Abstract
Background
The prognostic value of metastatic lymph node ratio (LNR) is still controversial in esophageal cancer.
Aim
This study aimed to compare the impact of AJCC N staging system (pN) and LNR on the prediction of long-term survival of patients with esophageal carcinoma.
Methods
A total of 496 patients were retrospectively analyzed who underwent esophageal resection at Henan Tumor Hospital from January 2006 to December 2010. The Kaplan–Meier method and log-rank test were used to estimate survival curves. Univariate and multivariate analyses were performed to compare prognostic factors for long-term survival. The difference between pN and LNR with overall survival (OS) was compared by receiver operating characteristic (ROC) curve and area under the curve (AUC).
Results
The 1-, 3-, 5-year overall survival rates of 496 patients were 73.6, 47.1 and 34.2 %, respectively. Univariate analyses showed that diseased region, tumor length, depth of tumor invasion, pN and LNR affected the prognosis, and multivariate analyses demonstrated that depth of tumor invasion, pN and LNR were independent risk factors. Among the three significant variables verified by multivariate analyses, LNR was the best for inadequately staged patients (<12 examined LNs). ROC analyses showed that compared with pN (AUC = 0.579, p = 0.037), LNR (AUC = 0.680, p = 0.002) had better predictive value (z = 2.275, p = 0.029).
Conclusions
LNR has greater prognostic value than pN for esophageal squamous cell carcinoma, especially for patients with <12 LNs removed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, esophageal cancer has a higher incidence in China than in any other country [1]. Radical esophagectomy and subsequent thorough lymph node dissection remain the major treatments to reduce the recurrence and improve the survival for patients with esophageal cancer. The status of lymph node metastases is considered as the most important prognostic factor for patients suffering esophageal carcinoma and undergoing surgical resection. American Joint Committee on Cancer (AJCC) recommended that the cutoff points for the pathologic classification (pN) should be as follows: pN1 refers to 1–2 involved regional lymph nodes; pN2 refers to 3–6 involved regional lymph nodes; and pN3 refers to 7 or more involved regional lymph nodes [2]. Accurate estimation of prognosis for esophageal cancer patients would help compare therapeutic effects of different adjuvant therapy.
However, making an accurate lymph node staging should be based on a large number of lymph node resections. Likewise, there has been some controversy regarding the number of lymph nodes (LNs) required for accurate staging [3, 4]. LNs may directly affect the prognostic assessment because of the insufficiency of detecting numbers. However, whether a ratio-based stratification would substitute for an inadequate pN classification is unclear. In addition, few studies have focused on comparing the prognostic value of lymph node ratio (LNR) with the new AJCC N staging system.
In this study, we aimed to compare the prognostic value of LNR and pN for long-term survival and determine the optimal cutoff point of LNR. Our results demonstrated that the stratification of the patients with LNR according to our cutoff points may bypass the staging migration for the patients with fewer than 12 harvested lymph nodes.
Materials and Methods
Eligibility
A total of 496 patients were recruited from Henan Tumor Hospital from January 2006 to December 2010. All patients underwent esophagectomy and had complete clinical and pathological data, and all resected specimens from these patients were submitted for pathologic examination to confirm the diagnosis of esophageal squamous cell carcinomas (ESCC). Patients who underwent neoadjuvant chemotherapy or chemoradiation therapy were excluded. Lymphatic metastasis was found in 59.3 % of the patients (n = 294). A total of 4017 lymph nodes were dissected (8.1 per patient), and 952 of them were positive (23.7 %).
Follow-Up
The follow-up period was 5 years (range 9–62 months, mean 33 months). The survival status of patients was ascertained in May 2014. We used a combination of phone calls, e-mail and letters to determine the status. The main reason for loss to follow-up was the deficiency of the information for us to connect with them. Follow-up began on the first day after esophagectomy. The following data were collected: preoperative factors (age, gender), tumor factors (location, size, clinical and pathologic staging) and lymph node status (the total number of LNs recovered, the total number of positive LNs, the LNR). Survival time was determined from the date of esophageal resection to the date of death. For patients who survived, survival time was determined from the date of surgery to the date of last follow-up.
