With the aims of screening more antibacterial activity metabolites, in this study, two new (1 and 2), together with five known (3–7) isoquinolines were isolated from the cigar tobacco-derived endophytic fungi Aspergillus puniceus. Their structures were determined by means of HR-ESI-MS and extensive 1D and 2D NMR spectroscopic studies. The new compounds were evaluated for antibacterial activities against Pseudomonas syringae (the main pathogenic sources of tobacco angular leaf spot disease), and compounds 1 and 2 exhibited inhibitory effects with MIC50 values of 8.5 and 5.4 μg/mL respectively. These rates are lower than that of positive control (agricultural streptomycin, with MIC50 of 2.2 μg/mL).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Among the numerous existing endophytic fungi, Aspergillus species constitute one of the most prolific sources of natural products with diverse chemical classes and interesting biological activities [1, 2]. In our previous work, bioactive metabolites such as alkaloids were described [3,4,5,6,7]. As characteristic chemical components of Aspergillus, alkaloids are important molecules, not only for chemical reasons but also for their diverse biological functions [8]. The isoquinolines are a large group of alkaloids that show diverse pharmacological activities [9, 10], and some isoquinolines have been isolated from Aspergillus strains [11–13].
As a part of our continuing search on bioactive compounds from tobacco-derived endophytic fungi, a chemical study on the culture broth of an Aspergillus puniceus from cigar tobacco was carried out. As a result, two new (1 and 2) and five known isoquinolines (3–7) were isolated from the EtOAc extract of its solid rice fermented medium. This paper describes the isolation, structural elucidation, and the antibacterial activities against Pseudomonas syringae (the main pathogenic source of tobacco angular leaf spot disease) of the isolated compounds.
The whole culture broth of A. puniceus was extracted with EtOAc. The extract was partitioned between EtOAc and 3% tartaric acid. The aqueous layer was adjusted to pH 9 with saturated Na2CO3 aq. and extracted with EtOAc again. The EtOAc-soluble alkaloidal materials was subjected repeatedly to column chromatography on silica gel, MCI, RP-18 and preparative HPLC to afford compounds 1–7, including two new isoquinolines, 1-(8-methoxy-3-methylisoquinolin-6-yl)propan-1-one (1) and 3-hydroxy-1-(8-methoxy-3-methylisoquinolin-6-yl)propan-1-one (2), along with five known ones (3–7). The NMR data of 1 and 2 were listed in Table 1. The new compounds were confirmed by the search of the newly updated SciFinder database (an electronic database for chemical structures published by the American Chemical Society). The known compounds, TMC-120A (3) [14], 6,7-dioxo-6,7-dihydropyrrolo[1,2-b]isoquinoline-3-carboxylic acid (4) [15], spathullin B (5) [15], skimmianine (6) [16], and puniceusine F (7) [17], were identified by the comparison of their spectroscopic data with the literatures.

Compound 1 was obtained as an orange gum. Its molecular formula C14H15NO2 was deduced from its HR-ESI-MS, which showed a pseudo-molecular [M + Na]+ ion at m/z 252.1002, with 8 degrees of unsaturation. Its infrared spectrum exhibited bands owing to carbonyl (1682 cm–1) and aromatic rings (1615, 1467, and 1378 cm–1), and the UV spectrum showed absorption maxima at 238 and 354 nm, also suggesting the presence of an aromatic ring in the molecule.
