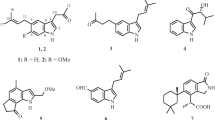
Two new (1 and 2) and four known (3–6) CPA-type indole alkaloids were isolated from the fungus Aspergillus versicolor. Their structures were determined by means of HR-ESI-MS and extensive 1D and 2D NMR spectroscopic studies. Compounds 1 and 2 were tested for their anti-tobacco mosaic virus (anti-TMV) activity. The results showed that compounds 1 and 2 demonstrated potential anti-TMV activity with inhibition rates within the range 29.2 and 30.1% respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aspergillus versicolor is a highly ubiquitous species of fungus commonly isolated from soil, plant debris, marine environments, and indoor air environments [1, 2]. Many metabolites produced by A. versicolor exhibit multiple biological activities, such as anti-bacterial, fungicidal, insecticidal, anti-viral, and cytotoxic properties [3, 4]. Prenylated indole alkaloids constitute one of the characteristic components in Aspergillus fungi, and they have attracted much attention from chemists and biologists owing to their complex structures and interesting bioactivity. Great efforts have been made to synthesize and identify new prenylated indole alkaloids [5,6,7,8,9,10].
The α-cyclopiazonic acid (α-CPA) is an indole-tetramic acid that was initially isolated from the fungus Penicillium cyclopium as a toxic metabolite [11]. It was then found to be widely distributed in the ubiquitous genera of molds Aspergillus and Penicillium. To date, some naturally occurring CPA analogs have been discovered with minor structural variations, and most are indole or oxindole alkaloids containing the tetramic acid unit [12,13,14,15]. Those alkaloids possess attractive biological activities such as anti-TMV activity [9], cytotoxicity [9, 13, 14], anti-oxidant activity [15], anti-bacterial activity [12], pronounced neurite outgrowth-promoting effects [8], and especially the CPA is one of the few inhibitors of the sarco(endo)plasmic reticulum Ca2+-ATPase [16, 17]. For the above reason, the CPA and its analogs have drawn widespread attention as potential drug candidates.
As part of our efforts to discover novel secondary metabolites from tobacco-derived microorganisms, the fungus Aspergillus versicolor, isolated from the root Nicotiana tabacum (tobacco) collected at Jiangchuan County, Yuxi City, China, was investigated and resulted in the yield of two new (1 and 2), and four known (3–6) CPA-type indole alkaloids. The new compounds were elucidated by means of spectroscopic methods, whereas the known compounds were identified by comparison with data in the literature. Compounds 1 and 2 were also evaluated for their anti-tobacco mosaic virus (anti-TMV) activity. Herein, we report their isolation, structure determination, and the bioassay screening.

A 70% aqueous Me2CO extract prepared from the fermentation products of the endophytic fungus A. versicolor was partitioned with EtOAc. The EtOAc-soluble materials were subjected repeatedly to column chromatography on silica gel and preparative HPLC to afford two new CPA-type indole alkaloids, aspergillines K and L (1 and 2), together with four known CPA-type indole alkaloids (3–6). The 1H and 13C NMR data of compounds 1 and 2 are listed in (Table 1). The known compounds, compared with the literature, were identified as cyclopiamide (3) [18], cyclopiamide D (4) [19], speradine G (5) [20], and amycocyclopiazonic acid (6) [13].