Statistical Analysis
Statistical analyses were performed using SPSS, version 17.0. Univariate and multivariate analyses were performed using Cox proportional models. The survival curves were evaluated using the Kaplan–Meier method, and the log-rank test was used to make statistical comparisons of different factors. The association of pN and LNR with OS was examined by ROC curve and the area under the curve (AUC), and statistical comparisons were tested by z-test. The hazard ratios (HR), the odds ratio (OR) and the 95 % confidence interval were used to assess the relationship between survival time and independent factors. p < 0.05 was considered significant.
Results
Patients and Survival
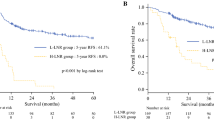
A total of 496 patients (mean age 56 years; range 34–80 years) underwent resection for esophageal cancer. Most patients were male (n = 340, 68.5 %) and 57 % of 496 patients had a history of smoking (n = 282). A total of 4017 lymph nodes were resected (median 7; mean 8.1), of which 952 lymph nodes (23.7 %) were found to be metastatic. Lymph node involvement was classified according to the 7th edition AJCC N staging system as follows: N0: no metastasis (n = 202, 40.7 %); N1: 1–2 metastatic lymph nodes (n = 168, 33.9 %); N2: 3–6 metastatic lymph nodes (n = 89, 17.9 %); N3: ≥7 metastatic lymph nodes (n = 37, 7.5 %). Patients with no involved LN had a 5-year survival of 41.9 %, while patients with 1–2 positive LNs, 3–6 positive LNs and ≥7 positive LNs had 5-year survivals of 33.5, 10.9 and 0 %, respectively. Figure 1 presents the Kaplan–Meier survival curves for the patients with different N stages. The difference in survival among these four groups was significant (p < 0.001).
Determine the Optimal Cutoff Point of LNR
We determined the additional appropriate cutoff points for classifying the LNR to make our cutoff points comparable with those for AJCC N staging. Initially, patients without involved lymph nodes (LNR = 0) were entered into one group. Subsequently, we used ROC curve to determine the best cutoff point for LNR as 0.3 which made Youden index (Youden index = sensitivity + specificity − 1) to be the maximum. The Youden index could be defined as a function of sensitivity and specificity and ranged between 0 and 1, with values close to 1 indicating relatively large effectiveness. Next, we found that another cutoff point associated with prognosis was 0.15 with intervals of 0.01 which was the minimum difference of LNR in different individuals in our study. Therefore, on the basis of optimal cutoff points, we divided patients into four groups as follows: no metastasis (n = 202, 40.7 %), LNR <0.15 (n = 113, 22.8 %), LNR between 0.15 and 0.30 (including 0.15 and 0.30, n = 125, 25.2 %) and LNR greater than 0.30 (n = 56, 11.3 %). Their 5-year OS rates were 41.9, 38.2, 27.1 and 11.6 %, respectively (p < 0.001). Corresponding Kaplan–Meier survival curves are shown in Fig. 2.
Univariate Analysis of Prognostic Factors
All prognostic factors were considered at univariate analyses. The results showed that tumor size (p = 0.009), tumor location (p = 0.020), pT (p = 0.011), pN (p < 0.001) and LNR (p < 0.001) were correlated with the prognosis of ESCC, but age (p = 0.345), total dissected lymph nodes (p = 0.720) and gender (p = 0.921) were not significantly correlated with the prognosis of ESCC (Table 1).
