Abstract
The aim of the study was to investigate the effects of rebamipide on symptom, histology, endogenous prostaglandin, and mucosal oxygen free radicals in chronic erosive gastritis (CEG) patients by using sucralfate as a control. The trial also examined whether Helicobacter pylori infection would affect rebamipide-induced protection. A total of 453 endoscopy-confirmed CEG patients from 11 hospitals in China were enrolled in the study. They randomly received either rebamipide (100 mg t.i.d) or sucralfate (1.0 t.i.d) for 8 weeks with a ratio of 3:1. Per-protocol analysis (n = 415) showed the accumulated symptom score in the rebamipide group dropped from 5.54 ± 0.97 to 0.80 ± 0.47 after 8 weeks (P < 0.001 versus control). The endoscopic inflammation score in rebamipide group also decreased from 2.65 ± 0.09 to 0.60 ± 0.10, which showed better effects than sucralfate. It was shown a significant improvement (P < 0.01) in prostaglandin E2 (PGE2) contents in rebamipide-treated subjects mucosa (225.4 ± 18.3 pg/g versus 266.7 ± 14.7 pg/g) compared with that in sucralfate group after 8 weeks of treatment. Malondialdehyde (MDA) contents were significantly depressed both in the trial and control group. When Helicobacter pylori infection was considered, no statistically difference was found in the effect of rebamipide on either symptom or inflammation scores. In conclusion, Rebamipide demonstrated a stronger suppressive effect on the mucosal inflammation in chronic erosive gastritis than sucralfate. The gastroprotection induced by rebamipide is not influenced by H. pylori infection, which indicates its usage in the treatment of H. pylori-associated CEG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic erosive gastritis is a very common disease puzzling clinicians, especially in Asian areas such as China, Japan, and Korea. Even without risk of carcinogenesis as atrophic gastritis, chronic erosive gastritis (CEG) always presents with various GI symptoms and histological change in gastric mucosa leading to decreased quality of life. The drugs to be selected to treat the disease are less effective than those for peptic ulcer. Traditional gastroprotective agents such as sucralfate can inhibit inflammation while the symptom release and duration was not satisfied. Previous data from our center showed that endoscopy-confirmed superficial, atrophic, and erosive gastritis cases per year changed from 529, 881, and 2,891 in 2000 to 1,605, 686, and 5,357 in 2006, respectively. Against this trend in China, we tried an application of rebamipide, a gastroprotective drug in the treatment of CEG.
Rebamipide is one of the most commonly used gastroprotective agents in East Asia [1]. It has been widely proven in animal model that the drug exhibits preventive effects in gastric mucosa by increasing endogenous prostaglandin or by suppressing oxygen free radicals, as well as increasing blood flow [2–5]. Though much evidence has demonstrated that rebamipide improves histological gastritis in vivo, more clinical evidence is needed to confirm its effects on chronic gastritis. Unlike in Western countries, gastric atrophy is more prominent in Asia, which indicates that mucosal protection is more important than mono anti-acid therapy. Furthermore, Helicobacter pylori and nonsteroidal anti-inflammatory drug (NSAID) are two major causes related to gastric injury. Preclinical data has demonstrated various mechanisms involved in rebamipide effects on H. pylori-associated gastritis, including disturbance of the adhesion of H. pylori to gastric epithelial cells and inhibitory effects on H. pylori-induced neutrophil activation or interleukin-8 secretion [6–8]. A few reports have even suggested that rebamipide may have a potential anti-H. pylori role [9] while this was not confirmed in large clinical trials [10]. However, we still lack clinical data on rebamipide for the treatment of H. pylori-related gastritis.
Although up to 50% of nonulcer dyspepsia (NUD) patients have H. pylori infection and underlying chronic gastritis, it remains controversial whether the bacteria influences the pattern of gastric symptoms [11]. However, in one recent published meta-analysis from China, the summary odds ratio for improvement in dyspeptic symptoms in patients with functional dyspepsia in whom H. pylori was eradicated was 3.61 (95% CI: 2.62–4.98, P < 0.00001) and the difference in the follow-up period did not influence the final outcomes [12]. Thus H. pylori status needs to be determined first in the current study before the effect of rebamipide could be evaluated. Moreover, chronic gastritis is characterized by the accumulation of oxidative DNA damage [13] and antral prostaglandin E2 basal levels appear to be important for the development of aspirin-induced gastric damage in subjects without H. pylori infection [14]. In this study, two indicators of oxygen free radicals and gastroprotective agents were investigated after rebamipide administration. Sucralfate, a traditional gastroprotective agent, has shown affirmative effects on chronic gastritis by means of anti-acid. Without the molecular mechanism of PG and oxidation adjustment, it becomes an ideal candidate for the control of rebamipide.
