Abstract
Due to the widespread use of high-quality cross-sectional imaging, pancreatic cystic neoplasms are being diagnosed with increasing frequency. Clinicians are therefore asked to counsel a growing number of patients with pancreatic cysts diagnosed incidentally at an early, asymptomatic stage. Over the last two decades, accumulating knowledge on the biologic behavior of these neoplasms along with improved diagnostics through imaging and endoscopic cyst fluid analysis have allowed for a selective therapeutic approach toward these neoplasms. On one end of the management spectrum, observation is recommended for typically benign lesions (serous cystadenoma), and on the other end, upfront resection is recommended for likely malignant lesions (main duct IPMN, mucinous cystadenoma, solid pseudopapillary tumor, and cystic pancreatic neuroendocrine tumors). In between, management of premalignant lesions (branch duct IPMN) is dictated by the presence of high-risk features. In general, resection should be considered whenever the risk of malignancy is higher than the risk of the operation. This review aims to describe the evolution and current status of evidence guiding the selection of patients with pancreatic cystic neoplasms for surgical resection, along with a specific discussion on the type of resection required and expected outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The first large retrospective reports describing surgical resection for rare, cystic pancreatic neoplasms were published in the 1950s–1970s [1–3]. Back in this era of no cross-sectional imaging, symptoms at presentation led to resection in most cases. Occasionally, invasion of adjacent structures or metastatic lesions were discovered intraoperatively [4]. The introduction of computed tomography (CT) in the late 1970s allowed for a more accurate preoperative characterization of pancreatic cystic neoplasms and revealed their true prevalence [5–7]. Currently, the prevalence of pancreatic cysts is 1–3% by CT and ultrasound (US), and approaches 10–20% by magnetic resonance imaging (MRI) [8–12]. By comparison, autopsy studies have shown the prevalence of pancreatic cysts to be around 25% in the general population, with the majority of cysts being smaller than 1 cm [13].
As a result, selective resection of cystic pancreatic neoplasms has become an increasingly acceptable strategy [14]. Diagnostic imaging with CT and/or MRI and endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) of cyst contents play a crucial role in the selection of the pancreatic cysts requiring surgical resection [7, 15, 16]. The decision to proceed with surgery for a pancreatic cyst requires the surgeon to weigh the risk of operative management against the risk of observing a potentially malignant lesion [17, 18]. Herein, we discuss the factors prompting surgical resection, methods of resection, and outcomes following resection for the most common pancreatic cystic neoplasms.
Serous Cystadenoma
Serous cystadenomas (SCA) have four morphological variants including the microcystic, macrocystic (oligocystic), mixed, and solid type, with less than half of SCA appearing in the “typical” microcystic form [19]. On diagnostic imaging, a typical SCA is characterized by a central stellate scar, calcifications, and small cysts in a honeycomb appearance. Establishing a diagnosis by imaging alone is often, but not always, possible [20, 21]. FNA has been shown to be a useful adjunct in the diagnosis of SCA, because SCAs contain fluid low in amylase (no communication with the pancreatic duct) and low in carcinoembryonic antigen (CEA, serous nature) [22]. Nonetheless, a considerable portion of patients with SCA may eventually undergo resection because of an unclear preoperative diagnosis [19]. The development of novel molecular markers in the cyst fluid is urgently needed to improve the accuracy of preoperative diagnosis for these benign pancreatic cystic neoplasms [23].
SCAs are slow-growing tumors, but can in very rare occasions have malignant potential. There have been approximately 30 reported cases of malignant transformation of SCA in the world literature, and these malignant SCA reports typically describe locally invasive lesions rather than metastatic disease [19, 24–26]. If the diagnosis can be accomplished preoperatively with certainty, surgical resection is not warranted. However, there are a few circumstances where surgical resection may be indicated for SCAs:
-
Symptoms SCAs comprise less than 10% of all symptomatic pancreatic cysts. They can occasionally be associated with abdominal pain, weight loss, nausea, fatigue, jaundice, or a palpable mass [27]. Typically, larger cyst size correlates with symptoms and can occasionally lead the treating physician to recommend resection.
