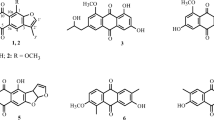
Two new indole alkaloids, 6-(4-methoxyphenoxy)-4-methoxy-2-methyl-1H-indole (1) and 6-(3,5-dimethoxyphenoxy)-4-methoxy-2-methyl-1H-indole (2), along with four known ones (3–6), were isolated from the fermentation products of the cigar tobacco-derived endophytic fungus Aspergillus oryzae. