Abstract
Introduction
Antithrombotic drugs, such as low-dose aspirin (LDA) and clopidogrel, can cause upper gastrointestinal complications.
Aim
The goal of the present study was to investigate whether a mucosal-protective agent, rebamipide, could prevent gastric mucosal injuries induced by LDA with or without clopidogrel in healthy subjects.
Materials and Methods
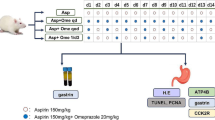
A randomized, double-blind, placebo-controlled trial was performed with 32 healthy male volunteers. Subjects were randomly assigned to a 14-day course of one of the following regimens: group A, placebo (tid) + LDA; group B, rebamipide (100 mg tid) + LDA (100 mg once-daily); group C, placebo + LDA + clopidogrel (75 mg once-daily); or group D, rebamipide + LDA + clopidogrel. The grade of gastric mucosal injuries was evaluated by esophagogastroduodenoscopy before and after dosing (on day 0 and day 14), and the grade of gastric mucosal injury was assessed according to the modified Lanza score. Subjective symptoms were assessed using the Gastrointestinal Symptom Rating Scale (GSRS). A rapid urease test was performed on day 0, and blood tests were performed on day 0 and day 14.
Results
Rebamipide significantly inhibited gastric mucosal injury induced by LDA alone or by LDA plus clopidogrel when compared with placebo in healthy subjects. GSRS score and hemoglobin level were not significantly different among the four groups.
Conclusions
Rebamipide is useful for the primary prevention of gastric mucosal injury induced by LDA alone or by LDA plus clopidogrel in healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspirin is now widely administered at relatively low doses as an antithrombotic drug for the prevention of cerebrovascular and cardiovascular diseases [1]. Despite the definite benefits from its antithrombotic effects, even low-dose aspirin can cause upper gastrointestinal (GI) complications, such as hemorrhagic gastritis and gastroduodenal ulcers [2]. Clopidogrel is a potent inhibitor of platelet adhesion and aggregation [3], and it is used worldwide to reduce thrombotic events. The most common adverse event associated with clopidogrel administration is bleeding [4]. The rate of bleeding with clopidogrel is similar to that with aspirin, although the rate of GI bleeding is less due to the agent’s lower gastrotoxicity [5]. The combination of aspirin and clopidogrel is clearly effective for the prevention of cardiovascular disease [6, 7]. However, the use of dual antiplatelet therapy (DAT), combining aspirin and clopidogrel, may confer an approximately twofold–fourfold increase in the risk of upper GI bleeding when compared with aspirin monotherapy or clopidogrel monotherapy [8–10].
Rebamipide is a well-known mucosal-protective agent that enhances defense mechanisms in the gastric mucosa by increasing gastric mucus and stimulating the production of endogenous prostaglandins. This drug has been reported to reduce gastric mucosal injury [11]. The efficacy of rebamipide in preventing LDA-induced gastric injury has been reported in healthy subjects [12]. However, no study has investigated whether rebamipide is useful for the prevention of gastric mucosal injuries induced by concomitant use of LDA and clopidogrel.
Therefore, the purpose of this study was to investigate whether rebamipide could prevent gastric mucosal injuries induced by LDA with or without clopidogrel in healthy subjects.
Materials and Methods
Study Design
A randomized, double-blind, placebo-controlled trial was performed in 32 healthy male volunteers. Subjects were randomly assigned to a 14-day course of one of the following regimens: group A, placebo (100 mg tid) + LDA (enteric-coated aspirin tablet, 100 mg once-daily) (Bayaspirin; Bayer Pharmaceutical Co., Ltd., Tokyo, Japan); group B, rebamipide (100 mg tid) (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) + LDA; group C, placebo + LDA and clopidogrel (75 mg once-daily) (Sanofi K.K., Tokyo, Japan); or group D, rebamipide + LDA and clopidogrel. Placebo or rebamipide was enclosed in a capsule (Size No. 2, Matsuya, Osaka, Japan), and two capsules tid were administered. Subjective symptoms were assessed using the Gastrointestinal Symptom Rating Scale (GSRS, Japanese version), and grade of gastric mucosal injuries was evaluated by esophagogastroduodenoscopy (EGD) before and after dosing (on day 0 and day 14). The grade of gastric mucosal injury was assessed according to the modified Lanza score (MLS). Anemia was evaluated by assessment of hemoglobin level on day 0 and day 14. The study protocol is shown in Fig. 1. The study protocol was approved by the Ethics Committee of Hyogo College of Medicine, and written informed consent was obtained from each subject.