-
Rapid growth Increased growth rates have been observed for SCAs greater than 4 cm in diameter or with a macrocystic (oligocystic) morphology [28, 29]. A previous study by Tseng and colleagues reported that the growth rate for tumors < 4 cm in size was approximately 0.5 cm/year, but increased to almost 2 cm/year for tumors ≥4 cm (Fig. 1). These investigators also reported that cysts >4 cm were more likely to be symptomatic; hence, they recommended resection of SCA that exceeded this size threshold. An additional observational study of 145 patients by Malleo and colleagues showed that the growth rate of SCAs was higher for the macrocystic (oligocystic) category. The growth rate of SCAs in this study also appeared to correlate with the age of the cyst and dramatically increased after the first 7 years of surveillance, irrespective of size at presentation.
Fig. 1 Serous cystadenomas larger than 4 cm appear to have a higher annual growth rate, often prompting surgical resection (adapted with permission from Tseng et al. [28])
-
Lesions that are marginally resectable at presentation Lesions whose resection may be significantly more demanding, if the tumor were to grow further, in terms of impending vascular involvement or change in the type of pancreatectomy required (Whipple vs. distal) may be preemptively approached surgically to avoid a more morbid operation in the future, especially in younger patients.
The operation required for SCAs is a segmental pancreatectomy targeting the site of the lesion. In a recent, large retrospective series of 1590 operations for SCA, distal pancreatectomy (54%) was more common than the Whipple procedure (29%) and operative mortality in this series was 0.6% [19]. Central pancreatectomy has also been described for resection of mid-gland lesions located at a safe distance from the common bile duct. Fistula rate is slightly higher than distal pancreatectomy (44 vs. 29%), but mortality is similar and central pancreatectomy patients had significantly reduced rates of long-term diabetes mellitus and pancreatic exocrine insufficiency [30]. Enucleation of SCA is also described for smaller lesions [19, 31]. Duodenum-preserving pancreatic head resection can also be performed with no recurrence in limited, small series [32].
Postoperative surveillance is not necessary, unless invasive carcinoma is found pathologically [33]. One study followed patients for a median of 49 months after resection and found no disease recurrence [34].
In general, once the diagnosis of a SCA is confirmed radiographically and/or by EUS, observation is sufficient. Resection is recommended for lesions that are indeterminate, clearly symptomatic, rapidly growing, or marginally resectable at presentation, especially in younger patients.
Mucinous Cystic Neoplasm
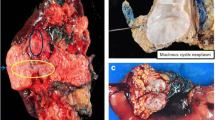
Resection of mucinous cystic neoplasms (MCNs) is recommended for all surgically fit patients [16], because current understanding suggests that all MCNs will transform to malignancy if not resected [35]. In addition, MCNs are commonly located the body or tail of the pancreas in middle-aged women, so the decision to operate is relatively straightforward as in most cases the operation is a laparoscopic left pancreatectomy and the patient is a good surgical candidate (Fig. 2).
Unilocular mucinous pancreatic cyst discovered incidentally by CT (left) in a 32-year-old woman. The patient underwent a laparoscopic spleen-preserving distal pancreatectomy. The surgical specimen is shown on the right. Note the location of the lesion (outlined with the dashed line) in relation to the pancreatic transection staple line (marked with the arrows). Pathology demonstrated a mucinous cystic neoplasm without dysplasia
MCNs are very difficult to differentiate from branch duct intraductal papillary mucinous neoplasms (BD-IPMNs) radiographically. Both lesions have high carcinoembryonic antigen (CEA) level on cyst fluid analysis (mucin production), but MCN fluid tends to have low amylase levels (no communication with the pancreatic duct). The hallmark of pathologic diagnosis for MCNs is the presence of ovarian-type stroma surrounding the cyst epithelium [15]. Carcinoma or atypia is present in 80% of patients when looking into early surgical series of symptomatic patients [36]. However, more recent series suggest a much lower rate of malignancy (mucinous cystadenocarcinoma), perhaps in the range of 5–20%, as the majority of lesions are found incidentally [37, 38]. Advanced age, tumor diameter greater than 4 cm, and mural nodularity have been proposed as predictors of malignancy [38–40].
As mentioned, the method of resection for the overwhelming majority of patients with MCN is distal pancreatectomy [38, 39]. Laparoscopic surgery is preferable provided the specimen can be removed without violation of the cyst wall and can be left intact for histological analysis [16, 35, 41]. Larger MCNs can be aspirated inside a specimen bag in the abdominal cavity and can be removed through a laparoscopic port site, especially if the suspicion for invasive malignancy is low. Lymphadenectomy including the nodes immediately adjacent to the pancreatic parenchyma should be performed [35]. The spleen can be preserved if there is no evidence of direct invasion [35, 42]. Limited pancreatic resections, such as enucleation and central pancreatectomy, can be performed [30–32, 43]. As mentioned, while central pancreatectomy has a higher postoperative pancreatic fistula rate, there are significantly lower rates of long-term endocrine and exocrine pancreatic insufficiency [30, 44].
