Abstract
Background
Vulnerable populations are disproportionately affected by hepatitis C virus (HCV) infection and experience high rates of health disparity. There are no data on real-world experience with highly efficacious direct-acting anti-HCV treatment in this population.
Aims
We aimed to evaluate the real-world experience with sofosbuvir-based regimens among a vulnerable HCV-infected population.
Methods
HCV treatment response was assessed among 204 patients who completed 12–24 weeks of sofosbuvir-based regimens (in combination with pegylated interferon and ribavirin, simeprevir, ledipasvir, or daclatasvir) at the San Francisco safety-net healthcare system liver specialty clinic between January 2014 and December 2015. Virologic response during therapy was assessed at weeks 4 and 8, end of therapy, and 12-week treatment discontinuation (SVR 12).
Results
Patient characteristics were median age 58 years, 60 % male, 42 % Caucasian (21 % black, 19 % Hispanic), 72 % had genotype 1 (23 % genotype 2 or 3), and the median baseline log10 HCV viral load was 6.1 IU/ml and alanine transaminase 63 U/l. Cirrhosis was present in 36 % (of whom 40 % were decompensated), and 18 % were HCV treatment-experienced. Overall, SVR 12 was achieved in 97 % (99 % genotype 1, 100 % genotype 2, 84 % genotype 3). Five of six (83 %) patients who relapsed had decompensated cirrhosis, and 67 % were also non-adherent to therapy. On-treatment virologic response did not impact SVR.
Conclusions
High rates of sustained virologic response can be achieved in safety-net HCV-infected patients. Access to DAA-based regimens is critical to addressing HCV-related health disparity in this at-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 2.7–3.9 million people are infected with chronic hepatitis C virus (HCV) in the USA [1], with reported cases of acute HCV increasing among young intravenous drug users (IVDU) [2]. Currently, HCV remains the leading cause of cirrhosis, hepatocellular carcinoma, and death due to liver disease [3] and is associated with considerable healthcare costs as well as lost productivity [4]. Moreover, vulnerable populations who predominantly receive care within safety-net healthcare systems are disproportionally affected by HCV and are at risk of experiencing health disparities associated with this infection [5–8]. Therefore, management of chronic HCV is a public health priority. Fortunately, we are currently faced with an unprecedented opportunity to address HCV in the USA. Recommendations for one-time age-cohort screening in addition to screening at-risk populations [9], and the introduction of the Patient Protection and Affordable Care Act (ACA) that expands access to health insurance coverage [10], provide an opportunity to better identify patients and potentially provide anti-HCV treatment to vulnerable populations. However, vulnerable populations accessing care within safety-net healthcare systems remain marginalized and socioeconomically disadvantaged. These groups often include patients with low health literacy as well as minority populations that may have limited English proficiency and also include those with increased rates of psychosocial and medical comorbidities. Thus, despite new access to Medicaid/Medicare public insurance through ACA, they continue to experience barriers to navigating healthcare system resources that are integral to accessing health care including but not limited to transportation, language interpretation, stable housing, and psychosocial support [11–14].
The development of interferon-sparing, well-tolerated, and highly effective oral direct-acting antiviral (DAA) regimens has recently greatly expanded the pool of HCV treatment candidates, many of whom were not eligible for or who preferred not to take interferon-based treatment. However, high cost limits access to these medications. In addition, treatment of HCV-infected former IVDU and other at-risk groups who rely on public healthcare systems remains challenging due to the high prevalence of underlying comorbid psychiatric disorders, substance abuse, and unstable housing that complicate care delivery [15]. Adherence to therapy is critical in light of the potential for emergence of viral resistance with drug interruption. Optimal delivery of HCV care in this population will require the integration of interventions such as enhanced patient education to address the unique needs of these populations. For example, in the San Francisco safety-net health system (now referred to as San Francisco Health Network), introduction of a mandatory formal HCV patient education class not only improved patient knowledge [16] but also increased both access to liver specialty care and improved sustained virologic response (SVR) rates to HCV therapy in the pegylated interferon (PEG) era [17]. It is therefore likely that patient-centered interventions will remain effective in improving HCV patient outcomes in the era of DAA-based therapy.
While there are limited real-world data among various cohorts emerging in the literature, to date there are no data on DAA-based treatment outcomes among the vulnerable population. Understanding success of DAA-based HCV therapy is critical to reducing health disparity in this population and provides support to further expanding treatment access among this traditionally difficult to treat group. We therefore aimed to evaluate the real-world experience with DAA-based treatment regimens and patient outcomes among a vulnerable safety-net HCV-infected population.
Materials and Methods
Study Design
This is a retrospective observational cohort study of patients treated with sofosbuvir-based regimens for HCV at the San Francisco General Hospital (SFGH) liver specialty clinic. This clinic is the specialty referral clinic for the San Francisco Health Network (SFHN), the traditionally designated safety-net healthcare system in San Francisco, which provides services to over 150,000 patients annually including most of the county’s uninsured and underinsured population [18]. The SFHN is administered by the San Francisco Department of Public Health and includes a network of 15 primary care clinics, San Francisco General Hospital (an academic medical center that serves as an acute care and referral facility), and the San Francisco Community Clinic Consortium, which includes 11 federally qualified health centers [18]. Prior to evaluation in the liver specialty clinic patients also attended a formal HCV education class offered by this specialty service [16, 17]. This study was approved by the University of California San Francisco Committee on Human Research.
Patient Population
In this study, all patients referred to the SFGH liver specialty clinic underwent evaluation for eligibility for HCV therapy. All patients who initiated HCV therapy with DAA-based regimens by the liver specialty clinic between January 1, 2014, and December 31, 2015, were included in the data analysis. Since the enactment of the ACA, the majority of these patients received public insurance (Medicaid and Medicare), and HCV therapy was approved through either public insurance or the drug company patient assistance programs if insurance denied access. All HCV treatment regimens during the study period were selected based on standard of care recommendations for the HCV genotype, were sofosbuvir (SOF)-based, and included combination with ribavirin (RBV), pegylated interferon (PEG) with RBV, simeprevir (SIM) with or without RBV, or ledipasvir (LDV) with or without RBV. The patients were followed in the clinic with clinical and laboratory evaluation every 2–4 weeks during therapy and at 4 and 12 weeks following completion of therapy. The planned duration of therapy ranged from 12 to 24 weeks depending on baseline characteristics of the patient; however, in some patients who had a planned treatment duration of 12 weeks, the duration was extended to up to 24 weeks based on virologic response during therapy if approved by insurance or through compassionate use by the drug company.
Data Extraction
Data were extracted from the electronic medical record with respect to demographic, clinical, laboratory, and imaging studies prior to, during, and following discontinuation of HCV therapy. Detailed hepatitis C virologic characteristics including HCV mode of acquisition, viral load, genotype, coinfection with hepatitis B or human immunodeficiency virus (HIV), and prior HCV treatment experience were captured. Severity of liver disease was determined by either noninvasive aspartate transaminase (AST) to platelet ratio index score (APRI), liver biopsy if available, or imaging studies documenting presence and complications of cirrhosis. History of decompensated liver disease was determined by biochemical or clinical parameters (such as ascites, hepatic encephalopathy, and variceal hemorrhage). Additionally, medical and psychiatric comorbidities were assessed and documented. As per standard practice of the liver specialty clinic, compliance to HCV therapy was documented in the clinic notes and these data were extracted.
Hepatitis C virologic response was determined during therapy. Rapid virologic response (RVR) was defined as an undetectable HCV viral load at week 4 during treatment. End of treatment (EOT) response was defined as an undetectable HCV viral load at the end of treatment. Sustained virologic response (SVR 12) was determined by undetectable HCV viral load at week 12 post-treatment. Virologic relapse was defined as detectable virus following EOT.
Statistical Analysis
Descriptive statistics were generated using median (range) and frequency (%). In order to assess factors associated with achieving RVR and week 8 response, univariate and multivariate logistic regression modeling was used. Multivariate logistic regression modeling included a priori compiled list of variables (age, sex, race, genotype). Statistical significance was assessed at a p value of <0.05 (two-sided). All analyses were performed using Stata version 12 statistical software, Stata Corp LP, College Station, TX.