Therefore, the major aim of the trial was to evaluate the effect of rebamipide on CEG in Chinese patients. Due to the less common use of NSAID in China than in Western countries, NSAID-induced gastric injury was excluded from the study. There is a great deal of evidence showing rebamipide’s protection on NSAID-induced gastric injury, so the current study was designed to illustrate the role of the drug on NSAID-unrelated gastric pathology.
Materials and Methods
Patients
The study was carried out as an open, randomized, positive drug parallel-controlled and subgrouped clinical trial performed in 11 centers between October 2004 and December 2005. Each center was expected to complete 60 cases of CEG therapy with either rebamipide or sucralfate (with a ratio of 3:1). The sample size and ratio were determined by a power study by statistics specialists.
Criteria for inclusion were age 18–65 years, diagnosed chronic erosive gastritis by endoscopy within 1 week, and having at least two symptoms of abdominal pain, distension, acid reflux, and belching. Exclusion criteria were: (i) patients with malignancy diseases, (ii) peptic ulcer, (iii) patients who has been administrated with drugs that may affect evaluation during two weeks before enrolled (NSAID, proton pump inhibitor, H2-antagonist, anti-acid regents, and antibiotics, etc.), (iv) severe heart or pulmonary disease, (v) pregnancy, (vi) allergic habitus, and (vii) other situations that the investigators considered unsuitable for the study. Previous H. pylori infection history and any NSAID usage during 1 month were also recorded. All patients signed written informed consent prior to the study and the whole protocol was approved by the ethics committee in each participating institute.
Erosive gastritis was determined by endoscopy according to the Sydney system and modified endoscopic chronic gastritis classification consensus (2003, China). Three biopsy samples were taken from antrum during endoscopy, one from the erosion area and another from a normal area with an extra sample for rapid urease testing. H. pylori positivity was defined as positive for both the rapid urease test and 13C or 14C urea-breath test. In two centers, an extra two samples were taken in each subject for quantification of mucosal PGE2 and the oxygen free radical product malondialdehyde (MDA).
Study Design
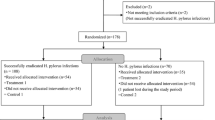
Finally 453 patients were enrolled, 15 of which did not have a definite H. pylori status. To evaluate the effect of rebamipide on H. pylori-associated gastritis, 438 patients were classified to three kinds of conditions before administration of gastroprotective agents: (i) H. pylori positive but not eradicated; (ii) H. pylori positive but eradicated, patients received a base therapy consisting of 1 week omeprazole 20 mg b.i.d. plus amoxicillin 1.0 b.i.d. and clarithromycin 500 mg b.i.d. prior to randomization; (iii) H. pylori negative. This subgrouping will lead to a clear interpretation of the effect of the gastroprotective agents beyond the influence of H. pylori status. The homogeneity between each subgroup was first analyzed as the 1 week eradication therapy in H. pylori-positive subjects may affect the dyspeptic symptom during the baseline periods. If there was any significant difference between the baselines of the subgroups, multivariant analysis was conducted. All 453 patients were randomized into two groups, the REB group receiving rebamipide 300 mg/d (100 mg po t.i.d.) (Mucosta®, Otsuka, Japan) and the SUC group receiving sucralfate 3.0/d (1.0 po t.i.d.) (Shu Ke Fei®, Hefeng Pham. Ltd, Shanghai) for 8 weeks (Fig. 1).
GI symptom changes in the first week were recorded daily on diary paper by patients and the following 2, 4, 6, and 8 weeks of information was written by investigators. Cure of H. pylori infection was determined by repeated 13C or 14C UBT at the end of study. Moreover, aiming to obtain evidence for the rebamipide-induced molecular mechanism, mucosal PGE2 and MDA concentration were detected before and after 8 weeks therapy in 2 of the 11 centers (Changhai & Zhongshan Hospitals; the other centers did not have the facilities to test this).
Randomization
Randomization was designed to determine the subjects to receive rebamipide or sucralfate therapy. Randomization was performed after the H. pylori status of subjects had been determined. The subjects were then recruited according to a randomization schedule produced by statistics software. A randomization number associated with either rebamipide or sucralfate was assigned to each patient in the study. An allocation ratio of 3:1 for the two treatment groups was set according to the power study, and each center used its own randomization number. Randomization numbers were generated by using the SAS program.