In terms of long-term outcomes, two separate series reporting on a total of 234 patients showed no recurrence in the absence of invasive malignancy; however, the 5-year disease-specific survival ranged from 57 to 75%, if mucinous cystadenocarcinoma was present [39, 40]. Postoperative surveillance is not required for noninvasive MCNs as they are considered—in contrast to IPMNs—to be a unifocal process and the remnant pancreas is not at risk for developing a metachronous lesion [40, 45]. However, for mucinous cystadenocarcinoma, radiographic surveillance should be instituted in a way similar to resected pancreatic adenocarcinoma [16].
Observation of suspected MCN has been reported in the literature in the context of neoplastic pancreatic cysts in general, due to an indeterminate diagnosis [46]. Unfortunately, without pathologic confirmation, we cannot know whether these lesions were BD-IPMN or MCN. Some argue in favor of observing small lesions (<3 cm) with no nodularity and a normal serum CA19-9 [39]. MRI may be the most useful imaging study to follow MCN, as it can accurately identify cystadenocarcinoma within an MCN in 91% of cases based on cyst size (>7 cm), septal thickness (>3 mm), wall thickness (>3 mm), number of loculations (>4), nodules, T1 hyperintensity, invasion into adjacent structures, and evidence of metastases [42]. Observation of MCN is not currently the first-line strategy, but could be reserved for elderly patients with co-morbidities and lesions without any of the above worrisome features.
Intraductal Papillary Mucinous Neoplasms
IPMNs are a heterogeneous group of mucin-producing pancreatic cystic neoplasms, characterized by intraluminal growth of papillary epithelium, either involving the main duct (MD) or a branch duct (BD). Since their recognition 20 years ago, these lesions have attracted considerable attention as the most common radiographically identifiable precursor lesion of pancreatic adenocarcinoma. Approximately 10–20% of pancreatic cancers are felt to be arising in the setting of an IPMN. Interestingly, this subset of IPMN-related pancreatic cancers appears to have a better prognosis than conventional pancreatic adenocarcinoma [47]. Therefore, identifying an IPMN at a pre-invasive or early invasive stage appears to be an opportunity to prevent or cure pancreatic cancer.
The rates of malignancy (in situ or invasive carcinoma) are much lower for BD-IPMNs (7–51%) compared with MD-IPMNs (70%) [16]. Therefore, the main question when managing a patient with a BD-IPMN is “when is an operation necessary?” Conversely, for MD-IPMNs an operation is almost always necessary, but the question to answer is “which operation is necessary?”
Branch Duct IPMN: When Is an Operation Necessary?
Per the 2012 Fukuoka consensus guidelines for IPMN, resection is justified in patients with “high-risk stigmata,” including obstructive jaundice in the presence of a cystic lesion in the head of the pancreas, main pancreatic duct dilation to 10 mm or more, or a cystic lesion with an enhancing solid component. The presence of “worrisome features,” including pancreatitis, main pancreatic duct dilation of 5–9 mm, a non-enhancing mural nodule, cyst wall thickening or enhancement, cyst greater than 3 cm in size, and an abrupt change in the caliber of the pancreatic duct with distal pancreatic atrophy, should prompt further investigation with EUS. If EUS shows a definitive mural nodule or main duct involvement or FNA shows suspicious or malignant cytology, then resection is warranted. In the absence of worrisome features, cysts less than 1 cm should be followed with a CT or MRI in 2–3 years and if no change the frequency of radiographic observation can be determined on an individual basis; cysts that are 1–2 cm should have yearly CT or MRI for 2 years and then a longer observation interval if no change is noted. Last, cysts 2–3 cm in size without worrisome features should be followed with alternating EUS and MRI every 3–6 months; however, surgery may be considered in young, fit patients to avoid many years of prolonged radiographic surveillance [16].