Results
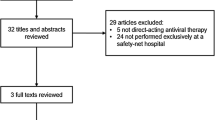
Between January 1, 2014, and December 31, 2015, 469 patients were evaluated for HCV treatment at the liver specialty clinic of the San Francisco safety-net healthcare system, and 204 patients were initiated on a SOF-based HCV antiviral treatment regimen. Of the remaining 262 patients evaluated during the study period in the clinic, 3 were treated with ombitasvir/paritaprevir/ritonavir/dasabuvir ± ribavirin (all had SVR 12), 101 were initiated on HCV therapy after the study period, 65 were either awaiting newer HCV treatment regimens or in whom treatment was deferred due to medical or psychiatric reasons (e.g., end-stage cancer, uncontrolled psychiatric disease) or adherence concerns, 43 were lost to follow-up, 35 did not receive medication approval from either insurance or drug compassionate use programs, 11 deferred therapy, and 8 relocated to another city. The characteristics of patients who did or did not receive HCV therapy during the study period were similar with respect to mean age (56 vs. 55 years, p = 0.8), sex (61 vs. 69 %, p = 0.08), race (non-White 58 vs. 66 %, p = 0.08), English language proficiency (79 vs. 83 %, p = 0.2), and HCV genotype (genotype 1, 72 vs. 68 %, p = 0.4).
Table 1 provides information on baseline demographic, clinical, and laboratory parameters of the treatment cohort. The majority of patients were male, with a median age of 58 years and more than 50 % were non-Caucasian. Half of the patients identified intravenous drug use (IVDU) as the most likely mode of HCV transmission, and nearly 40 % had psychiatric comorbidity. With respect to HCV disease parameters, 72 % were infected with genotype 1, median log10 HCV viral load was 6.1 IU/ml, median alanine aminotransferase (ALT) was 63 U/l, and more than one-third of patients had cirrhosis with 40 % of cirrhotic patients having decompensated liver disease. Of the 68 patients who underwent liver biopsy, the majority had stage 2 or greater fibrosis (72 %), grade 2 or greater inflammation (66 %), and steatosis was present in 51 %. Eighteen percent of patients were treatment-experienced (PEG/RBV with or without protease inhibitor), and all but one patient received full duration of therapy (12 or 24 weeks). This patient discontinued therapy at 10 weeks.
Of the 204 treated patients, 189 had blood tests at SVR 12 time point. Of these 97 % (183/189) achieved SVR 12 and 6 (3 %) relapsed following treatment. Of the 15 patients who did not return for SVR 12 evaluation, seven had undetectable HCV viral load at 4 weeks following completion of therapy, an additional three had achieved ETR, and five were lost to follow-up. By genotype, SVR 12 rates were 99 % for genotype 1, 100 % for genotype 2, 84 % for genotype 3, and 92 % for other genotypes (4 and 6). Figure 1 shows SVR 12 results based on cirrhosis and history of prior HCV treatment status. Virologic testing during therapy showed that 26 % (50/193) of patients achieved rapid virologic response (RVR) defined as undetectable HCV viral load at 4 weeks following initiation of therapy, while 92 % had ETR. Among 71 patients with detectable HCV viral load at week 8, 24 patients who were initially prescribed 12 weeks of therapy extended therapy to 24-week duration (two patients received 14 weeks of therapy and two received 16 weeks of therapy), but there was no difference in rates of SVR 12 among those who did or did not have extension of therapy based on virologic response at this treatment time point (p = 0.3). Further analysis showed that five of six with virologic relapse had decompensated cirrhosis and three were also treatment-experienced. Additionally, four had reported non-adherence to therapy. With respect to genotype and treatment regimen, two of the relapsers had genotype 1 and both were treated with SIM/SOF for 12–24 weeks, three had genotype 3, two treated with SOF/RBV and one treated with DCV/SOF (all for 24 weeks), and one had genotype 6 and was treated with SOF/LDV for 12 weeks.
Table 2 shows symptoms reported by patients during therapy. Fatigue and headache were the most common symptoms with all regimens. Pruritus was also commonly reported with SIM-containing regimens. Fifteen patients required dose adjustment of RBV during therapy due to anemia. No patient discontinued therapy due to side effects.
