Abstract
N staging predicting esophageal cancer patient prognosis has been studied. Lymph node ratio, which is considered to show metastatic lymph node status more accurately, is found to have prognostic significance in several tumors. We investigated whether lymph node ratio (LNR) was associated with the prognosis of esophageal cancer in this study. Esophageal cancer patients who underwent esophagectomy at Qilu Hospital of Shandong University from January 2007 to December 2008 were studied. A total of 209 cases were evaluated in this study. The median disease-free survival (DFS) of this cohort was 35.2 months, and 5-year DFS rate was 32.1 %. The median overall survival (OS) was 46.4 months, and 5-year OS rate was 40.0 %. Kaplan–Meier survival analysis revealed that patients with LNR higher than 0.2 had significantly poorer DFS (p < 0.001) and OS (p < 0.001) than those with LNR less than 0.2. In a multivariate analysis, LNR was found to be an independent prognostic factor for DFS (p = 0.008, HR 1.863, 95 % CI 1.180–2.942) and OS (p = 0.025, HR 1.708, 95 % CI 1.068–2.731). N stage (p = 0.028, HR 1.626, 95 % CI 1.055–2.506) was also found to be an independent prognostic factors for OS. Subgroups analysis revealed significant difference in OS and DFS rates between different LNR categories within the same N stages (p < 0.05) but not between different N stages within the same LNR category (p > 0.05). LNR was recognized as an independent factor in both OS and DFS in esophageal cancer. Besides, LNR showed a better prognostic value than N stage for esophageal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is the sixth leading cause of cancer deaths worldwide [1]. The overall survival of esophageal cancer remains poor although some improvements have been achieved in treatment [1, 2]. Lymph node status is one of the factors closely related with the long-term survival of esophageal cancer patients. Esophageal carcinoma patients with nodal metastases generally have a poor prognosis after surgical resection. Patients without involvement of regional nodes (pN0) had a significant improvement in 5-year survival [3].
The seventh American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) staging system includes primary tumor status, lymph node status, and metastatic status. N staging predicting esophageal cancer patient prognosis has been studied [4–7]. However, N stage is categorized by the number of positive lymph node retrieved, which can be influenced by many factors, such as variation in examining pathologists, the adequacy of the surgical resection, obesity, and so on [8–11].
The concept of lymph node ratio (LNR) was discussed by some reports recently. LNR, the ratio of metastatic to total retrieved nodes, which is considered to reflect metastatic lymph node status more accurately, has been found to have prognostic significance in several kinds of tumors [12–16]. However, in esophageal cancer, there are limited studies about the prognostic significance of LNR. Hence the aim of our study was to investigate whether LNR can be functioned as an independent prognostic factor of esophageal cancer.
Patients and methods
Patients
We collected all esophageal cancer patients who underwent esophagectomy at the Department of Thoracic Surgery, Qilu Hospital of Shandong University, from January 2007 to December 2008. Patients were excluded from the present study when they (1) died within 30 days after operation, (2) underwent preoperative chemotherapy or radiotherapy, and (3) could not be contacted during follow-ups. A total of 209 cases were included in this study. All patients included in our study had histologic documentation from at least two independent pathologists. Among 209 cases, 111 patients did not receive any therapy after operation, 45 cases received postoperative radiotherapy, 22 cases received postoperative chemotherapy, and 31 cases received postoperative chemoradiotherapy. Clinicopathological data was obtained from the hospital electronic recording system, such as gender, tumor location, tumor stage, lymph node metastasis, treatment regimen, and so on.
Lymph node ratio (LNR)
LNR was the ratio of metastatic lymph nodes to total retrieved nodes. Patients were divided into two groups according to LNR for analysis. To determine the optimal cutoff point for the number of the lymph node ratio as predictors of survival, we simulated the log-rank test for groups defined by LNR < c) and LNR > c for observed values of the covariate for the entire data set. The largest log-rank test statistic was then applied to detect the optimal cutoff point [17].