A segmental pancreatectomy targeting the lesion is the operation of choice for BD-IPMN. These lesions are distributed relatively uniformly throughout the gland, with a slight predilection for the head (58%) versus body/tail (35%) [48]. Multifocality of BD-IPMN occurs in up to 25% of cases [48, 49]. In such cases, the surgeon should try to avoid a total pancreatectomy. Instead, the index high-risk lesion should be resected with a partial pancreatectomy and regional lymphadenectomy, leaving less worrisome lesions behind in the remnant pancreas for postoperative surveillance. The benefit of this approach is based on preservation of pancreatic parenchyma and avoidance of the apancreatic state with associated brittle diabetes. When total pancreatectomy has been performed for non-malignant IPMN, the 5-year survival has been reported to be only 74% and the 90-day mortality as high as 7% [50]. Central pancreatectomy and enucleation can been performed in selected cases of BD-IPMN without high-risk stigmata [30, 51].
The 2012 guidelines may actually lead to overtreatment of BD-IPMN. A recent retrospective multicenter analysis showed that surveillance of patients with worrisome features was associated with a 96% 5-year disease-specific survival [52]. For BD-IPMN specifically, the 5-year overall survival was 86% when considering an initial strategy of surveillance for both high-risk stigmata and worrisome features. Radiographic evidence of progression was seen in 52% over a median follow-up of 51 months, but only 14% of the cohort underwent resection. Along the same lines, the rate of malignancy within BD-IPMNs appears to be variable depending on the study methodology used: In retrospective surgical series, associated with obvious selection bias toward resection, the malignancy rate is approximately 25%, but in prospective series of non-operative management for BD-IPMN the malignancy rate has been reported to be as low as 5% [16, 53].
Main Duct IPMN: Which Operation Is Necessary?
Approximately 70% of MD-IPMN lesions contain high-grade dysplasia or invasive cancer [54]. Given this high prevalence of malignancy, the decision to proceed with surgical resection is more straightforward for MD-IPMNs; however, determining the type of resection remains an important consideration.
Segmental ectasia of the main pancreatic duct or the presence of a radiographically high-risk lesion (typically a solid mass) can guide the surgeon to perform a right (Whipple) or left (distal) pancreatectomy (Fig. 3). However, if the main duct is diffusely dilated without a focal high-risk lesion (complex cysts or nodules seen on CT, MRI, or EUS), the surgical community is divided regarding the most appropriate approach: On one hand, a total pancreatectomy can be considered to extirpate all radiographically visible disease (Fig. 4); on the other hand, the majority of pancreatic surgeons would recommend a Whipple (right) pancreatectomy with frozen section of the pancreatic neck margin. If high-grade dysplasia or invasive cancer is noted at the margin, the resection is extended to include an additional segment of pancreatic body until the margin is negative (for high-grade dysplasia or carcinoma). If this is not accomplished, the resection can be extended to a completion pancreatectomy. In a recent single-institutional series of 173 patients undergoing resection for MD-IPMN at a high-volume center, total pancreatectomy was only required in about 10% of cases [55]. Central pancreatectomy is rarely adequate for this disease due to the high likelihood of positive transection margins [56].
In the case of MD-IPMN, the operative approach may vary depending on the morphology of the lesion: Segmental ectasia of the main pancreatic duct should prompt the surgeon to perform targeted partial pancreatectomy to remove the affected part of the pancreas with either a Whipple (top right) or a distal (top left) pancreatectomy. If the main duct is diffusely dilated, the method of resection depends on the presence or not of a focal high-risk lesion (complex cyst or nodule seen on CT, MRI, or EUS). If one is present (bottom right), a partial targeted pancreatectomy should performed (in this case a Whipple) with frozen section of the neck margin (and additional resection if the margin is positive for high-grade dysplasia or carcinoma); if no high-risk lesion is present and the duct is diffusely dilated (bottom left), the options include a total pancreatectomy or a Whipple pancreaticoduodenectomy with frozen section of the neck margin (as described above). Most surgeons would favor the latter approach in this situation
MD-IPMN case necessitating a total pancreatectomy. Abdominal CT (top left) showing massive diffuse dilatation of the main pancreatic duct with extensive calcifications involving the pancreatic parenchyma. ERCP images (top right) showing dilated bile and pancreatic ducts, with irregular-shaped filling defects which were noted to change shape in real time, suggestive of mucin within the pancreatic duct. Operative specimen (bottom left) of total pancreatectomy that was necessary to extirpate the tumor replacing the entire pancreas. Gross inspection of the sectioned surgical specimen (bottom right) shows diffuse replacement of the entire gland by mucin-filled cysts
Lymph node positivity occurs in approximately 13% of MD-IPMN cases and is associated with decreased survival [55]. In addition, a higher number of positive lymph nodes is associated with worse survival [57]. Therefore, a regional lymphadenectomy should be performed for all resections of MD-IPMN, in the context of an oncologically complete operation [56].