Multivariate Analysis of Prognostic Factors
Multivariate analyses of the OS using Cox regression model identified the following variables as independent predictors of survival: pT (p = 0.047), pN (p = 0.018) and LNR (p = 0.011) (Table 2). The LNR categories had better prognostic value than AJCC pN categories because the LNR categories had a higher hazard ratio (HR 2.354) than the AJCC N categories (HR 1.985). The reason may be that stage migration resulted in an inaccurate N stage when <12 lymph nodes were examined. Under this situation, we divided patients into two groups (number of examined LNs < 12 and number of examined LNs ≥ 12). Multivariate analysis was performed in two groups, and the results showed that AJCC pN stage was not statistically significant (p = 0.068) when the number of examined LNs was <12. However, both pN and LNR were statistically significant (p = 0.040, OR 1.7; p = 0.034, OR 2.4; respectively) when the number of examined LNs was greater than or equal to 12 (Table 3).
Comparison of LNR and pN
Multivariate analysis showed that LNR was significantly associated with the prognosis compared to pN. In addition, ROC curve analysis indicated that LNR (AUC = 0.680, p = 0.002) had larger area than pN (AUC = 0.579, p = 0.037). Then we used z-test to compare the area under the curve, and the results (z = 2.275) showed that the differences between pN and LNR were statistically significant (p = 0.029). LNR had better predictive value for the prognosis of ESCC patients than pN (Fig. 3).
Discussion
The aim of our retrospective study was to compare the prognostic value of total number of LNs with the ratio of positive lymph nodes in Chinese patients with esophageal cancer. The 7th edition AJCC TNM staging system recommended that a minimum of 12 nodes should be removed to ensure the accuracy of staging, but there is no general consensus on the exact number of LNs that should be retrieved, and surgeons should generally remove as many LNs as possible [2]. The total number of LNs retrieved may be affected by many factors such as tumor length, the experience of both the surgeons and pathologists and the location of the tumor [5]. For patients with fewer than 12 LNs recovered, N stage may be incorrect because of stage migration [6]. Although all the patients in our study underwent radical esophagectomy, the number of LNs retrieved was <12 in the majority of patients (66.9 %). Therefore, the N stage of these patients may be classified inaccurately according to the 7th edition AJCC staging system. Considering all the variables that could affect the total number of LNs dissected, the ratio of metastatic lymph nodes can compensate for inadequate LN dissection. The use of LNR could overcome several limitations including surgical and pathological techniques, tumor locations, and even the number of LNs that should be retrieved.
The LNR has proven to be an important prognostic factor in several malignancies, such as breast, colon and gastric cancer [7–9]. To our knowledge, few documents have compared the pN stage with the LNR for the determination of the prognosis of esophageal carcinoma. In the present study, the LNR showed clear advantage over the pN stage. Univariate and multivariate analyses showed that patients with more than 0.3 LNR were much worse than patients with 0.3 or fewer LNR. Multivariate analyses showed that the LNR categories had a higher hazard ratio than the AJCC N categories (HR 2.354 vs. 1.985). We used ROC curve to compare the area under the curve, and the results indicated that LNR categories had a larger area than AJCC N categories (0.680 vs. 0.579, p = 0.029). These data indicate that the pN and LNR are both appropriate for adequately staged patients (≥12 examined LNs), whereas the LNR should be used for inadequately staged patients (<12 examined LNs). Currently, this difference is not shown in the AJCC staging system. Our results are consistent with the report by Mariette et al. [10] who compared the number and the ratio of LNMs and showed the difference between the ratio of LNMs and esophageal cancer outcomes in a cohort of 1214 consecutive patients with esophageal carcinoma. However, Wilson et al. [11] found that the use of LNRs was not related to the outcome. The different results are most likely due to the stratification of the groups according to the inappropriate cutoff point of LNR.