Compound 1, an orange gum, had a molecular formula of C17H16N2O2, as revealed from HR-ESI-MS (m/z 303.1115 [M + Na]+), suggesting 11 degrees of unsaturation. The IR spectrum showed absorption bands amine (3189 cm–1), carbonyl (1692 cm–1), and aromatic ring (1640, 1482, 1367 cm–1), and the UV spectrum showed absorption maxima at 215, 264, 320, 354, and 398 nm. It also showed the existance of an aromatic ring. Its 1H, 13C, and DEPT NMR data (Table 1) displayed resonances for 17 carbons and 16 hydrogen atoms, which were ascribed to a 1,2,3,4-tetrasubstituted naphthalene ring (C-3, C-4, C-8–C-15, H-9, H-11–H-13), a –CO-N- moiety (C-2 and N-1) [18], a –C(CH3)2-N-CO- moiety (C-5, NH-6, C-7, C-16, C-17, H3-16, and H3-17) [19], and an N-ethyl group (C-18, C-19, H2-18, and H3-19). On the basis of the carbon chemical shifts of these resonances, the naphthalene ring and two carbonyl groups in compound 1 accounted for 9 of the 11 degrees of unsaturation. The –CO-N- moiety should be fused to a naphthalene ring to form a 1H-pyrrol-2(3H)-one ring (N-1, C-2, C-3, C-14, and C-15), and the –C(CH3)2-NH-CO- moiety should also be attached to the naphthalene ring to form a dimethyl-1H-pyrrol-2(5H)-one (C-4, C-5, NH-6, C-7, C-8, C-16, C-17, H3-16, H3-17) to meet 11 degrees of unsaturation. This deduction was supported the HMBC correlations (Fig. 1) from H-9 to C-4, C-7, C-8, from H3-16 and H3-17 to C-8, and from NH-6 to C-4, C-8. The NMR data of 1 (Table 1) showed great similarity to those of the known compound, cyclopiamide (3) [18], with the only obvious difference in the extra presence of an N-ethyl moiety and disappearance of a N-methyl group, which suggested that 1 was a cyclopiamide analog. The HMBC spectrum showed correlations of H-18 with C-14, C-2, which suggested that the N-ethyl group attached to N-1 of the cyclopiamide skeleton. Thus, the structure of 1 was determined as shown, and gave the trivial name of aspergilline K.
Aspergilline L (2) was also obtained as orange gum with a molecular formula C19H16N2O4, according to the ion peak of m/z 359.1002 ([M + Na]+) in the HR-ESI-MS. The 1H NMR, 13C NMR, UV, and IR spectra of 2 were highly similar to those of 1. The chemical shift differences resulted from the disappearance of an N-ethyl group and appearance of a N-3-oxobutana moiety (C-18–C-21, H2-19, and H3-21) in 2. The existence of an N-3-oxobutana moiety was also supported by HMBC correlations from H2-19 to C-18, C-20, from H3-21 to C-19, C-20. In addition, the absence of N-1 proton, and the observation of the HMBC correlations from N-6 proton to C-5, C-14 also supported the N-3-oxobutana moiety attached to N-1 of the cyclopiamide skeleton. The structure of 2 was therefore defined.
As certain CPA-type indole alkaloids from fungus A. versicolor exhibit potential anti-TMV activity [9], compounds 1 and 2 were tested for their anti-TMV activities. The anti-TMV activities were tested with the half-leaf method, using ningnanmycin (C16H25N7O8, CAS#: 156410-09-2, a commercial new cytosine nucleoside peptide antibiotic for plant viral diseases in China, with an inhibition rate of 33.0%) as a positive control [21, 22]. The results showed that compounds 1 and 2 demonstrated potential anti-TMV activity, with inhibition rates within the range 29.2 to 30.1%.
EXPERIMENTAL
General. UV spectra were obtained using a Shimadzu UV-1900 spectrophotometer. A Bio-Rad FTS185 spectrophotometer was used for scanning IR spectra. 1D and 2D NMR spectroscopic data were recorded on a DRX-500 NMR spectrometer with TMS as internal standard. ESI-MS and HR-ESI-MS analyses were measured on an Agilent 1290 UPLC/6540 Q-TOF mass spectrometer. Semipreparative HPLC was performed on an Agilent 1260 preparative liquid chromatograph with Zorbax PrepHT GF (2.12 mm × 25 cm) or Venusil MP C18 (2.0 mm × 25 cm) columns. Column chromatography was performed using silica gel (200–300 mesh, Qingdao Marine Chemical, Inc., Qingdao, China), LiChroprep RP-18 gel (40–63 μm, Merck, Darmstadt, Germany), Sephadex LH-20 (Sigma-Aldrich, Inc, USA), or MCI gel (75–150 μm, Mitsubishi Chemical Corporation, Tokyo, Japan). Column fractions were monitored by TLC visualized by spraying with 5% H2SO4 in ethanol and heating.