Follow-up
The follow-up was updated to October 2013. Survival time was calculated from the date of surgery to the event or the last follow-up. The reexamination included clinical examination, chest X-ray, abdominal CT scan, colonoscopy, and evaluation of CEA and CA19-9.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package (version 20.0; SPSS Inc, Chicago, Ill). Survival analyses were performed by Kaplan–Meier curves with log-rank tests for significance. Statistical analyses included univariate analysis and multivariate analysis. Univariable Cox regression analyses were performed using disease recurrence or death as the outcomes with a significance level of p < 0.05. Multivariate analysis was carried out with a Cox proportional hazards model to evaluate LNR and other prognostic factors with respect to disease-free survival (DFS) and overall survival (OS). Two-sided p values of <0.05 were considered statistically significant.
Results
Clinicopathological characteristics of patients
Among the 209 cases, 80.4 % was male and 19.6 % was female. The median age of this cohort was 60.6 years. Most of primary tumor sites were middle thorax (56.5 %) and lower thorax (33.5 %). Patients with a LNR less than 0.2 accounted for 76.6 % of the whole cohort. Table 1 shows the clinicopathological parameters of the studied patients.
Prognostic impact of LNR and N stage
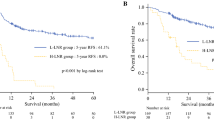
The median DFS of this cohort was 35.2 months, and 5-year DFS rate was 32.1 %. The median OS of this cohort was 46.4 months, and 5-year OS rate was 40.0 %. Kaplan–Meier survival analysis revealed a correlation between LNR and overall and disease-free survival times. Patients with LNR higher than 0.2 had significantly poorer DFS (p < 0.001) and OS (p < 0.001) than those with LNR less than 0.2. Kaplan–Meier curves of DFS and OS based on LNR are shown in Figs. 1 and 2.
Survival analysis showed significant difference in OS (p < 0.001) and DFS (p < 0.001) among different N categories in this entire data. Kaplan–Meier curves of DFS and OS based on N stage are shown in Figs. 3 and 4.
Univariate and multivariate survival analyses
The results of univariate analyses are shown in Table 2. The log-rank test revealed that factors significantly correlated with OS and DFS, including T stage, N stage, LNR, TNM stage, histological grade, and treatment regimen (all p < 0.05).
Multivariate analyses were performed using Cox proportional-hazards regression. Table 3 shows the results of multivariate analyses. In multivariate analysis, LNR was found to be an independent prognostic factor for DFS (p = 0.008, HR 1.863, 95 % CI 1.180–2.942) as well as TNM stage (p < 0.001, HR 2.215, 95 % CI 1.424–3.444). With respect to OS, LNR was also an independent prognostic factor (p = 0.025, HR 1.708, 95 % CI 1.068–2.731). Besides, N stage (p = 0.028, HR 1.626, 95 % CI 1.055–2.506), TNM stage (p = 0.002, HR 2.051, 95 % CI 1.304–3.226), and treatment regimen (p = 0.047, HR 1.198, 95 % CI 1.002–1.432) were independent prognostic factors for OS.
Subgroup analysis
Significant differences in OS and DFS rates were found between different LNR categories within the same N category (p < 0.05, Table 4) but not between different N categories within the same LNR category (p > 0.05, Table 5). These results confirmed that LNR showed better prognostic value than N stage for esophageal cancer.
Discussion
Esophageal cancer staging relies on the TNM system designed jointly by the Union International Against Cancer (UICC), and the AJCC. TNM staging system is the international unifying classification for malignancy, including primary tumor status (T), metastatic lymph node status (N), and distant metastatic status (M). The UICC and the AJCC have provided a more accurate estimation of prognosis than the sixth edition, proposing an updated classification (seventh edition) for N categories based on the number of metastatic lymph nodes [18], which emphasizes the significance of metastatic lymph node on the clinical staging. Thus, more and more attention has been focused on its prognostic value of esophageal cancer.
Under this circumstance, the definition of lymph node ratio (LNR) emerges to describe the lymph node status of patients more accurately. Until now, the association between low LNR and improvement in survival for patients has been proved in various malignancies, such as gastric cancer [19, 20] and breast cancer [14], as we have mentioned before. Meanwhile, LNR was also proposed to be an independent prognostic factor in gallbladder cancer [21] and pancreatic cancer [22]. A longitudinal study from a single cancer center in Taiwan found that high LNR was an independent poor prognostic factor in colorectal cancer [23].