Inclusion/Exclusion Criteria
Eligible subjects were males aged 24–40 years, had taken no medications within 4 weeks of the start of the study, and had normal physical examination and laboratory results. The exclusion criteria were as follows: (1) subjects with tumors, ulcers, ulcer scars, or bleeding in the upper GI tract; (2) subjects who had a history of ulcers, gastric surgery, or GI bleeding; (3) hemoglobin levels <13 g/dl; and (4) subjects who had an aspirin allergy.
Endoscopic Evaluation of Gastric Mucosal Injury
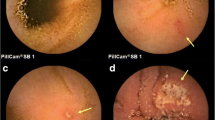
The grade of gastric mucosal injury was assessed according to the MLS [13, 14]. In this scoring system, gastric mucosal injury is graded into six categories from 0 to 5: Grade 0 is no erosion/hemorrhage; grade 1 is 1–2 lesions of erosion and/or hemorrhage localized in one area of the stomach; grade 2 is 3–5 lesions of erosion and/or hemorrhage localized in one area of the stomach; grade 3 is 6–9 lesions of erosion and/or hemorrhage localized in one area of the stomach, or no more than 10 lesions in two areas of the stomach; grade 4 is erosions and/or hemorrhage in three areas of the stomach, or no fewer than 10 lesions in the whole stomach; and grade 5 is a gastric ulcer, defined as a mucosal defect larger than 5 mm in diameter.
During endoscopy, more than 60 endoscopic pictures covering the whole area of the stomach were saved in the database, and later, the MLS was graded independently by two endoscopists (K.T., T.O.) after they had been blinded to any information about the subjects.
Helicobacter pylori Determination
Subjects underwent EGD with biopsies for diagnosis and assessment of Helicobacter pylori (HP) infection using the rapid urease test.
Gastrointestinal Symptom Rating Scale (GSRS)
The GSRS is a Swedish disease-specific and self-administered questionnaire designed to evaluate the perceived severity of GI symptoms during the previous week [15]. The questionnaire includes 15 items and uses a seven-grade Likert scale. This gives a total range value between 15 and 105, where the highest score (seven) represents the most pronounced symptom and the lowest score (one) represents no symptoms. The items are divided into five dimensions representing reflux syndrome, abdominal pain syndrome, indigestion syndrome, diarrhea syndrome, and constipation syndrome.
Statistical Analysis
Characteristics were compared using the Fisher’s exact test. The results are expressed as mean ± SD values. Statistical analyses were conducted using SPSS software version 11.0J (SPSS, Inc., Chicago, IL, USA). Fisher’s exact test, Mann–Whitney’s U test, and Kruskal–Wallis test were used for comparisons. Differences were considered significant at p < 0.05.
Results
Thirty-two healthy male subjects were enrolled. None of them were excluded. The study flow diagram is shown in Fig. 2. Subjects were divided into four groups, and two subjects dropped out of the study due to missed dose or illness. This was not correlated with adverse event. The characteristics of the subjects are shown in Table 1. HP infection was found four of 30 subjects, with one affected subject in each of the four groups.