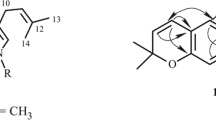
The 1H, 13C, and DEPT NMR data of 1 displayed resonances for 14 carbons and 15 hydrogen atoms, which were ascribed to a 1,2,3,5-tetrasubstituted benzene ring (C-5–C-10, H-5, and H-7), an aromatic methine (C-4, H-4), an N-bearing aromatic methine (C-1, H-1), an N-bearing aromatic quaternary carbon (C-3), a methyl group (C-1′, H3-1′), a propionyl group (–(C=O)CH2CH3, C-2′–C-4′, H2-3′, and H3-4′) [18], and a methoxy group (δC 55.6, δH 3.82 s). By comparing with the published literature, the 1H and 13C NMR data for 1 were highly similar to those of known compounds, 3-methylisoquinoline- 6-carboxylic acid at C-1–C-10 and C-1′ [19] or TMC-120A at C-1–C-4, and C-1′ [14], and this led us to speculate that 1 should be a 3-methylisoquinoline. By further analysis of the above NMR data, two aromatic methines (C-1, C-4, H-1, and H-4), a quaternary carbon (C-3), a methyl (C-1′, H3-1′), and a nitrogen atom (N-2) could be incorporated with the benzene to form an 3-methylisoquinoline to support the existence of two N-bearing aromatic carbons (C-1 and C-4) and 8 degrees of unsaturation. In addition, the existence of isoquinoline moiety can further be confirmed by the HMBC correlations from H-1 to C-3/C-8/ C-9/C-10, from H-4 to C-5/C-10, and from H-5 to C-4/C-9/C-10. The HMBC correlations from H3-1′ to C-3/C-4, from H-4 to C-1′ also supported that the methyl was located at C-3 (Fig. 1).
As the skeleton (3-methyl-isoquinoline) of the compound was determined unambiguously, the remaining signals (propionyl and methoxy groups) could be considered as a substituents, and the existence of propionyl was supported by the comparison of typical propionyl signals (δC 9.66 (-CH3), 32.4 (-CH2-) and 200.4 (-C=O; δH 1.35 (t, J = 7.2, -CH3), and 3.50 (m, -CH2-) with known compounds [18], and the HMBC correlation from H2-3′ to C-2′/C-4′, from H-4′ to C-2′/C-3′ in NMR spectra. Moreover, the propionyl group linked to C-6 was established by HMBC correlations from H-5 and H-7 to C-2′, and from H2-3′ to C-6. Finally, the position of the methoxy group at C-8 was supported by an HMBC correlation from the methoxy protons (δH 3.82 s) to C-8. Additionally, the typical proton signals on the benzene ring 8.02 (d, J = 1.8 Hz) and 7.50 (d, J = 1.8 Hz) were also consistent with the 6,8-substitution on the isoquinoline. Hence, the structure of 1 was fully assigned, and given the systematic name 1-(8-methoxy-3-methylisoquinolin-6-yl)propan-1-one.
3-Hydroxy-1-(8-methoxy-3-methylisoquinolin-6-yl)propan-1-one (2) was also obtained as a pale yellow gum, and its molecular formula was determined to be C14H15NO3 by HR-EI-MS (m/z 268.0940 [M + Na]+). The 1H and 13C spectral data of 2 depict a similar structure to compound 1. The obvious chemical shift differences resulted from the disappearance of a methyl resonance (δC 9.66 q and δH 1.35 (t, J = 7.2 Hz), and the appearance of a hydroxymethyl signal (δC 58.9 t, δC 4.06 (t, J = 6.6 Hz). These changes led us to speculate that the propionyl group at C-6 in 1 should be converted into a 3-hydroxypropanoyl group (-(C=O)CH2CH2OH, C-2′–C-4′, H2-2′–H2-4′) [20] in 2. In addition, the position of the 3-hydroxypropanoyl group at C-6 and methoxy group at C-8 can also be determined by further analysis of its HMBC correlations. Therefore, the structure of 2 was determined.
As certain of the isoquinolines exhibit potential antibacterial activity [12, 21], and the bacteria, Pseudomonas syringae pv. angulata is the main cause of tobacco angular leaf spot disease [22], compounds 1 and 2 were tested for their anti-P. syringae activity. The compounds were assayed for their antimicrobial activities in 96-well plates according to the literatures [12, 23]. Compounds 1 and 2 showed activity with MIC50 values of 8.5 and 5.4 μg/mL, respectively, whereas the MIC50 value of the positive control (agricultural streptomycin) is 2.2 μg/mL.