In the previous studies, concerns were focused on comparing the accuracy or advantage between N stage and LNR in predicting prognosis of patients with esophageal cancer. Some studies [24] examined whether involvement of a higher number of lymph nodes was associated with worse survival among esophageal cancer patients. They found that disease-specific survival rates decreased with higher LNR and a higher LNR was independently associated with worse disease-specific survival. A prospective study of 224 patients was conducted to assess predictors of survival after esophagectomy for esophageal and gastroesophageal junction malignancy [25]. In the present study, our results showed a significant decrease in survival as the percentage of positive nodes increased. LNR was pointed to predict survival more accurately than current staging systems. The prognostic value of LNR in 700 esophageal squamous cell carcinoma (ESCC) patients after tri-incisional esophagectomy without neoadjuvant therapy was evaluated by making comparisons with N categories in the UICC/AJCC classification system (seventh edition), and the researchers concluded that LNR was an independent prognostic factor after tri-incisional esophagectomy, regardless of the number of retrieved lymph nodes [26]. They demonstrated that LNR might reduce stage migration and had more potential for predicting patient outcomes in ESCC.
In the present study, we evaluated the prognostic value of LNR in esophageal cancer. We divided the whole cases into two groups based on their LNR for survival analysis. We found that patients with LNR higher than 0.2 had a better OS and DFS than patients with LNR lower than 0.2. Our result was consistent with many previous studies, which clarified the significance of LNR in overall survival and disease-free survival of patients in esophageal cancer. One of the selected criteria of our study was esophageal cancer patients not receiving any therapy before esophagectomy. We intended to investigate the long-term outcome of patients after esophagectomy, and preoperative therapy can affect the outcome after esophagectomy to a certain extent. Preoperative therapy helps in downstaging esophageal cancer, thus enhancing the chance of curative resection and improving the prognosis of patients with esophageal cancer [27, 28]. So we excluded patients who had preoperative therapy from our study to eliminate this effect.
However, Mariette C and his colleagues [29] retrospectively analyzed 536 esophageal cancer patients taking into account neoadjuvant therapy and lymphadenectomy extent. Two hundred seventy-six patients who received neoadjuvant chemoradiation were included in this study. They found that the prognostic role of both the number and the ratio of metastatic lymph nodes was maintained regardless of patients receiving neoadjuvant chemoradiation or not. Besides, they had additional conclusion that LNR was shown to be more accurate for inadequately staged patients (<15 examined lymph nodes), whereas the number of metastatic lymph nodes was pertinent for adequately staged patients (≥15 examined lymph nodes).
Coincidentally, we also hold the opinion that it is difficult to demonstrate whether LNR is better than the number of metastatic lymph nodes. We need to take the results in the exact context to evaluate. In the present study, we have figured out that both LNR and N stage were associated with DFS and OS in esophageal cancer. But after comparison of survival rates between patient subsets in either N classification, we found significant difference in OS and DFS rates between different LNR categories within the same N stages but not between different N stages within the same LNR category. These results confirmed that LNR showed better prognostic value than N stage for esophageal cancer. This can be explained by the fact that LNR, determined by both metastatic lymph nodes and retrieved lymph nodes, is recognized as a better tool for revealing the lymphatic status of EC patients, compared with N stage. Lymph node metastasis is closely related with the recurrence and survival of esophageal cancer. Therefore, closer relation was found between LNR and survival rates.
As we all know, lymphadenectomy should take various factors into consideration, such as completeness of the resection, procedure-related morbidity and mortality, and disease-free survival [30]. In addition, many factors could influence the number of retrieved lymph nodes [31]. Thus, N staging, which was determined only by the number of metastatic lymph node, could not reflect the status of lymph node metastasis as accurately as LNR.
In this study, LNR >0.2 was associated with poorer OS and DFS in esophageal cancer. However, our study did not provide the evidence that the lower the LNR gained, the better the prognosis achieved. It has been proved that postoperative complications occurred more frequency with increasing number of lymph nodes retrieved [32, 33]. In other words, optimal LNR can increase the likelihood of proper staging and improve patient outcome with minimal complications.