Clinical Effect Evaluation
The symptoms during the first week and at the end of 2, 4, 6, and 8 weeks were monitored by scoring symptoms including pain, distension, reflux, and belching. Each symptom was graded as 0 (none), 1 (mild), 2 (obvious, partially disturbing daily life), and 3 (severe, disturbing daily life and needing drugs). The total scores of symptoms were then calculated to evaluate the effect on symptom change by treatment. Each patient was taught how to evaluate and make a record on a diary card.
Endoscopic images before and after therapy were considered another indicator of effective treatment. Alterations in gastric mucosa images could be quantified according to the modified Lanza standard as following: 0 (no erosion), 1 (one or two erosive lesions limited in one area as antrum, corpus or fundus), 2 (three to five lesions but in the same area), 3 (lesions involving two areas but fewer than ten), and 4 (extensive lesions or more than ten). Endoscopists in each center received training before the trial started and two copies of pictures from every patient were sent to the leading center for repeat reviewing.
The specimens taken from antrum were used to study the histological effects of rebamipide on inflammation in the gastric mucosa. The strategy of taking both erosive and nonerosive lesions at the same time avoided miscounting of the inflammatory score. Histological findings on the activity and chronic inflammation were graded as 0 (normal), 1 (mild), 2 (moderate), or 3 (marked) according to the updated Sydney system [15]. Specimens were treated with hematoxylin and eosin, and Giemsa stains. One pathologist, having no information on the subjects, performed uniform histological grading. Safety monitoring was also conducted according to the recorded adverse events.
Mucosal PGE2 and MDA Measuring
Two mucosal specimens taken from erosive and nonerosive lesion (within 2 cm of the erosive area) in each patient were collected before and after treatment. Mucosal PGE2 and MDA concentration were measured by the RIA and thiobarbituric acid (TBA) methods as previously published, respectively.
End Points
The primary endpoint was symptom change and histological remission at 8 weeks. The secondary endpoint was the change of PGE2 and MDA concentration in the gastric mucosa after the therapy. To exclude the effect of H. pylori infection, subgroup analysis was carried out after the analysis of the whole group.
Statistical Analysis
All the case report forms (CRFs) were sent to the Department of Statistics, Second Military Medical University by the end of study. Data were then computerized and analyzed with SAS 8.2 software (SAS Institute Inc., North Carolina, US). The full analysis set (FAS) consists of the randomized 453 patients. The per-protocol (PP) analysis was performed by using data only from subjects characterized by the criteria: (i) completion of the whole treatment; (ii) availability of results useful for the primary aim; and (iii) no major protocol violations. The demographic characteristics of the two groups before treatment were compared using the Student’s t-test, chi-squared test, and Mann–Whitney U test according the character of index. Within each group, symptom scoring, endoscopic, and histological grading were compared before and after therapy by using the Wilcoxon rank sum test for their categorical character. For the PGE2 and MDA results, the paired t-test was used to compare the alteration. When comparing the effects between two groups or three subgroups, we used covariance analysis or Cochran-Mantel-Haenszel (CMH) method. All statistical tests were two-sided, with a 5% level of significance.
Results
Demographic Characteristics of Subjects
A total of 453 CEG patients were enrolled in the study and randomized into two groups at the beginning as follows: rebamipide 342 cases and sucralfate 110 cases. One patient’s grouping information was not recorded on the CRF and could not be put in FAS. H. pylori status was not determined for another 14 patients in the rebamipide group, which hence could not be included in the PP analysis (Fig. 2). The number of cases in the subgroups according to H. pylori status was 150 (eradicated), 129 (not eradicated), and 159 (negative). Then 438 patients were randomized as 331 cases in rebamipide group and 107 in sucralfate group. Twenty-three patients (REB: 13; SUC: 10) were excluded from the PP analysis for the following reasons: incomplete histology results (n = 16); failure of eradication assessment (n = 2); lost to follow-up (n = 2); adverse events (n = 3). Finally the PP set consisted of 415 patients (REB: 318; SUC: 97). The adverse events (AE) occurring during study includes one case of eczema, one case of abnormal hepatic function, and another hospitalization for acute appendicitis.
The comparison of demographic characteristics showed that there were no significant differences between the REB and SUC groups in terms of age, sex, stature, body weight, disease course, smoking or alcohol habit and current H. pylori status, as shown in Table 1.