Fungus Material. The culture of Aspergillus versicolor was isolated from the root Nicotiana tabacum (tobacco), collected from Jiangchuan County, Yuxi City, People′s Republic of China, in 2016. The strain was identified by one of authors (Gang Du) based on the analysis of the ITS sequence. It was cultivated at room temperature for 7 days on potato dextrose agar at 28°C. Agar plugs were inoculated into 250 mL Erlenmeyer asks each containing 100 mL potato dextrose broth and cultured at 28°C on a rotary shaker at 180 rpm for 5 days. Large-scale fermentation was carried out in 100 Fernbach asks (500 mL), each containing 100 g of rice and 120 mL of distilled H2O. Each flask was inoculated with 5.0 mL of cultured broth and incubated at 25°C for 45 days.
Extraction and Isolation. The fermentation products were extracted four times with 70% aqueous Me2CO (4 × 10 L) at room temperature and filtered. The filtrate was concentrated to a small volume under reduced pressure until Me2CO free, and extracted with an equal volume of EtOAc three times to obtain an EtOAc extract of mycelia. The EtOAc extracts were combined and concentrated to dryness under reduced pressure to give 45.2 g of crude product. The crude extract was applied to silica gel (200–300 mesh) column chromatography, eluting with a CHCl3–MeOH gradient system (20:1, 8:2, 7:3, 6:4, 5:5), to give five fractions A–E. The further separation of fraction B (8:2, 21.6 g) by silica gel column chromatography, eluted with CHCl3–Me2CO (9:1, 8:2, 7:3, 6:4, 1:1), yielded subfractions B1–B5. Subfraction B1 (9:1, 1.86 g) was subjected to preparative HPLC (65% MeOH, flow rate 20 mL/min) to give 1 (12.2 mg), 2 (14.5 mg), and 4 (13.8 mg). Subfraction B2 (8:2, 6.38 g) was subjected to preparative HPLC (55% MeOH, flow rate 12 mL/min) to give 3 (15.2 mg), 5 (16.3 mg), and 6 (10.6 mg).
Anti-TMV Assays. The anti-TMV activities were tested using the half-leaf method [21, 22], and ningnanmycin (2% water solution), a commercial product for plant disease in China, was used as a positive control. The virus was inhibited by mixing with the solution of tested compounds (20 μM in DMSO). After 30 min, the mixture was inoculated on the left side of the leaves of N. glutinous, whereas the right side of the leaves was inoculated with the mixture of DMSO solution and the virus as a control. The local lesion numbers were recorded 3–4 days after inoculation. Three repetitions were conducted for each compound. The inhibition rates were calculated according to the formula:
where C is the average number of local lesions of the control and T is the average number of local lesions of the treatment. Ningnanmycin (20 μM in DMSO), a commercial virucide for plant disease in China, was used as a positive control.
Aspergilline K (1). C17H16N2O2, obtained as a pale-yellow gum. UV (MeOH, λmax, nm) (log ε): 215 (4.48), 264 (4.26), 320 (2.92), 354 (3.38), 398 (3.12). IR (KBr, νmax, cm–1): 3189, 1692, 1640, 1482, 1367, 1180, 1142, 778. For 1H and 13C NMR data (500 and 125 MHz, CDCl3), see Table 1. ESI-MS m/z 303 [M + Na]+; HR-ESI-MS m/z 303.1115 [M + Na]+ (calcd for C17H16N2NaO2, 303.1109).
Aspergilline L (2). C19H16N2O4, obtained as a pale-yellow gum. UV (MeOH, λmax, nm) (log ε): 215 (4.42), 268 (4.29), 359 (3.42), 400 (3.26). IR (KBr, νmax, cm–1): 3240, 2924, 1715, 1702, 1642, 1485, 1323, 1180, 1084, 1035, 785. For 1H and 13C NMR data (500 and 125 MHz, CDCl3), see Table 1. ESI-MS m/z 359 [M + Na]+; HR-ESI-MS m/z 359.1002 [M + Na]+ (calcd for C19H16N2NaO4, 359.1008).
References
F. Bongomin, C. R. Batac, M. D. Richardson, and D. W. Denning, Mycopathologia, 183, 485 (2018).
J. M. Restrepo-Florez, A. Bassi, and M. R. Thompson, Int. Biodeterior. Biodegrad., 88, 83 (2014).
X. L. Zhang, Z. Li, and J. T. Gao, Nat. Prod. J., 8, 275 (2018).
R. Orfali, M. A. Aboseada, N. M. Abdel-Wahab, H. M. Hassan, S. Perveen, F. Ameen, E. Alturki, and U. R. Abdelmohsen, RSC Adv., 11, 17116 (2021).