There are some limitations about our study. First of all, the cutoff point of LNR lacks an acknowledged value at present. We selected 0.2 as the cutoff value according to the statistic method from the previous studies. Moreover, the sample size was small since there were only 209 cases included. Large sample clinical analysis is required for further study.
In conclusion, LNR was recognized as an independent factor in both OS and DFS in esophageal cancer. Besides, LNR showed better prognostic value than N stage for esophageal cancer. Further studies are needed to verify whether LNR could be used to select the appropriate preventive measures for an individual with poor prognosis to improve outcomes.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: Cancer J Clin. 2013;63(1):11–30. doi:10.3322/caac.21166.
Dubecz A, Gall I, Solymosi N, Schweigert M, Peters JH, Feith M, et al. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2012;7(2):443–7. doi:10.1097/JTO.0b013e3182397751.
Lee PC, Port JL, Paul S, Stiles BM, Altorki NK. Predictors of long-term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg. 2009;88(1):186–92. doi:10.1016/j.athoracsur.2009.03.079. discussion 92–3.
Peyre CG, Hagen JA, DeMeester SR, Van Lanschot JJ, Holscher A, Law S, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg. 2008;248(6):979–85. doi:10.1097/SLA.0b013e3181904f3c.
Rizk N, Venkatraman E, Park B, Flores R, Bains MS, Rusch V, et al. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006;132(6):1374–81. doi:10.1016/j.jtcvs.2006.07.039.
Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Liu DT, et al. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2013;8(4):495–501. doi:10.1097/JTO.0b013e3182829e2c.
Zhu Z, Chen H, Yu W, Fu X, Xiang J, Li H, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol. 2014;21(9):2857–63. doi:10.1245/s10434-014-3665-y.
Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99(6):433–41. doi:10.1093/jnci/djk092.
Gorog D, Nagy P, Peter A, Perner F. Influence of obesity on lymph node recovery from rectal resection specimens. Pathol Oncol Res: POR. 2003;9(3):180–3.
Fujita H. The history of lymphadenectomy for esophageal cancer and the future prospects for esophageal cancer surgery. Surg Today. 2014. doi:10.1007/s00595-014-0841-4.
Sisic L, Blank S, Weichert W, Jager D, Springfeld C, Hochreiter M, et al. Prognostic impact of lymph node involvement and the extent of lymphadenectomy (LAD) in adenocarcinoma of the esophagogastric junction (AEG). Langenbeck’s Arch Surg/Deut Ges Chir. 2013;398(7):973–81. doi:10.1007/s00423-013-1101-6.
Lee YC, Yang PJ, Zhong Y, Clancy TE, Lin MT, Wang J. Lymph node ratio-based staging system outperforms the seventh AJCC system for gastric cancer: validation analysis with National Taiwan University Hospital Cancer Registry. Am J Clin Oncol. 2014. doi:10.1097/COC.0000000000000110.
Shida A, Fujioka S, Kawamura M, Takahashi N, Ishibashi Y, Nakada K, et al. Prediction of lymph node metastasis in patients with submucosa-invading early gastric cancer. Anticancer Res. 2014;34(8):4471–4.
Park J, Byun BH, Noh WC, Lee SS, Kim HA, Kim EK, et al. Lymph node to primary tumor SUV ratio by 18F-FDG PET/CT and the prediction of axillary lymph node metastases in breast cancer. Clin Nucl Med. 2014;39(4):e249–53. doi:10.1097/RLU.0b013e3182a75477.
Jiang K, Zhu Y, Liu Y, Ye Y, Xie Q, Yang X, et al. Lymph node ratio as an independent prognostic indicator in stage III colorectal cancer: especially for fewer than 12 lymph nodes examined. Tumour Biol: J Int Soc Oncodev Biol Med. 2014. doi:10.1007/s13277-014-2484-x.
Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg. 2011;253(1):82–7. doi:10.1097/SLA.0b013e3181ffa780.