Effects of Rebamipide on GI Symptoms
The records on daily and weekly symptoms revealed that treatment with rebamipide or sucralfate could dramatically alleviate the four common CEG symptoms of pain, reflux, distension, and belching, as shown in Fig. 3. Improvement in symptoms showed a tendency to be acceptable (score lower than one) after 1 week therapy and be continuous for 8 weeks time. Subsequently, when compared with the baseline, the accumulated scores were significantly reduced both in the REB (2.49 ± 0.54 versus 5.54 ± 0.97, P < 0.001) and SUC (3.11 ± 0.47 versus 5.95 ± 0.83, P < 0.001) groups at the end of week 1. However, the score difference between the baseline and week 8 in the REB group was significantly larger than that in the SUC group (P < 0.001, Fig. 4), while there was no significant difference between the two baselines (P = 0.189). Though the median together with 25% and 75% intervals instead of the mean should be used to represent the symptom score for its categorical native, the mean value was preferred to show the trend clearly.
The trend of CEG-associated GI symptoms in the rebamipide (REB) and sucralfate (SUC) groups. Time courses of the mean value of symptom scores are shown in the graphs. The X-axis represents days from baseline and the Y-axis represents symptom score. (The lines represent the trend rather than a continuous variable)
The reduced total scores of CEG-associated GI symptoms with rebamipide (REB) and sucralfate (SUC) treatment by PP analysis. The X-axis indicates the time courses after therapy while the Y-axis represents accumulated symptom score. The error bars represent the standard deviation (SD) and the height of the bars represents the mean score. * P-value produced by comparing with the baseline within each group (Wilcoxon signed rank test), ** P-value between the two groups by Wilcoxon rank sum analysis
Effects of Rebamipide on Gastric Mucosal Inflammation
The visible improvement of mucosal inflammation under endoscopy is one of the most important indexes in the evaluation of the therapeutic effect on CEG. Both REB and SUC could significantly decrease the endoscopic score with 8 weeks therapy as shown in Fig. 5a. Before therapy the median endoscopic score in both the REB and SUC groups was 3. At 8 weeks, the median scores became 0 in the REB and 1 in the SUC group (0.60 ± 0.10 versus 2.65 ± 0.09, P < 0.001 in the REB group and 1.05 ± 0.19 versus 2.53 ± 0.14, P < 0.001 in the SUC group, mean ± SEM, Wilcoxon signed rank test). The Wilcoxon rank sum analysis showed that the difference between the two groups was statistically significant (P < 0.001) after 8 weeks therapy while no significant difference could be observed at baseline (P = 0.219). Furthermore, Fig. 5b, c shows the mean inflammation and activity scores by the updated Sydney system in the histological examination of the gastric mucosa at baseline and at the end of week 8. Both the inflammation and activity scores of the two groups at baseline were comparable (P = 0.078 and P = 0.851). In both groups, the scores of chronic inflammation significantly decreased after treatment (median dropped from 3 to 1.5 in the REB group and from 2 to 2 in the SUC group, P < 0.001 by Wilcoxon signed rank test, respectively) and no significant differences could be observed between the REB and SUC groups (P = 0.545). However, the REB group showed a significant inhibition on inflammatory activity (median of 1 versus 0, P < 0.001 by Wilcoxon signed rank test) while the SUC group did not, even though the difference between the two groups remained nonsignificant (P = 0.877 by Wilcoxon rank sum analysis).
Inhibition of gastric mucosal inflammation with rebamipide (REB) and sucralfate (SUC) treatment by PP analysis. The three regions in the graph represent endoscopic inflammation score (a), histological chronic inflammation score (b), and histological activity score (c). The X-axis indicates the time courses before and after 8 weeks therapy while the Y-axis represents the inflammation score. The error bars represent the standard error of the mean (SEM) and the height of bars represents the mean score. * P-value produced by comparing with the baseline in the REB group (Wilcoxon signed rank test), ** P-value produced by comparing with the baseline in the SUC group (Wilcoxon signed rank test), *** P-value between the two groups by Wilcoxon rank sum analysis
Rebamipide Increased PGE2 and Depressed MDA Content in Gastric Mucosa
The PGE2 content in gastric mucosa has been wildly accepted as an indicator of mucosal protecting agent level, which indeed reflects the effect of REB or SUC. Considering that the distribution of inflammation in antrum was not homogeneous, we compared the effect of REB and SUC on the PGE2 level in the erosive and nonerosive regions, respectively. In contrast to expectations, the nonerosive area had a slightly but nonsignificantly higher PGE2 level than the erosive area before therapy. After 8 weeks of treatment, REB induced a significantly increased PGE2 level in both areas, from 236.2 ± 17.9 pg/g to 261.2 ± 21.6 pg/g in the nonerosive area and from 225.4 ± 18.3 pg/g to 266.7 ± 14.7 pg/g in the erosive area (P < 0.001 by paired t-test, Table 2). SUC also increased PGE2 level in both areas. However the difference between REB and SUC on PGE2 induction was only evident in the erosive area (P = 0.002 by covariance analysis).