As mentioned, frozen section analysis of the pancreatic margin is critical when performing a segmental pancreatectomy for MD-IPMN. Positive transection margins for high-grade dysplasia or carcinoma increase the risk of local recurrence and decreased survival [55, 58]. Intraoperative frozen section analysis is recommended to guide the extent of resection [59]. Additional resection should be pursued if histological examination shows at least high-grade dysplasia [60]. A recent study on MD-IPMN patients showed that the positivity of intraopertative frozen section analysis of the pancreatic margin for high-grade dysplasia or invasive carcinoma was 16% [55].
Laparoscopic surgery may lead to improved immediate postoperative outcomes with no compromise on the ultimate oncological outcome for pancreatic adenocarcinoma [61–63]. Minimally invasive techniques have been established for distal pancreatectomy and are recently being applied selectively to Whipple and total pancreatectomy [64].
Long-Term Outcome and Follow-Up
Prognosis and surveillance after resection of IPMN heavily depends on whether invasive carcinoma is found within the lesion. If this is the case, adjuvant therapy and follow-up strategies should resemble those for conventional pancreatic adenocarcinoma. Interestingly, IPMN-associated pancreatic cancer appears to have better prognosis than de novo pancreatic cancer (Fig. 5). Among IPMN-associated cancers, colloid carcinoma (arising from intestinal-type IPMN) behaves more favorably than tubular carcinoma (arising from pancreaticobiliary IPMN, Fig. 5) [47, 65]. Despite this improved prognosis, most oncologists would recommend adjuvant gemcitabine-based chemotherapy and cross-sectional imaging every 3–6 months after resection of invasive IPMN. It should be noted that there is no evidence supporting this approach, but this recommendation is based on data extrapolated from trials on standard pancreatic adenocarcinoma.
IPMN-associated pancreatic cancers tend to have a better prognosis after resection than standard pancreatic adenocarcinoma (left). This is particularly true for colloid IPMN carcinoma (arising from intestinal-type IPMN) as opposed to tubular IPMN carcinoma (arising for pancreaticobiliary type IPMN). This favorable prognosis appears to be related to a lower rate of lymph node metastasis (adapted with permission from Poultsides et al. [47])
After partial pancreatectomy for noninvasive IPMN, close radiographic follow-up is still recommended, as the process is felt to represent a “field defect” affecting the entire gland and additional lesions can appear in the remnant pancreas over time. In this situation, the recurrence rates in the pancreatic remnant have been reported to be between 5 and 25% [58, 66–69]. A retrospective review from Johns Hopkins demonstrated that following surgery for noninvasive IPMN 25% of patients developed a new IPMN over a period of 5 years [67]. The risk of developing pancreatic cancer was 7% at 5 years and 38% at 10 years. A similar study from Memorial Sloan Kettering reported that after resection for noninvasive IPMN 8% of patients experienced recurrence in the pancreatic remnant within a median follow-up of 36 months [58]. Based on the aforementioned data, surveillance imaging should occur at 2 and 5 years for completely resected IPMN with no dysplasia at the margin. If low-grade or moderate-grade dysplasia is noted at the transection margin, imaging should occur at 6-month intervals for 2 years followed by annual surveillance [16, 68]. Long-term follow-up beyond 5 years is also justified, as the chance of developing a new IPMN after surgery for noninvasive IPMN can be as high as 62% at 10 years [67].
Solid Pseudopapillary Neoplasm
Solid pseudopapillary neoplasms (SPNs) are uncommon cystic lesions of the pancreas, typically seen in the body and tail of the gland in young women. They comprise roughly 2% of pancreatic cysts [46]. They tend to have low malignant potential. A recent meta-analysis including 2744 patients revealed vascular involvement in 4.6%, lymph node metastasis in 1.6% and distant metastasis in 7.7% of cases [70]. Preoperative diagnosis by cross-sectional imaging is usually feasible, as these tumors usually appear as large, well-demarcated, solitary, mixed solid-cystic heterogeneous masses. EUS/FNA typically confirms the diagnosis, especially with beta-catenin immunostaining [71, 72].