Bollschweiler E, Baldus SE, Schroder W, Schneider PM, Holscher AH. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94(5):355–63. doi:10.1002/jso.20569.
Talsma K, van Hagen P, Grotenhuis BA, Steyerberg EW, Tilanus HW, van Lanschot JJ, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol. 2012;19(7):2142–8. doi:10.1245/s10434-012-2218-5.
Lorenzon L, Mercantini P, Ferri M, La Torre M, Sparagna A, Balducci G, et al. Lymph-node ratio classification strongly correlates with cancer survivals of patients who underwent R0 resection for gastric cancer with more than 15 nodes harvested. Eur Surg Res Europaische Chir Forsch Rech Chir Europeennes. 2014;53(1–4):1–10. doi:10.1159/000360937.
Ke B, Song XN, Liu N, Zhang RP, Wang CL, Liang H. Prognostic value of the lymph node ratio in stage III gastric cancer patients undergoing radical resection. PLoS One. 2014;9(5):e96455. doi:10.1371/journal.pone.0096455.
Birnbaum DJ, Vigano L, Russolillo N, Langella S, Ferrero A, Capussotti L. Lymph node metastases in patients undergoing surgery for a gallbladder cancer. Extension of the lymph node dissection and prognostic value of the lymph node ratio. Ann Surg Oncol. 2014. doi:10.1245/s10434-014-4044-4.
Smith BJ, Mezhir JJ. An interactive Bayesian model for prediction of lymph node ratio and survival in pancreatic cancer patients. J Am Med Inf Assoc: JAMIA. 2014;21(e2):e203–11. doi:10.1136/amiajnl-2013-002171.
Chen YL, Wang CY, Wu CC, Lee MS, Hung SK, Chen WC, et al. Prognostic influences of lymph node ratio in major cancers of Taiwan: a longitudinal study from a single cancer center. J Cancer Res Clin Oncol. 2014. doi:10.1007/s00432-014-1810-4.
Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg. 2008;206(2):239–46. doi:10.1016/j.jamcollsurg.2007.09.003.
Kelty CJ, Kennedy CW, Falk GL. Ratio of metastatic lymph nodes to total number of nodes resected is prognostic for survival in esophageal carcinoma. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2010;5(9):1467–71.
Tan Z, Ma G, Yang H, Zhang L, Rong T, Lin P. Can lymph node ratio replace pN categories in the tumor-node-metastasis classification system for esophageal cancer? J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2014;9(8):1214–21. doi:10.1097/JTO.0000000000000216.
Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26(7):1086–92. doi:10.1200/JCO.2007.12.9593.
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74. doi:10.1245/s10434-011-2049-9.
Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247(2):365–71. doi:10.1097/SLA.0b013e31815aaadf.
D’Journo XB, Doddoli C, Michelet P, Loundou A, Trousse D, Giudicelli R, et al. Transthoracic esophagectomy for adenocarcinoma of the oesophagus: standard versus extended two-field mediastinal lymphadenectomy? Eur J Cardio-Thorac Surg: Off J Eur Assoc Cardio-Thorac Surg. 2005;27(4):697–704. doi:10.1016/j.ejcts.2004.12.022.
Chandarana M, Jiwnani S, Karimundackal G, Pramesh CS. Lymphadenectomy in esophageal cancer: the real issues. Ann Thorac Surg. 2014;98(1):389–90. doi:10.1016/j.athoracsur.2014.01.040.
Davies AR, Sandhu H, Pillai A, Sinha P, Mattsson F, Forshaw MJ, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg. 2014;101(5):511–7. doi:10.1002/bjs.9456.
Ye T, Sun Y, Zhang Y, Zhang Y, Chen H. Three-field or two-field resection for thoracic esophageal cancer: a meta-analysis. Ann Thorac Surg. 2013;96(6):1933–41. doi:10.1016/j.athoracsur.2013.06.050.
Acknowledgment
This study was supported by the Academics Independent Innovation Project Fund (201401252).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, N., Jia, Y., Wang, J. et al. Prognostic significance of lymph node ratio in esophageal cancer. Tumor Biol. 36, 2335–2341 (2015). https://doi.org/10.1007/s13277-014-2840-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2840-x