Rebamipide significantly reduced the MLS in subjects receiving LDA monotherapy at day 14 compared with placebo (placebo + LDA, day 14) (Fig. 3a, the range of MLS, 0–4 vs. 0–3, p < 0.05). In group A (placebo + LDA), the MLS was significantly aggravated at day 14 compared with day 0 (the range of MLS, 0–4 vs. 0–3, p < 0.05). Moreover, rebamipide significantly reduced the MLS in subjects receiving LDA plus clopidogrel at day 14 compared with placebo (placebo + LDA plus clopidogrel, day 14) (Fig. 3b, the range of MLS, 3–4 vs. 0–3, p < 0.01). In group C (placebo + LDA plus clopidogrel), the MLS was significantly aggravated at day 14 compared with day 0 (the range of MLS, 3–4 vs. 0–3, p < 0.01). Rebamipide did not aggravate the MLS after 14-day LDA or LDA plus clopidogrel administration (Fig. 3a, b).
a The MLS was shown before and after 2 weeks of placebo + LDA and rebamipide + LDA administration. b The MLS was shown before and after 2 weeks of placebo + LDA + clopidogrel and rebamipide + LDA + clopidogrel administration. *p < 0.05 versus placebo + LDA (day 14); † p < 0.05 versus placebo + LDA (day 0); **p < 0.01 versus placebo + LDA + clopidogrel (day 0); ‡‡ p < 0.01 versus placebo + LDA + clopidogrel (day 14), NS not significant
The GSRS score was not significantly different among the four groups (Table 2). Moreover, there was no correlation between GSRS and MLS.
Discussion
This is the first randomized, double-blinded, placebo-controlled trial to show the protective effect of rebamipide in subjects with LDA- and/or clopidogrel-related gastric injuries. Rebamipide was superior to placebo in the prevention of gastric mucosal injuries.
Today, aspirin is the first-line antiplatelet drug for secondary cardiovascular and cerebrovascular prevention, as it produces a 25 % reduction in serious vascular events when compared with placebo [16]. Based on clinical findings, combination therapy with LDA and clopidogrel is recommended for the treatment of acute coronary syndromes and the prevention of coronary events after placement of a stent [17, 18]. In addition, LDA and clopidogrel are more effective than aspirin alone in reducing asymptomatic embolization [19]. The use of DAT is associated with an approximately twofold–fourfold increase in the risk of GI bleeding when compared with aspirin monotherapy [8–10]. A meta-analysis showed that the risk for GI bleeding in aspirin users increased with concomitant use of clopidogrel and anticoagulant therapies, but decreased in patients who received proton pump inhibitors (PPI) [20, 21]. However, some studies described an interaction between omeprazole and clopidogrel that resulted in a reduction in the efficacy of clopidogrel [22].
Rebamipide is a gastroprotective agent that induces the production of intracellular prostaglandins [23], improves blood flow [24], suppresses increases in permeability [25], anti-inflammatory action [26], and scavenges free radicals [27]. This drug has been used across Asia for the treatment of various gastric lesions, such as ulcers, erosions, and edema. Several previous reports have shown that rebamipide is effective in the treatment of gastric injuries [23, 28–30] as well as for small intestinal injuries [31] induced by LDA. Kawai et al. [30] reported that short-term administration of LDA induced mild gastric injuries and that rebamipide prevented these injuries despite continuous dosing of LDA. However, no study has investigated whether rebamipide is useful for the prevention of gastric mucosal injuries induced by concomitant use of LDA and clopidogrel. In the present study, we demonstrated that rebamipide significantly inhibited upper GI mucosal injury induced by LDA alone or by LDA plus clopidogrel in healthy subjects.
However, PPI is superior to a mucosal-protective drug, gefarnate, to reduce the recurrence risk of gastric ulcer in patients with a history of ulcers who are taking LDA [32]. Therefore, PPI might be better than mucosal-protective agents, such as rebamipide, for the subjects who have a history of gastric ulcers.
Regarding gastric injuries caused by LDA plus clopidogrel, median MLS for LDA and LDA plus clopidogrel groups were similar in our study, which is consistent with results described by Uotani et al. [33]. On the other hand, in regard to upper GI symptoms, Cayla et al. [34] reported that 15.4 % of patients on daily LDA have upper GI symptoms, among which gastroesophageal reflux was the most frequent symptom. As shown in Table 2, we evaluated five subscale parameters related to GI symptoms before and after medication dosing. Unfortunately, we could not detect changes in the specific symptoms among the four groups, though there are some reports suggesting that use of rebamipide can result in alleviation of GI symptoms [35]. In regard to the hemoglobin level, there were no significant differences among the four groups, meaning that serious bleeding complications did not occur during the 14-day study period.