Experimental
General. UV and IR (KBr) spectra were obtained on an UV-1900 spectrophotometer (Shimadzu, Kyoto, Japan) and a FTS185 spectrophotometer (Bio-Rad, Hercules, CA, USA). NMR experiments were carried out on Bruker DRX-500 NMR spectrometer (Bruker, Karlsruhe, Germany) with TMS as the internal standard. ESI-MS and HR-ESI-MS analyses were performed on a 6540 Q-TOF mass spectrometer equipped with Agilent 1290 UPLC (Agilent Technologies, Wilmington, DE, USA). 80–100 mesh or 200–300 mesh silica gel (Qingdao Marine Chemical, Inc., Qingdao, China) and 75–150 μm MCI CHP20P gel (Mitsubishi Chemical Corporation, Tokyo, Japan) were used for normal column chromatography. The fractions were monitored by thin-layer chromatography (Qingdao Marine Chemical, Qingdao, China), and the spots were visualized by heating silica gel plates (approximately 120°C) sprayed with 5% H2SO4 in ethanol. Semipreparative HPLC was performed with an Agilent 1260 preparative liquid chromatography (Agilent Technologies, Wilmington, DE, USA) with a Venusil MP C18 column (5 μm, 2.0 cm × 25 cm, Bonna-Agela, Tianjin, China) or a Zorbax PrepHT GF C18 column (5 μm, 2.12 cm × 25 cm; Agilent, Palo Alto, CA, USA).
Fungal Material. The culture of Aspergillus puniceus YNNI-21-35 was isolated from the leaves of cigar tobacco, which was collected from the fermentation plant of Yuanjiang County, Yuxi Prefecture, Yunnan Province, in 2021. The strain was identified by one of authors (Dr. Yin-Ke Li) based on the analysis of the ITS sequence. It was cultivated at room temperature for 7 days on potato dextrose agar at 28°C. Agar plugs were inoculated into 250-mL Erlenmeyer flasks each containing 100 mL potato dextrose broth and cultured at 28°C on a rotary shaker at 180 rpm for 5 days. Large scale fermentation was carried out in 100 Fernbach flasks (1.0 L) each containing of 500 g of rice and 300 mL nutrient solution (glucose 5%; peptone 0.15%; yeast 0.5%; KH2PO4 0.05%; urea 0.1%; MgSO4 0.05% in 1.0 L of deionized water; pH 6.5 before autoclaving). Each flask was inoculated with 5.0 mL of cultured broth and incubated at 27°C for 20 days.
Extraction and Isolation. The whole culture broth of A. puniceus was extracted four times with EtOH (4 × 10 L) at room temperature and filtered. The extract was partitioned between EtOAc and 3% tartaric acid. The aqueous layer was adjusted to pH 9 with saturated Na2CO3 aq. and extracted with EtOAc again. The crude extract (98.4 g) was applied to silica gel column chromatography, eluting with a CHCl3–MeOH gradient system (9:1, 8:2, 7:3, 6:4, 5:5). Five fractions were obtained from the silica gel column and individually decolorized on MCI gel to yield fractions A–E. The further separation of Fr. A (9:1, 8.24 g) by silica gel column chromatography, eluted with CHCl3–(Me)2CO (9:1, 8:2, 7:3, 6:4, 1:1), yielded mixtures subfractions A1–A5. Subfraction A2 (8:2, 1.63 g) was subjected to RP-18 column chromatography (MeOH–H2O, 40:60–80:20 gradient) and HPLC to give 1 (12.2 mg), 3 (16.8 mg), and 6 (10.6 mg); subfraction A3 (7:3, 1.28 g) was subjected to RP-18 column chromatography (MeOH–H2O, 30:70–70:30 gradient) and HPLC to give 2 (14.3 mg) and 5 (16.8 mg). The further separation of Fr. C (7:3, 10.28 g) by silica gel column chromatography, eluted with CHCl3–(Me)2CO (7:3, 6:4, 1:1, 4:6, 3:7), yielded mixture subfractions C1–C5. Subfraction C-2 (6:4, 1.85 g) was subjected to RP-18 column chromatography (MeOH–H2O, 20:80–60:40 gradient) and HPLC to give 4 (18.3 mg) and 7 (15.2 mg).
Antibacterial Assays. The strain of bacteria (Pseudomonas syringae pv. angulata) was obtained from Yunnan Academy of Tobacco Agricultural Sciences. The antibacterial activities were tested by a serial dilution technique using 96-well microtiter plates [12, 23], using agricultural streptomycin (a commercial product for plant bacteria disease in China) as a positive control. The tested compounds and positive control were dissolved in DMSO to give a stock solution.