SPNs are most commonly resected by distal pancreatectomy (49%), followed by Whipple procedure (24%). Parenchymal-sparing resections including central pancreatectomy and enucleation are less common (13%) [70]. Since 2005, there has been a significant increase in minimally invasive resections [73]. Splenic preservation seems to have no effect on recurrence [74].
Recurrence after resection of SPN is between 1 and 9% over approximately 50 months of follow-up [70, 75]. Disease-specific mortality is 1.5% over the same follow-up period [70]. In cases of recurrence or metastatic disease, survival can be prolonged significantly with repeat surgery, metastasectomy, radiation, or radiofrequency ablation of liver metastases [73].
There are no current recommendations for surveillance, but annual surveillance has been suggested for at least 5 years [76]. Tumor size greater than 8 cm, microscopic malignant features, and initial stage IV disease are significant predictors of recurrence and should prompt active surveillance. Median time to recurrence was 18.6 months in one study [73].
Cystic Pancreatic Neuroendocrine Tumors
Cystic pancreatic neuroendocrine tumors (cPNETs) represent a small minority of both pancreatic cysts and pancreatic neuroendocrine tumors (PNETs). cPNETs comprise approximately 7% of cystic pancreatic neoplasms [7]. They are typically non-functioning. These lesions are distinguished by other cysts on cross-sectional imaging based on their enhancing hypervascular rim and lack of septations (Fig. 6). EUS is a useful tool to obtain tissue that can be stained for synaptophysin and chromogranin A [77]. Most lesions are found incidentally and are less than 3 cm in diameter [78].
The degree of cystic component appears to correlate with clinicopathologic features and prognosis for cPNETs, with cystic tumors exhibiting a more favorable behavior. A recent retrospective review of 214 PNET patients from the authors’ institution included eight patients with purely cystic tumors, seven with mostly cystic tumors, 15 with mostly solid tumors, and 184 with purely solid tumors [79]. As the degree of cystic component decreased, the rate of several unfavorable factors increased, such as tumor size (1.5 ± 0.5, 3.0 ± 1.7, 3.7 ± 2.6, and 4.0 ± 3.5 cm), lymph node metastasis (0, 0, 26.7, and 34.2%), intermediate or high grade (0, 16.7, 20.0, and 31.0%), synchronous liver metastases (0, 14.3, 20.0, and 26.6%), and need for pancreaticoduodenectomy (0, 0, 6.7, and 25.0%). No cases of purely cystic PNETs were associated with synchronous liver or lymph node metastasis, intermediate/high grade, recurrence, or death due to disease. Among patients presenting without metastatic disease, 10-year recurrence-free survival was 100% in patients with purely and mostly cystic tumors versus 53.0% in patients with purely and mostly solid tumors. The authors hypothesized that perhaps purely cystic PNETs may represent a subset of PNETs pancreatic cystic neoplasms that can be safely observed without the need for immediate resection.
Conclusion
Surgical management of pancreatic cystic neoplasms continues to evolve toward a more selective resection strategy, as our ability to preoperatively diagnose their histopathologic type is gradually improving. Once the diagnosis is established, the recommended management algorithm is summarized in Fig. 7. On one end of the management spectrum, observation is recommended for typically benign lesions (serous cystadenoma), and on the other end, upfront resection is recommended for likely malignant lesions (main duct IPMN, mucinous cystadenoma, solid pseudopapillary tumor, and cystic pancreatic neuroendocrine tumors). In between, management of premalignant lesions (branch duct IPMN) is dictated by the presence of high-risk features. In general, an operation is justified when its risk is lower than the risk of the lesion harboring malignancy. Although modern cross-sectional imaging and endoscopic ultrasonography can lead to the correct diagnosis in the majority of cases, the accurate identification of the degree of dysplasia within the cyst preoperatively still remains challenging. Future scientific discovery in this direction will further allow for an even more successful selection of patients who truly require an operation and will avoid observation of patients with cysts harboring an occult malignancy.
References
Mozan AA. Cystadenoma of the pancreas. Am J Surg. 1951;81:204–214.
Becker WF, Welsh RA, Pratt HS. Cystadenoma and cystadenocarcinoma of the pancreas. Ann Surg. 1965;161:845–863.
Barbee CL, DeMello FB, Grage TB. Cystadenoma and cystadenocarcinoma of the pancreas. J Surg Oncol. 1976;8:1–10.