This study has several limitations. First, the study population was small, and the study was performed in a single center. Second, participants were younger healthy subjects and were administered only a 14-day course of the drugs. In the clopidogrel in unstable angina to prevent recurrent events (CURE) trial that studied DAT [6], adding clopidogrel to aspirin increased the relative risk of GI bleeding by over 85 % over 1 year. In the clinical setting, DAT is often given in older population, in which the rate of HP infection is high; this may aggravate LDA-induced gastric lesions in the gastric body [36].
In conclusion, rebamipide significantly inhibited upper GI mucosal injury induced by LDA alone or by LDA plus clopidogrel in healthy subjects. These data suggest that rebamipide is useful for the primary prevention of low-dose aspirin-induced gastric mucosal injury in low-risk subjects.
References
Thomson RM, Anderson DC. Aspirin and clopidogrel for prevention of ischemic stroke. Curr Neurol Neurosci Rep. 2013;13:327.
Serrano P, Lanas A, Arroyo MT, Ferreira IJ. Risk of upper gastrointestinal bleeding in patients taking low-dose aspirin for the prevention of cardiovascular diseases. Aliment Pharmacol Ther. 2002;16:1945–1953.
Umemura K, Ishihara H, Nakashima M. Anti-platelet effects of clopidogrel in rat middle cerebral artery thrombosis model. Thromb Res. 1995;80:209–216.
Grove EL, Würtz M, Schwarz P, Jørgensen NR, Vestergaard P. Gastrointestinal events with clopidogrel: a nationwide population-based cohort study. J Gen Intern Med. 2013;28:216–222.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339.
Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502.
Sadanandan S, Singh IM. Clopidogrel: the data, the experience, and the controversies. Am J Cardiovasc Drugs. 2012;12:361–374.
Coleman CI, Sobieraj DM, Winkler S, et al. Effect of pharmacological therapies for stroke prevention on major gastrointestinal bleeding in patients with atrial fibrillation. Int J Clin Pract. 2012;66:53–63.
ACTIVE Investigators, Connolly SJ, Pogue J, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078.
García Rodríguez LA, Lin KJ, Hernández-Díaz S, Johansson S. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation. 2011;123:1108–1115.
Yamamoto T, Isono A, Mishina Y, et al. Gastroduodenal mucosal injury in patients taking low-dose aspirin and the role of gastric mucoprotective drugs: possible effect of rebamipide. J Clin Biochem Nutr. 2010;47:27–31.
Ono S, Kato M, Imai A, et al. Preliminary trial of rebamipide for prevention of low-dose aspirin-induced gastric injury in healthy subjects: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Biochem Nutr. 2009;45:248–253.
Lanza FL, Graham DY, Davis RE, Rack MF. Endoscopic comparison of cimetidine and sucralfate for prevention of naproxen-induced acute gastroduodenal injury. Effect of scoring method. Dig Dis Sci. 1990;35:1494–1499.
Iijima K, Ara N, Abe Y, et al. Gastric acid secretion level modulates the association between Helicobacter pylori infection and low-dose aspirin-induced gastropathy. J Gastroenterol. 2011;46:612–619.
Svedlund J, Sjödin I, Dotevall G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134.
Fowkes FG, Price JF, Stewart MC, et al. Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848.
Faxon DP, Lawler E, Young M, Gaziano M, Kinlay S. Prolonged clopidogrel use after bare metal and drug-eluting stent placement: the Veterans Administration drug-eluting stent study. Circ Cardiovasc Interv. 2012;5:372–380.
Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617.
Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial. Circulation. 2005;111:2233–2240.
Yeomans N, Lanas A, Labenz J, et al. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Am J Gastroenterol. 2008;103:2465–2473.
Depta JP, Bhatt DL. Antiplatelet therapy and proton pump inhibition: cause for concern? Curr Opin Cardiol. 2012;27:642–650.