P. Zhang, Q. Wei, X. L. Yuan, and K. Xu, Bioorg. Chem., 99, 103840 (2020).
N. Netz and T. Opatz, Mar. Drugs., 13, 4814 (2015).
P. C. Zhao, Y. Xue, J. H. Li, X. Li, X. Y. Zu, Z. Q. Zhao, C. S. Quan, W. N. Gao, and S. X. Feng, Biotechnol. Lett., 41, 651 (2019).
L. Liu, L. Bao, L. Wang, K. Ma, J. J. Han, Y. L. Yang, R. X. Liu, J. W. Ren, W. B. Yin, and W. Z. Wang, J. Org. Chem., 83, 812 (2018).
M. Zhou, M. M. Miao, G. Du, X. N. Li, S. Z. Shang, W. Zhao, Z. H. Liu, G. Y. Yang, C. T. Che, Q. F. Hu, and X. M. Gao, Org. Lett., 16, 5016 (2014).
J. B. Roque, E. V. Mercado-Marin, S. C. Richter, D. P. de Sant′Ana, K. Mukai, Y. D. Ye, and R. Sarpong, Chem. Sci., 11, 5929 (2020).
C. W. Holzapfel, Tetrahedron, 24, 2101 (1968).
Y. Xiang, Q. Zeng, Z. M. Mai, Y. C. Chen, X. F. Shi, X. Y. Chen, W. M. Zhong, X. Y. Wei, W. M. Zhang, S. Zhang, and F. Z. Wang, Fitoterapia, 150, 104839 (2021).
Y. Kwon, S. H. Kim, Y. Shin, M. Bae, B. Y. Kim, S. K. Lee, K. B. Oh, J. Shin, and D. C. Oh, Mar. Drugs, 12, 2326 (2014).
X. H. Ma, J. X. Peng, G. W. Wu, T. J. Zhu, G. Q. Li, Q. Q. Gu, and D. H. Li, Tetrahedron, 71, 3522 (2015).
W. H. Chen, K. L. Li, X. P. Lin, S. R. Liao, B. Yang, X. F. Zhou, J. J. Wang, Y. H. Liu, and J. F. Wang, Nat. Prod. Res., 35, 5266 (2021).
M. T. Tsuda, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami, M. Shiro, M. Hirai, Y. Ohizumi, and J. Kobayashi, Tetrahedron, 59, 3227 (2003).
B. Climent, E. Santiago, A. Sanchez, M. Munoz-Picos, F. Perez-Vizcaino, A. Garcia-Sacristan, L. Rivera, and D. Prieto, Biochem. Pharmacol., 182, 114222 (2020).
C. W. Holzapfel, M. W. Bredenkamp, M. S. Snyman, J. C. A. Boeyens, and C. C. Allen, Phytochemistry, 29, 639 (1990).
X. Y. Xu, X. Y. Zhang, X. H. Nong, X. Y. Wei, and S. H. Qi, Tetrahedron, 71, 610 (2015).
X. Hu, Q. W. Xia, Y. Y. Zhao, Q. H. Zheng, Q. Y. Liu, L. Chen, and Q. Q. Zhang, Chem. Pharm. Bull., 62, 942 (2014).
Q. F. Hu, B. Zhou, J. M. Huang, X. M. Gao, L. D. Shu, G. Y. Yang, and C. T. Che, J. Nat. Prod., 76, 292 (2013).
M. Zhou, M. M. Miao, G. Du, S. Z. Shang, W. Zhao, Z. H. Liu, G. Y. Yang, C. T. Che, Q. F. Hu, and X. M. Gao, Org. Lett., 16, 5016 (2014).
Acknowledgment
This project was supported by the National Natural Science Foundation of China (No. 21967021), the Yunnan Applied Basic Research Projects for Excellent Young Scholars (grant to M.Z.), and the Foundation of Yunnan Innovative Research Team (2019HC020).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2022, pp. 603–606.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, YN., Liu, MX., Cai, BB. et al. Aspergillines K and L, Two New Anti-TMV Indole Alkaloids from Fungus Aspergillus versicolor Derived from Tobacco. Chem Nat Compd 58, 712–716 (2022). https://doi.org/10.1007/s10600-022-03774-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03774-y