Multiple cutoff points of LNR have been presented in different studies based on the extent of lymph node dissection and the pathological type of esophageal cancer. Hsu et al. [12] found that patients with LNR > 0.20 had poorer prognosis than those with LNR < 0.2. Liu et al. [13] divided 1325 patients into four groups based on LNR and showed that LNR was an independent prognostic factor. Wijnhoven et al. [14] defined LNR of 0.2 as the optimal cutoff point to be highly discriminative in predicting the survival by using a Cox proportional hazards regression model. In the present study, we determined LNR of 0.30 as the best cutoff point by using ROC curve which makes Youden index to be the maximum point and defined LNR of 0.15 as another cutoff point in intervals of 0.01. Consequently, we divided patients into four groups based on LNRs and compared the prognoses among these four groups using the Kaplan–Meier method. The difference in the survival of these groups was significant (p < 0.05). To our knowledge, few reports used the ROC curve by Youden index for calculating the optimal cutoff point of LNR in patients with ESCC. While different authors have used diverse cutoff points of LNR, it is necessary to replicate these findings in an independent population before they can be applied in clinical practice.
As a retrospective study, some limitations of this study should be pointed out. First, the location of the involved LNs affected patient outcomes [15, 16]. Because of the retrospective nature, the LNs were not divided into groups according to different regions. In future studies, more clinical and pathological information should be provided to evaluate the effects of LNRs at different sites on the prognosis of ESCC. Second, the cutoff points of LNR have not been discussed or recognized adequately in the datasets. Future clinical trials should prospectively provide further evidence evaluating the use of the optimal cutoff point of LNR as a standard for the N staging of ESCC.
In conclusion, our findings strongly suggest that the LNR and pN are more appropriate as important prognostic factors for ESCC. LNR may reduce the amount of stage migration, which is superior to pN when the pathologist examines an inadequate number of LNs (<12 examined LNs).
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: Cancer J Clin. 2013;63:11–30.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474.
Yang H-X, Xu Y, Fu J-H, et al. An evaluation of the number of lymph nodes examined and survival for node-negative esophageal carcinoma: data from China. Ann Surg Oncol. 2010;17:1901–1911.
Groth SS, Virnig BA, Whitson BA, et al. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the Surveillance Epidemiology and End Results database. J Thorac Cardiovasc Surg. 2010;139:612–620.
Wang KN, Xu QR, Chen LQ. Impact of lymph-node metastasis site in patients with thoracic esophageal cancer: Does the lymph node metastasis site matter? J Surg Oncol. 2011;104:334.
Dutkowski P, Hommel G, Bottger T, et al. How many lymph nodes are needed for an accurate pN classification in esophageal cancer? Evidence for a new threshold value. Hepato-gastroenterology. 2002;49:176–180.
Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712.
Maduekwe UN, Lauwers GY, Fernandez-del-Castillo C, et al. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol. 2010;17:1267–1277.
Vinh-Hung V, Verschraegen C, Promish DI, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6:R680–R688.
Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365–371.
Wilson M, Rosato EL, Chojnacki KA, et al. Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res. 2008;146:11–15.
Hsu W-H, Hsu P-K, Hsieh C-C, et al. The metastatic lymph node number and ratio are independent prognostic factors in esophageal cancer. J Gastrointest Surg. 2009;13:1913–1920.
Liu Y, Ma L, Wang S, et al. Prognostic value of lymph node metastases and lymph node ratio in esophageal squamous cell carcinoma. Eur J Surg Oncol (EJSO). 2010;36:155–159.
Wijnhoven BP, Tran KT, Esterman A, et al. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717.
Shimada H, S-i Okazumi, Matsubara H, et al. Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three-field lymph node dissection. World J Surg. 2006;30:1441–1449.
Xu Q-R, Zhuge X-P, Zhang H-L, et al. The N-classification for esophageal cancer staging: should it be based on number, distance, or extent of the lymph node metastasis? World J Surg. 2011;35:1303–1310.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, C., Deng, Wy., Li, N. et al. Lymph Node Ratio as an Alternative to the Number of Metastatic Lymph Nodes for the Prediction of Esophageal Carcinoma Patient Survival. Dig Dis Sci 60, 2771–2776 (2015). https://doi.org/10.1007/s10620-015-3681-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3681-1