Furthermore, MDA, a metabolite of oxygen free radicals, showed a lower concentration in the nonerosive area than in the erosive area at baseline (203.8 mmol/g versus 316.5 mmol/g, P < 0.01). Depressed MDA level could be observed in both the erosive and nonerosive areas in the REB group (216.5 ± 61.5 mmol/g and 177.6 ± 32.5 mmol/g, Table 2). The MDA content in the SUC group was decreased in both areas but only significantly in the erosive area. When compared with SUC, REB showed a statistically better effect on MDA inhibition in the erosive area (P = 0.046 by covariance analysis).
Further Analysis in Subgroups According to H. pylori Status
In the PP set (Fig. 2), at the start of the trial 201 of the 318 subjects in the REB group and 63 of the 97 subjects in the SUC group were H. pylori-infected patients, with an infection rate of 63.2% and 64.9%, respectively (P = 0.810 by chi-squared test). For study purposes, 54.7% (n = 110) of the H. pylori-positive patients in the RUB group and 47.6% (n = 30) of the H. pylori-positive patients in the SUC group received triple eradication therapy. There was no significant difference between the REB and SUC groups in terms of the ratio of antibiotics-treated patients before the formal treatment started (P = 0.386). While the H. pylori-positive rate became 24.5% (n = 78) in the REB group and 36.1% (n = 35) in the SUC group when the H. pylori status was redetected after 8 weeks of therapy. The positive rate in the REB group was significantly lower than that in the SUC group, with a P value of 0.027 by the chi-squared test.
A subgroup analysis by H. pylori status associated with REB or SUC effect on symptom change is shown in Fig. 6. The tendency cause by REB was not affected by whether H. pylori eradication was performed or not (P > 0.05). Interestingly, the significant difference between the REB and SUC groups could be observed 1 week earlier in H. pylori-negative patients than in H. pylori-positive patients, in whom the differences could only be observed after 1 month. Moreover, endoscopic scores revealed that, even in the H. pylori noneradicated subgroup, REB yielded improvement in inflammation at week 8 with a significantly lower score (0.57 ± 0.85, P < 0.001) compared with baseline (2.71 ± 0.84). REB also performed effectively in terms of reducing endoscopic scores both in the H. pylori natively or acquired negative subgroups (0.57 ± 0.77 and 0.64 ± 0.94, P < 0.001 compared with baseline, respectively). No significant difference could be found among the three subgroups by Wilcoxon rank sum analysis. The results from histological inflammation or activity paralleled the result from endoscopy. In 52 subjects tested for MDA and PGE2 in the REB group, the subgroups status was 18, 16, and 18 for H. pylori noneradicated, eradicated, and negative status, respectively. In the erosive lesions, the PGE2 content increased from 234.7 ± 27.0 pg/g to 254.8 ± 32.1 pg/g (P < 0.05) in H. pylori-eradicated subgroup, with no significant difference when compared with the noneradicated and negative subgroups (220.6 ± 25.3 pg/g to 263.8 ± 19.8 pg/g and 240.9 ± 24.5 pg/g to 260.6 ± 25.8 pg/g, respectively, P > 0.05). For MDA analysis, REB significantly decreased mucosal MDA in all three subgroups and no H. pylori-related effects were found.
The decrease of total symptom scores with rebamipide (REB) or sucralfate (SUC) therapies in subgroups according to H. pylori status. The time courses of the mean symptom score value are shown in the graphs. The X-axis represents days from baseline and the Y-axis represents mean symptom score. * P < 0.05 and ** P < 0.01 between the REB and SUC groups by Wilcoxon rank sum analysis. (The lines represent the trend rather than a continuous variable)
Safety Assessment
Only three cases of adverse effects (AEs) occurred, in 0.62% of the REB group (n = 2) and 1.03% (n = 1) of the SUC group (n = 1) and were excluded from PP analysis, including one case of rash, one case of abnormal hepatic function, and another hospitalization for acute appendicitis. All of the AEs developed in the period from the start to week 4. No gastrointestinal symptoms, including diarrhea, vomiting, and stomatitis, were found. All the events were judged nonserious and not relevant to the study. Two patients (one each in the REB and SUC groups) discontinued the study drug and were lost to the follow-up. These results demonstrated that treatment with rebamipide did not affect patient safety when compared with the previously proved safe drug sucralfate (P = 0.551).
