive dyspeptic patients. Am J Gastroenterol. 1998;93:1073–1079.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Rew JS, Joo YE, Kim HS, et al. Efficacy of rebamipide maintenance therapy after eradication of Helicobacter pylori infection in patients with chronic gastritis or gastric ulcer. Kor J Gastroenterol. 2000;36:175–184.
Adachi K, Furuta K, Miwa H, et al. A study on the efficacy of rebamipide for patients with proton pump inhibitor-refractory non-erosive reflux disease. Dig Dis Sci. 2012;57:1609–1617.
Hasegawa M, Horiki N, Tanaka K, et al. The efficacy of rebamipide add-on therapy in arthritic patients with COX-2 selective inhibitor-related gastrointestinal events: a prospective, randomized, open-label blinded-endpoint pilot study by the GLORIA study group. Mod Rheumatol. 2013;23:1172–1178.
Kawai T, Yamagishi T, Goto S. Circadian variations of gastrointestinal mucosal damage detected with transnasal endoscopy in apparently healthy subjects treated with low-dose aspirin (ASA) for a short period. J Atheroscler Thromb. 2009;16:155–163.
Mizukami K, Murakami K, Abe T, et al. Aspirin-induced small bowel injuries and the preventive effect of rebamipide. World J Gastroenterol. 2011;17:5117–5122.
Naito Y, Iinuma S, Yagi N, et al. Prevention of indomethacin-induced gastric mucosal injury in helicobacter pylori-negative healthy volunteers: a comparison study rebamipide vs famotidine. J Clin Biochem Nutr. 2008;43:34–40.
Tozawa K, Oshima T, Okugawa T, et al. A randomized, double-blind, placebo-controlled study of rebamipide for gastric mucosal injury taking aspirin with or without clopidogrel. Dig Dis Sci. 2014;59:1885–1890.
Kim HK, Kim JI, Kim JK, et al. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig Dis Sci. 2007;52:1776–1782.
Kim JH, Park SH, Cho CS, et al. Preventive efficacy and safety of rebamipide in nonsteroidal anti-inflammatory drug-induced mucosal toxicity. Gut Liver. 2014;8:371–379.
Du Y, Li Z, Zhan X, et al. Anti-inflammatory effects of rebamipide according to Helicobacter pylori status in patients with chronic erosive gastritis: a randomized sucralfate-controlled multicenter trial in China-STARS study. Dig Dis Sci. 2008;53:2886–2895.
Han X, Jiang K, Wang B, et al. Effect of rebamipide on the premalignant progression of chronic gastritis: a randomized controlled study. Clin Drug Investig. 2015;35:665–673.
Seearamroongruang T, Chunlertrith K, Mairiang P, et al. Effect of rebamipide combined with omeprazole on symptom improvement in non-helicobacter pylori gastritis. Thai J Gastroenterol. 2009;10:82–90.
Gagliano-Juca T, Moreno RA, Zaminelli T, et al. Rebamipide does not protect against naproxen-induced gastric damage: a randomized double-blind controlled trial. BMC Gastroenterol. 2016;16:58.
Park SH, Cho CS, Lee OY, et al. Comparison of prevention of NSAID-induced gastrointestinal complications by rebamipide and misoprostol: a randomized, multicenter, controlled trial-STORM STUDY. J Clin Biochem Nutr. 2007;40:148–155.
Song KH, Lee YC, Fan DM, et al. Healing effects of rebamipide and omeprazole in Helicobacter pylori-positive gastric ulcer patients after eradication therapy: a randomized double-blind, multinational, multi-institutional comparative study. Digestion. 2011;84:221–229.
Hong SJ, Park SH, Moon JS, et al. The benefits of combination therapy with esomeprazole and rebamipide in symptom improvement in reflux esophagitis: an international multicenter study. Gut Liver. 2016;10:910–916.
Kamada T, Sato M, Tokutomi T, et al. Rebamipide improves chronic inflammation in the lesser curvature of the corpus after Helicobacter pylori eradication: a multicenter study. Biomed Res Int. 2015;2015:865146.
Jin X, Li YM. Systematic review and meta-analysis from Chinese literature: the association between Helicobacter pylori eradication and improvement of functional dyspepsia. Helicobacter. 2007;12:541–546.
Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006. https://doi.org/10.1002/14651858.CD002096.pub4.
Du Y, Bai Y, Xie P, et al. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21.
Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566–1576.
Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
Wang WH, Huang JQ, Zheng GF, et al. Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials. Clin Gastroenterol Hepatol. 2007;5:178–185. (quiz 140).
Thomson AB, Sauve MD, Kassam N, et al. Safety of the long-term use of proton pump inhibitors. World J Gastroenterol. 2010;16:2323–2330.
Acknowledgments
MPT was a recipient of a University of Malaya Grand Challenge fund which also funded the salary of MHJ (GC002-14HTM).
Author information
Authors and Affiliations
Contributions
SM planned the study, contributed to data collection, and drafted the manuscript. MHJ and SZS performed data collection and preliminary data analysis. MPT planned the study and performed data collection. SR performed final data analysis and contributed to the drafting of the manuscript. All authors agreed on the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Fig. 6
Funnel plot demonstrating pooled analysis for RCT studies with continuous outcomes for dyspepsia based on symptom duration ≤ 4 weeks and > 4 weeks (JPEG 111 kb)

Fig. 7
Funnel plot demonstrating pooled analysis for RCT studies with categorical outcomes for dyspepsia based on various UGI symptom scales (JPEG 135 kb)

Fig. 8
Funnel plot demonstrating pooled analysis for RCT studies with categorical outcomes for dyspepsia based on placebo compared with active drug control (JPEG 109 kb)
Rights and permissions
About this article
Cite this article
Jaafar, M.H., Safi, S.Z., Tan, MP. et al. Efficacy of Rebamipide in Organic and Functional Dyspepsia: A Systematic Review and Meta-Analysis. Dig Dis Sci 63, 1250–1260 (2018). https://doi.org/10.1007/s10620-017-4871-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4871-9