Hodgkinson DJ, ReMine WH, Weiland LH. Pancreatic cystadenoma. A clinicopathologic study of 45 cases. Arch Surg. 1978;113:512–519.
Stanley RJ, Sagel SS, Levitt RG. Computed tomographic evaluation of the pancreas. Radiology. 1977;124:715–722.
Johnson CD, Stephens DH, Charboneau JW, Carpenter HA, Welch TJ. Cystic pancreatic tumors: CT and sonographic assessment. AJR Am J Roentgenol. 1988;151:1133–1138.
Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg. 2011;212:590–600.
Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553.
Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807.
de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811.
Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084.
Soroida Y, Sato M, Hikita H, et al. Pancreatic cysts in general population on ultrasonography: prevalence and development of risk score. J Gastroenterol. 2016;51:1133–1140.
Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206.
Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432–443.
Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32.
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197.
Al Efishat M, Allen PJ. Therapeutic approach to cystic neoplasms of the pancreas. Surg Oncol Clin N Am. 2016;25:351–361.
Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic cyst disease: a review. JAMA. 2016;315:1882–1893.
Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305–312.
Gouhiri M, Soyer P, Barbagelatta M, Rymer R. Macrocystic serous cystadenoma of the pancreas: CT and endosonographic features. Abdom Imaging. 1999;24:72–74.
Khurana B, Mortele KJ, Glickman J, Silverman SG, Ros PR. Macrocystic serous adenoma of the pancreas: radiologic-pathologic correlation. AJR Am J Roentgenol. 2003;181:119–123.
Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts. A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41–47.
Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501–1510.
Ohta T, Nagakawa T, Itoh H, Fonseca L, Miyazaki I, Terada T. A case of serous cystadenoma of the pancreas with focal malignant changes. Int J Pancreatol. 1993;14:283–289.
Matsumoto T, Hirano S, Yada K, et al. Malignant serous cystic neoplasm of the pancreas: report of a case and review of the literature. J Clin Gastroenterol. 2005;39:253–256.
Galanis C, Zamani A, Cameron JL, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820–826.
Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427-3.
Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–419.
Malleo G, Bassi C, Rossini R, et al. Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut. 2012;61:746–751.
Crippa S, Bassi C, Warshaw AL, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg. 2007;246:69–76.
Crippa S, Bassi C, Salvia R, Falconi M, Butturini G, Pederzoli P. Enucleation of pancreatic neoplasms. Br J Surg. 2007;94:1254–1259.
Beger HG, Schwarz M, Poch B. Duodenum-preserving total pancreatic head resection for benign cystic neoplastic lesions. J Gastrointest Surg. 2012;16:2160–2166.
Katz MH, Mortenson MM, Wang H, et al. Diagnosis and management of cystic neoplasms of the pancreas: an evidence-based approach. J Am Coll Surg. 2008;207:106–120.
Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P. Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg. 2003;27:319–323.
Fernandez-del Castillo C. Mucinous cystic neoplasms. J Gastrointest Surg. 2008;12:411–413.
Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573–580.
Crippa S, Fernandez-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–219.
Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026–1031.
Park JW, Jang JY, Kang MJ, Kwon W, Chang YR, Kim SW. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology. 2014;14:131–136.
Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579.
Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438–446.
Di Paola V, Manfredi R, Mehrabi S, et al. Pancreatic mucinous cystoadenomas and cystoadenocarcinomas: differential diagnosis by means of MRI. Br J Radiol. 2016;89:20150536.
Talamini MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg. 1998;227:896–903.
Iacono C, Verlato G, Ruzzenente A, et al. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873–885.
Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40:67–71.
Allen PJ, D’Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–582.
Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476.
Kim YI, Shin SH, Song KB, et al. Branch duct intraductal papillary mucinous neoplasm of the pancreas: single-center experience with 324 patients who underwent surgical resection. Korean J Hepatobiliary Pancreat Surg. 2015;19:113–120.
Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79.
Zakaria HM, Stauffer JA, Raimondo M, Woodward TA, Wallace MB, Asbun HJ. Total pancreatectomy: short- and long-term outcomes at a high-volume pancreas center. World J Gastrointest Surg. 2016;8:634–642.
Hwang HK, Park JS, Kim JK, Park CM, Cho SI, Yoon DS. Comparison of efficacy of enucleation and pancreaticoduodenectomy for small (< 3 cm) branch duct type intraductal papillary mucinous neoplasm located at the head of pancreas and the uncinate process. Yonsei Med J. 2012;53:106–110.