1-(8-Methoxy-3-methylisoquinolin-6-yl)propan-1-one (1), orange gum, C14H15NO2. UV (MeOH, λmax, nm) (log ε): 215 (4.02), 238 (3.86), 354 (3.64). IR (νmax, cm–1): 3079, 1682, 1615, 1467, 1378, 1350, 1272, 1139, 1042, 835. For 1H (500 MHz) and 13C (125 MHz) NMR data, see Table 1. ESI-MS m/z 252 [M + Na]+, HR-ESI-MS m/z 252.1002 (calcd for C14H15NNaO2, 252.0995).
3-Hydroxy-1-(8-methoxy-3-methylisoquinolin-6-yl)-propan-1-one (2), orange gum, C14H15NO3. UV (MeOH, λmax, nm) (log ε): 215 (4.08), 242 (3.72), 358 (3.60). IR (νmax, cm–1): 3405, 3068, 1684, 1618, 1462, 1370, 1346, 1278, 1145, 1056, 812. For 1H (500 MHz) and 13C (125 MHz) NMR data, see Table 1. ESI-MS m/z 268 [M + Na]+; HR-ESI-MS m/z 268.0940 (calcd for C14H15NNaO3, 268.0944).
References
A. Hagag, M. F. Abdelwahab, A. El-Kader, and M. A. Fouad, J. Appl. Microbiol., 132 (6), 4150 (2022).
S. S. El-Hawary, A. S. Moawad, H. S. Bahr, U. R. Abdelmohsen, and R. Mohammed, RSC. Adv., 10, 22058 (2020).
G. Y. Yang, J. M. Dai, Q. L. Mi, Z. J. Li, X. M. Li, J. D. Zhang, J. Wang, Y. K. Li, W. G. Wang, M. Zhou, and Q. F. Hu, Phytochemistry, 198, 113137 (2022).
J. M. Dai, Q. L. Mi. X. M. Li, D. Gang, G. Y. Yang, J. D. Zhang, J. Wang, Y. K. Li, H. Y. Yang, M. Dong, Z. J. Li, and Q. F. Hu, Phytochemistry, 205, 113485 (2023).
Y. N. Zhu, M. X. Liu, B. B. Cai, Y. Li, M. F. Li, H. S. Wang, M. Zhou, G. Y. Yang, Q. F. Hu, and Y. K. Li, Chem. Nat. Compd., 58, 712, (2022).
M. F. Li, D. Xiao, L. C. Zhu, L. Liu, J. N. Zheng, X. J. Gu, Y. N. Zhu, J. Xie, X. Wang, J. M. Dai, Q. L. Mi, Y. K. Yang, Q. F. Hu, Y. K. Li, and J. Q. Shi, Chem. Nat. Compd., 58, 1093 (2012).
J. M. Dai, L. C. Zhu, D. Xiao, J. Xie, X. Wang, Q. L. Mi, J. Q. Shi, G. Y. Yin, Y. K. Yang, G. Y. Yang, Q. F. Hu, and W. Kai, Chem. Nat. Compd., 58, 1005 (2022).
B. Debnath, W. S. Singh, M. Das, S. Goswami, M. K. Singh, D. Maiti, and K. Manna, Mater. Today Chem., 9, 56 (2018).
P. Yadav and K. Shah, Bioorg. Chem., 109, 104639 (2021).
G. Prabal and S. K. Gopinatha, Mini-Rev. Med. Chem., 10, 568 (2010).
Q. Li, C. M. Chen, Y. He, M. S. Wei, L. Cheng, X. Kang, J. P. Wang, X. C. Hao, H. C. Zhu, and Y. H Zhang, Phytochemistry, 169, 112177 (2020).
Acknowledgment
This project was supported by the Foundation of the China Tobacco Monopoly Bureau Grants and Yunnan Provincial Tobacco Monopoly Bureau Grants (110202103018, 2022530000241004), the National Natural Science Foundation of China (No. 32260111), and the Foundation of Yunnan Innovative Research Team (2019HC020).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2023, pp. 963–966.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, MS., Qiu, WY., Lin, ZL. et al. Two New Isoquinolines from Cigar Tobacco-Derived Endophytic Fungi Aspergillus puniceus and Their Antibacterial Activity. Chem Nat Compd 59, 1142–1146 (2023). https://doi.org/10.1007/s10600-023-04212-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-04212-3