Discussion
One of the major roles of rebamipide [2-(4-chlorobenzoylamino)-3[2(1H)-quinolonin-4-yl] propionic acid, MucostaTM] is to stimulate the generation of endogenous prostaglandins in the gastric mucosa and it has been reported to facilitate and accelerate ulcer healing. Depending on the extensive published studies in vitro or in vivo, this clinical trial represents a reliable result of rebamipide on chronic gastritis. Rebamipide shows satisfied CEG-associated symptom attenuation with 8 weeks therapy, with a rapid effect during 1 week. Most importantly, this effect has been proven by using sucralfate, a traditional gastroprotective regent as a positive control. In a previous placebo-controlled multicenter study [16], rebamipide showed no significant improvement in individual symptom scores, except for a significantly reduced belching score in rebamipide 100 mg and 200 mg groups at week 2 in Helicobacter pylori-positive patients. Though symptom scores are sometimes subjective and vary between studies, they may provide direct evidence in clinical trials. Rebamipide showed a better effect than sucralfate on symptom attenuation, including stomach ache and distension, as well as acid reflux and belching after the baselines were calibrated.
The mechanism by which rebamipide induces gastric mucosa protection was also proved in this study. Rebamipide leads to upregulated PGE2 and downregulated oxygen free radicals level in the whole area of antrum. Furthermore, the anti-inflammation effects were proved by both macro and micro inspection. The inflammation scores were significantly reduced. Rebamipide might also perform gastroprotection via more pathways, such as downregulation of intercellular adhesion molecule-1 (ICAM-1) expression, inhibition of mitochondrial damage, lipid peroxidation, and apoptosis in indomethacin-induced injuries [17–19]. Compared with sucralfate, rebamipide shows a more advanced role in the inhibition of activity, which is characterized by neutrophil infiltration. It has been reported that rebamipide decreased the susceptibility of gastric mucosa to acid-induced injury by inhibiting neutrophil activation in rats [20] while similar evidence in humans is rare. Rebamipide plays an extra role of stimulation of endogenous PG excretion and anti-oxide action compared with sucralfate, which led to the more obvious improvement of gastric inflammation in this study. Interestingly, rebamipide performed better than sucralfate on the level of PGE2 and MDA only in the erosive region in the stomach. The potential causes for this lesion-dependant effect might be the higher oxygen free radical level in the erosive area and that rebamipide has stronger effect on COX-2 synthesis and radical scavenging [21–23]. Moreover, other roles involved in rebamipide-induced gastric ulcer healing, such as stimulating angiogenesis, may also lead to the disappearance of erosive lesions in CEG [24]. Other than inflammation indexes, MDA and PGE2, epithelial barrier function or paracellular permeability have been considered new targets for rebamipide effect in animal models [25–27], although these indexes need to be designed practically before being used in future clinical trials.
Because predominant clinical CEGs are H. pylori associated, the bacterial factor should not be ignored in this study. As estimated, the H. pylori infection rate in the population was over 60% before randomization. H. pylori-associated gastritis or ulcer is always harder to cure than unassociated cases, as ulcers should be treated after anti-H. pylori triple therapy. However, rebamipide did not show a worse result with H. pylori infection compared with eradicated or negative subjects despite this concern. Actually rebamipide acted in a H. pylori-independent manner. However the results could not be explained by the hypothesis that rebamipide has the function of eliminating H. pylori, even though we have found the H. pylori infection rate in the rebamipide group to be lower than in the sucralfate group. What we can conclude from our study is that rebamipide may improve outcomes of H. pylori-associated gastritis, as previously published [28–31]. And more long-term, well-designed controlled trials need to be carried out in the future to confirm the drug's relationship with H. pylori status [32].
Importantly, no serious side-effects were experienced by any subjects in the study. Unlike misoprostol, rebamipide resulted in less adverse diarrhea side-effects. The data in the current study indicates the safety of rebamipide.
In conclusion, rebamipide leads to rapid, obvious, and long-term improvement of upper GI symptoms in CEG patients. Rebamipide can ameliorate inflammation in gastric mucosa by induction of gastric mucosal PGE2 synthesis and inhibition of free-radical activity. Most importantly, this effect was not influenced by H. pylori status and thus rebamipide can be used in the treatment of H. pylori-associated gastritis.