Crippa S, Bassi C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2016 (Epub ahead of print).
Marchegiani G, Fernandez-del Castillo C. Is it safe to follow side branch IPMNs? Adv Surg. 2014;48:13–25.
Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93–102.
Marchegiani G, Mino-Kenudson M, Sahora K, et al. IPMN involving the main pancreatic duct: biology, epidemiology, and long-term outcomes following resection. Ann Surg. 2015;261:976–983.
Crippa S, Partelli S, Falconi M. Extent of surgical resections for intraductal papillary mucinous neoplasms. World J Gastrointest Surg. 2010;2:347–351.
Partelli S, Fernandez-Del Castillo C, Bassi C, et al. Invasive intraductal papillary mucinous carcinomas of the pancreas: predictors of survival and the role of lymph node ratio. Ann Surg. 2010;251:477–482.
White R, D’Angelica M, Katabi N, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993.
Couvelard A, Sauvanet A, Kianmanesh R, et al. Frozen sectioning of the pancreatic cut surface during resection of intraductal papillary mucinous neoplasms of the pancreas is useful and reliable: a prospective evaluation. Ann Surg. 2005;242:774–778.
Falconi M, Salvia R, Bassi C, Zamboni G, Talamini G, Pederzoli P. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg. 2001;88:376–381.
Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010;210:779–785.
Stauffer JA, Coppola A, Mody K, Asbun HJ. Laparoscopic versus open distal pancreatectomy for pancreatic adenocarcinoma. World J Surg. 2016;40:1477–1484.
Stauffer JA, Coppola A, Villacreses D, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: long-term results at a single institution. Surg Endosc. 2016 (Epub ahead of print).
Chapman BC, Paniccia A, Ryan C, Schulick RD, Edil BH. Laparoscopic spleen-preserving total pancreatectomy for a main-duct intraductal papillary mucinous neoplasm. Ann Surg Oncol. 2016 (Epub ahead of print).
Yopp AC, Katabi N, Janakos M, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg. 2011;253:968–974.
Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Patterns of recurrence after resection of IPMN: who, when, and how? Ann Surg. 2015;262:1108–1114.
He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg. 2013;216:657–665.
Ohtsuka T, Kono H, Tanabe R, et al. Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am J Surg. 2012;204:44–48.
Ridtitid W, DeWitt JM, Schmidt CM, et al. Management of branch-duct intraductal papillary mucinous neoplasms: a large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest Endosc. 2016;84:436–445.
Law JK, Ahmed A, Singh VK, et al. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331–337.
Law JK, Stoita A, Wever W, et al. Endoscopic ultrasound-guided fine needle aspiration improves the pre-operative diagnostic yield of solid-pseudopapillary neoplasm of the pancreas: an international multicenter case series (with video). Surg Endosc. 2014;28:2592–2598.
Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369.
Kang CM, Choi SH, Kim SC, et al. Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea. Ann Surg. 2014;260:348–355.
Cavallini A, Butturini G, Daskalaki D, et al. Laparoscopic pancreatectomy for solid pseudo-papillary tumors of the pancreas is a suitable technique; our experience with long-term follow-up and review of the literature. Ann Surg Oncol. 2011;18:352–357.
Marchegiani G, Andrianello S, Massignani M, et al. Solid pseudopapillary tumors of the pancreas: Specific pathological features predict the likelihood of postoperative recurrence. J Surg Oncol. 2016;114:597–601.
Allen PJ, Brennan MF. The management of cystic lesions of the pancreas. Adv Surg. 2007;41:211–228.
Baker MS, Knuth JL, DeWitt J, et al. Pancreatic cystic neuroendocrine tumors: preoperative diagnosis with endoscopic ultrasound and fine-needle immunocytology. J Gastrointest Surg. 2008;12:450–456.
Gaujoux S, Tang L, Klimstra D, et al. The outcome of resected cystic pancreatic endocrine neoplasms: a case-matched analysis. Surgery. 2012;151:518–525.
Cloyd JM, Kopecky KE, Norton JA, et al. Neuroendocrine tumors of the pancreas: degree of cystic component predicts prognosis. Surgery. 2016;160:708–713.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Gerry, J.M., Poultsides, G.A. Surgical Management of Pancreatic Cysts: A Shifting Paradigm Toward Selective Resection. Dig Dis Sci 62, 1816–1826 (2017). https://doi.org/10.1007/s10620-017-4570-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4570-6