Tantry US, Kereiakes DJ, Gurbel PA. Clopidogrel and proton pump inhibitors: influence of pharmacological interactions on clinical outcomes and mechanistic explanations. JACC Cardiovasc Interv. 2011;4:365–380.
Kim HK, Kim JI, Kim JK, et al. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig Dis Sci. 2007;52:1776–1782.
Banan A, Fitzpatrick L, Zhang Y, Keshavarzian A. OPC-compounds prevent oxidant-induced carbonylation and depolymerization of the F-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic Biol Med. 2001;30:287–298.
Yoshikawa T, Naito Y, Tanigawa T, Kondo M. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance. Arzneimittelforschung. 1993;43:363–366.
Naito Y, Yoshikawa T, Iinuma S, et al. Rebamipide protects against indomethacin-induced gastric mucosal injury in healthy volunteers in a double-blind, placebo-controlled study. Dig Dis Sci. 1998;43:83S–89S.
Murakami K, Okajima K, Uchiba M, et al. Rebamipide attenuates indomethacin-induced gastric mucosal lesion formation by inhibiting activation of leukocytes in rats. Dig Dis Sci. 1997;42:319–325.
Park SH, Cho CS, Lee OY, et al. Comparison of prevention of NSAID-induced gastrointestinal complications by rebamipide and misoprostol: a randomized, multicenter, controlled trial—STORM study. J Clin Biochem Nutr. 2007;40:148–155.
Thong-Ngam D, Chayanupatkul M, Klaikeaw N, Rerknimitr R, Mahachai V. Effect of rebamipide on gastric ulcer healing caused by Helicobacter pylori and/or NSAIDs or non NSAIDs-non H. pylori. J Med Assoc Thail. 2009;92:1207–1212.
Kawai T, Yamagishi T, Goto S. Circadian variations of gastrointestinal mucosal damage detected with transnasal endoscopy in apparently healthy subjects treated with low-dose aspirin (ASA) for a short period. J Atheroscler Thromb. 2009;16:155–163.
Mizukami K, Murakami K, Abe T, et al. Aspirin-induced small bowel injuries and the preventive effect of rebamipide. World J Gastroenterol. 2011;17:5117–5122.
Sugano K, Choi MG, Lin JT, et al. Multinational, double-blind, randomised, placebo-controlled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: the LAVENDER study. Gut. 2013. doi:10.1136/gutjnl-2013-304722.
Uotani T, Sugimoto M, Nishino M, et al. Ability of rabeprazole to prevent gastric mucosal damage from clopidogrel and low doses of aspirin depends on CYP2C19 genotype. Clin Gastroenterol Hepatol. 2012;10:879–885.
Cayla G, Collet JP, Silvain J, Thiefin G, Woimant F, Montalescot G. Prevalence and clinical impact of upper gastrointestinal symptoms in subjects treated with low dose aspirin: the UGLA survey. Int J Cardiol. 2012;156:69–75.
Miwa H, Osada T, Nagahara A, et al. Effect of a gastro-protective agent, rebamipide, on symptom improvement in patients with functional dyspepsia: a double-blind placebo-controlled study in Japan. J Gastroenterol Hepatol. 2006;21:1826–1831.
Fukuzawa M, Kawai T, Watanabe M, Tomiyama H, Yamashina A, Moriyasu F. Correlation between Helicobacter pylori infection and low-dose aspirin use on damage of the upper gastrointestinal tract. J Gastroenterol Hepatol. 2012;27:76–81.
Acknowledgments
We thank Ms. Mayumi Yamada and Ms. Kazuko Nagase for their expert technical assistance. No external funding was used to carry out this investigation.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
During the preparation of this manuscript, our most admired colleague, Professor Takayuki Matsumoto, passed away.
Rights and permissions
About this article
Cite this article
Tozawa, K., Oshima, T., Okugawa, T. et al. A Randomized, Double-Blind, Placebo-Controlled Study of Rebamipide for Gastric Mucosal Injury Taking Aspirin With or Without Clopidogrel. Dig Dis Sci 59, 1885–1890 (2014). https://doi.org/10.1007/s10620-014-3108-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3108-4