Abbreviations
- CEG:
-
Chronic erosive gastritis
- PGE2 :
-
Prostaglandin E2
- MDA:
-
Malondialdehyde
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- REB:
-
Rebamipide
- SUC:
-
Sucralfate
- STARS:
-
Symptom Treatment and Anti-inflammatory Effect of Rebamipide and Sucralfate for gastritis
References
Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A (1998) Rebamipide, overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci 43:5S–13S
Arakawa T, Higuchi K, Fujiwara Y, Watanabe T, Tominaga K, Sasaki E, Oshitani N, Yoshikawa T, Tarnawski AS (2005) 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci 50:3S–11S
Genta RM (2003) Review article: the role of rebamipide in the management of inflammatory disease of the gastrointestinal tract. Aliment Pharmacol Ther 18:8S–13S
Murata H, Yabe Y, Tsuji S, Tsujii M, Fu HY, Asahi K, Eguchi H, Kawano S, Hayashi N (2005) Gastro-protective agent rebamipide induces cyclooxygenease-2 (COX-2) in gastric epithelial cells. Dig Dis Sci 50:70S–75S
Sakurai K, Osaka T, Yamasaki K (2005) Rebamipide reduces recurrence of experimental gastric ulcers: role of free radicals and neutrophils. Dig Dis Sci 50:90S–96S
Azuma T, Yamazaki S, Yamakawa A, Ito Y, Ohtani M, Dojo M, Yamazaki Y, Higashi H, Hatakeyama M (2003) The effects of cure of Helicobacter pylori infection on the signal transduction of gastric epithelial cells. Aliment Pharmacol Ther 18:39S–44S
Yoshida N, Ishikawa T, Ichiishi E, Yoshida Y, Hanashiro K, Kuchide M, Uchiyama K, Kokura S, Ichikawa H, Naito Y, Yamamura Y, Okanoue T, Yoshikawa T (2003) The effect of rebamipide on Helicobacter pylori extract-mediated changes of gene expression in gastric epithelial cells. Aliment Pharmacol Ther 18:63S–75S
Kim JS, Kim JM, Jung HC, Song IS (2003) The effect of rebamipide on the expression of proinflammatory mediators and apoptosis in human neutrophils by Helicobacter pylori water-soluble surface proteins. Aliment Pharmacol Ther 18:45S–54S
Hahm KB, Kim DH, Lee KM, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Joo HJ, Cho YK, Nam KT, Cho SW (2003) Effect of long-term administration of rebamipide on Helicobacter pylori infection in mice. Aliment Pharmacol Ther 18:24S–38S
Fujioka T, Arakawa T, Shimoyama T, Yoshikawa T, Itoh M, Asaka M, Ishii H, Kuwayama H, Sato R, Kawai S, Takemoto T, Kobayashi K (2003) Effects of rebamipide, a gastro-protective drug on the Helicobacter pylori status and inflammation in the gastric mucosa of patients with gastric ulcer: a randomized double-blind placebo-controlled multicentre trial. Aliment Pharmacol Ther 18:146S–152S
Talley NJ, Quan C (2002) Review article: Helicobacter pylori and nonulcer dyspepsia. Aliment Pharmacol Ther 16:58S–65S
Jin X, Li YM (2007) Systematic review and meta-analysis from Chinese literature: the association between Helicobacter pylori eradication and improvement of functional dyspepsia. Helicobacter 12:541–546
Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R (1998) Oxidative DNA damage accumulation in gastric carcinogenesis. Gut 42:351–356
Rokkas T, Simsek I, Ladas S (2007) Helicobacter pylori and non-malignant diseases. Helicobacter 12:20S–22S
Rugge M, Genta RM (2005) Staging and grading of chronic gastritis. Hum Pathol 36:228–233
Talley NJ, Riff DS, Schwartz H, Marcuard SP (2001) Double-blind placebo-controlled multicentre studies of rebamipide, a gastroprotective drug, in the treatment of functional dyspepsia with or without Helicobacter pylori infection. Aliment Pharmacol Ther 15:1603–1611
Hiratsuka T, Futagami S, Shindo T, Hamamoto T, Ueki N, Suzuki K, Shinji Y, Kusunoki M, Shinoki K, Wada K, Miyake K, Gudis K, Tsukui T, Sakamoto C (2005) Rebamipide reduces indomethacin-induced gastric injury in mice via down-regulation of ICAM-1 expression. Dig Dis Sci 50:84S–89S
Nagano Y, Matsui H, Muramatsu M, Shimokawa O, Shibahara T, Yanaka A, Nakahara A, Matsuzaki Y, Tanaka N, Nakamura Y (2005) Rebamipide significantly inhibits indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells. Dig Dis Sci 50:76S–83S