Discussion
This is the first study to date to report real-world outcomes of DAA-based therapy in a population accessing care in a liver specialty setting in the traditionally safety-net healthcare system. We demonstrate a very successful SOF-based treatment response with 97 % achieving SVR 12 irrespective of severity of liver disease and prior HCV treatment experience. These results highlight that this population, historically deemed difficult to treat, can be treated safely and effectively given an infrastructure that provides patient education and integrated care delivery. In fact, the minority (3 %) that experienced virologic relapse following therapy either were non-adherent to therapy, had decompensated liver disease, or were treatment-experienced. These results also confirm that on-treatment virologic assessment is not necessary, as it does not appear to be predictive of virologic relapse, as shown in other studies [19, 20].
Clinical trials have demonstrated significant improvement in SVR with SOF-based regimens [21, 22]; however, the controlled nature of a clinical trial limits application to real-world populations. Recently reported real-world data show variable SVR rates from those seen in clinical trials; while some show lower rates of response, others do not. In a study by Backus et al. among 4026 US veterans, the SVR rates were reported at 67 % in genotype 1 patients treated with SOF/PEG/RBV, 75.5 % in those treated with SIM/SOF, and 74.1 % in those treated with SIM/SOF/RBV. Among those with genotype 2, SVR was achieved in 79 % treated with SOF/RBV. However, over 90 % of the patients in this study were male, a large proportion had advanced liver disease by FIB-4, and the regimens did not include SOF/LDV that may make these findings less generalizable [23]. In contrast, another study from the VA population of over four thousand patients with genotypes 1–4 that included SOF/LDV regimens along with the other regimens reported by Backus et al., showed higher response rates of over 90 % among those treatment-naïve or treatment-experienced patients who had received SOF/LDV [24].
Studies of more diverse populations have also shown variable results. In a smaller study of 113 patients, SVR rates of 75 % were achieved in those with genotypes 1, 4, 5, and 6 treated with SOF/PEG/RBV, but the rates were higher among those with genotypes 2 (93 %) and 3 (81 %) [25]. The majority of patients in this study had genotypes 2 and 3 (67 %), 11 % of genotypes other than 2 and 3, and 36 % of patients with genotypes 2 and 3 missed doses during therapy, which may have influenced response rates. Recently, results from a real-world longitudinal, multicenter, prospective observational cohort study evaluating SOF/LDV treatment through the HCV-TARGET Registry were published [19]. Of the 2099 patients with genotype 1 who were treated with SOF/LDV ± RBV for 8-, 12-, or 24-week duration, 95–97 % of patients achieved SVR 12 [19]. Factors predictive of higher SVR included higher albumin, lower total bilirubin, absence of cirrhosis, and absence of proton pump inhibitors use. Similarly, our study shows high rates of SVR in the safety-net population, and half of the patients who had a virologic relapse had decompensated cirrhosis, consistent with lower response rates reported in this population.
Though consistent with results reported in larger and diverse populations, our study is limited by a relatively small sample size, and receipt of therapy within the liver specialty clinic setting, which may not be generalizable to the non-specialty setting. Moreover, as emphasized and recommended by the American Association for the Study of Liver Disease/Infectious Diseases Society of America [26] practice guidelines, the relative likelihood of adherence to HCV therapy was evaluated by the treating provider prior to initiation of therapy. Thus, these results represent a relatively adherent population, though still at risk for health disparities associated with low income, low health literacy, and medical and psychosocial comorbidities that are common in vulnerable populations. Nevertheless, assessment of adherence prior to initiation of HCV therapy is a standard practice in light of potential for the development of virologic resistance with DAA therapies.
In conclusion, despite the known presence of comorbidities and psychosocial challenges faced by this population, current DAA-based therapies are highly efficacious and well tolerated in vulnerable populations. In light of the disproportionately high rates of HCV in vulnerable populations and the fact that these patients are particularly at high risk of experiencing health disparity, access to HCV therapy with these regimens is especially critical in this population.
References
Centers for Disease Control. Viral hepatitis. http://www.cdc.gov/hepatitis/hcv/statisticshcv.htm. Accessed December 6, 2015.
Centers for Disease Control. Notes from the field: risk factors for hepatitis C virus infections among young adults—Massachusetts, 2010. MMWR. 2011;60:1457–1458.
Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521.
Razavi H, ElKhoury AC, Elbasha E, et al. Chronic hepatitis C disease burden and cost in the United States. Hepatology. 2013;57:2164–2170.
Saab S, Jackson C, Nieto J, Francois F. Hepatitis C in African Americans. Am J Gastroenterol. 2014;109:1576–1584.
Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. JGIM. 2012;27:861–867.
Nguyen GC, Segev DL, Thuluvath PJ. Racial disparities in the management of hospitalized patients with cirrhosis and complications of portal hypertension: a national study. Hepatology. 2007;45:1282–1289.
Quillin RC III, Wilson GC, Wima K, et al. Neighborhood level effects of socioeconomic status on liver transplant selection and recipient survival. Clin Gastroenterol Hepatol. 2014;12:1934–1941.
Moyer VA. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: a U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357.
Bumenthal D. Health care coverage under the Affordable Care Act—a progress report. N Engl J Med. 2014;17:275–281.
Centers for Disease Control and Prevention. CDC health disparities and inequalities report—United States, 2013. MMWR. 2013;62:1–186. http://www.cdc.gov/mmwr/pdf/other/su6203.pdf.
Guerra V, McMahon S. Charity care organizations as navigators: considerations for guiding consumers toward the best coverage options. Center for Health Care Strategies. February 2014. http://www.chcs.org/usr_doc/Charity_Care_as_Navigators_02414.pdf.
The U.S. Department of Housing and Urban Development. The 2013 Annual Homeless Assessment Report (AHAR) to congress. November 2013. http://www.onecpd.info/resources/documents/AHAR‐2013‐Part1.pdf.
Substance Abuse and Mental Health Services Administration. Current statistics on the prevalence and characteristics of people experiencing homelessness in the United States. July 2011. http://homeless.samhsa.gov/ResourceFiles/hrc_factsheet.pdf.
Sood S, Wong D, Holmes A, Everall I, Saling M, Nicoll A. Depression in a real world population of hepatitis C patients. J Gastroenterol Pancreatol Liver Disord. 2014;30:1–3.
Surjadi M, Torreullas C, Ayala C, Yee HF Jr, Khalili M. Formal patient education improves patients’ knowledge of hepatitis C in vulnerable populations. Dig Dis Sci. 2011;56:213–219.
Lubega S, Agbim U, Surjadi M, Mahoney M, Khalili M. Formal hepatitis C education enhances HCV care coordination, expedites HCV treatment and improves antiviral response. Liver Int. 2013;33:999–1007.
Bindman AB, Chen A, Fraser JS, Yee HF Jr, Ofman D. Healthcare reform with a safety net: lessons from San Francisco. Am J Manag Care. 2009;15:747–750.
Terrault N, Zeuzem S, Di Bisceglie AM, et al. Effectiveness of ledipasvir–sofosbuvir combination in patients with hepatitis C virus infection and factors associated of sustained virologic response. Gastroenterology. 2016. doi:10.1053/j.gastro.2016.08.004.
Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. The Lancet. 2014;384:1756–1765.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887.
Afdhal N, Zeuzem S, Kwo P, et al. for the ION-1 Investigators. Ledipasvir and Sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014. doi:10.1056/NEJMoa1402454.
Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans. Aliment Pharmacol Ther. 2015;42:559–573.
Butt AA, Yan P, Shaikh OS, Chung RT, Sherman KE, ERCHIVES study. Sofosbuvir-based regimens in clinical practice achieve SVR rates closer to clinical trials: results from ERCHIVES. Liver Int. 2016;36:651–658. doi:10.1111/liv.13036.
Wu CJ, Roytman MD, Hong LK, et al. Real-world experience with sofosbuvir-based regimens for chronic hepatitis C, including patients with factors previously associated with inferior treatment response. Hawaii J Med Public Health. 2015;74:3–7.
American Association for the Study of Liver Disease/Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. hcvguidelines.org. Accessed January 23, 2016.
Funding
This work was supported in part by National Institutes of Health K24AA022523 (to M.K.) and P30 DK026743 (UCSF Liver Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest in connection with the submitted manuscript. Dr. Khalili has research grant support from Gilead Inc and Intercept Pharmaceuticals and has participated in the advisory boards of Bristol Myers Squibb, Gilead Inc, and Intercept Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Beck, K.R., Kim, N. & Khalili, M. Sofosbuvir-Containing Regimens for Chronic Hepatitis C Are Successful in the Safety-Net Population: A Real-World Experience. Dig Dis Sci 61, 3602–3608 (2016). https://doi.org/10.1007/s10620-016-4340-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4340-x