Naito Y, Kajikawa H, Mizushima K, Shimozawa M, Kuroda M, Katada K, Takagi T, Handa O, Kokura S, Ichikawa H, Yoshida N, Matsui H, Yoshikawa T (2005) Rebamipide, a gastro-protective drug, inhibits indomethacin-induced apoptosis in cultured rat gastric mucosal cells: association with the inhibition of growth arrest and DNA damage-induced 45 alpha expression. Dig Dis Sci 50:104S–112S
Harada N, Okajima K, Liu W (2005) Rebamipide decreases the susceptibility of gastric mucosa to acid-induced injury in rats by inhibiting neutrophil activation. Dig Dis Sci 50:56S–62S
Hiratsuka T, Futagami S, Tatsuguchi A, Suzuki K, Shinji Y, Kusunoki M, Shinoki K, Nishigaki H, Fujimori S, Wada K, Miyake K, Gudis K, Tsukui T, Sakamoto C (2005) COX-1 and COX-2 conversely promote and suppress ischemia-reperfusion gastric injury in mice. Scand J Gastroenterol 40:903–913
Bamba H, Ota S, Kato A, Miyatani H, Kawamoto C, Yoshida Y, Fujiwara K (2003) Effect of rebamipide on prostaglandin receptors-mediated increase of inflammatory cytokine production by macrophages. Aliment Pharmacol Ther 18:113S–118S
Sakurai K, Sasabe H, Koga T, Konishi T (2004) Mechanism of hydroxyl radical scavenging by rebamipide: identification of mono-hydroxylated rebamipide as a major reaction product. Free Radic Res 38:487–494
Tarnawski AS, Chai J, Pai R, Chiou SK (2004) Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action? Dig Dis Sci 49:202–209
Haruma K, Ito M (2003) Review article: clinical significance of mucosal-protective agents: acid, inflammation, carcinogenesis and rebamipide. Aliment Pharmacol Ther 18:153S–159S
Matysiak-Budnik T, Heyman M, Megraud F (2003) Review article: rebamipide and the digestive epithelial barrier. Aliment Pharmacol Ther 18:55S–62S
Joh T, Takezono Y, Oshima T, Sasaki M, Seno K, Yokoyama Y, Ohara H, Nomura T, Alexander JS, Itoh M (2003) The protective effect of rebamipide on paracellular permeability of rat gastric epithelial cells. Aliment Pharmacol Ther 18:133S–138S
Terano A, Arakawa T, Sugiyama T, Yoshikawa T, Haruma K, Asaka M, Shimosegawa T, Sakaki N, Ishii H, Sakamoto C, Takahashi S, Kinoshita Y, Fujioka T, Kobayashi K (2006) A pilot study to evaluate a new combination therapy for gastric ulcer: Helicobacter pylori eradication therapy followed by gastroprotective treatment with rebamipide. J Gastroenterol Hepatol 21:103–109
Higuchi K, Tanigawa T, Hamaguchi M, Takashima T, Sasaki E, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Matsumoto T, Watanabe T, Arakawa T (2003) Comparison of the effects of rebamipide with those of cimetidine on chronic gastritis associated with Helicobacter pylori in Mongolian gerbils. Aliment Pharmacol Ther 18:1S–7S
Choi KW, Lee YC, Chung IS, Lee JJ, Chung MH, Kim NY, Kim SW, Kim JG, Roe IH, Lee SW, Jung HY, Choi MG, Hahm KB, Hong WS, Kim JH (2002) Effect of rebamipide in treatment of Helicobacter pylori-associated duodenal ulcer: attenuation of chemokine expression and nitrosative damage. Dig Dis Sci 47:283–291
Adachi K, Fujishiro H, Mihara T, Komazawa Y, Kinoshita Y (2003) Influence of lansoprazole, famotidine, roxatidine and rebamipide administration on the urea breath test for the diagnosis of Helicobacter pylori infection. J Gastroenterol Hepatol 18:168–171
Haruma K, Ito M, Kido S (2002) Long-term rebamipide therapy improves Helicobacter pylori-associated chronic gastritis. Dig Dis Sci 47:862–867
Acknowledgements
The authors would like to thank all the institutes participating this study, as well as Prof. Jia He, Yalin Sun M.D., and Na Liu M.D. from the Statistics Department of SMMU for data collection and analysis. Credits should also go to Otsuka Pharmaceutical Co., Ltd. for providing the drugs for the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, Y., Li, Z., Zhan, X. et al. Anti-inflammatory Effects of Rebamipide According to Helicobacter pylori Status in Patients with Chronic Erosive Gastritis: A Randomized Sucralfate-Controlled Multicenter Trial in China—STARS Study. Dig Dis Sci 53, 2886–2895 (2008). https://doi.org/10.1007/s10620-007-0180-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-0180-z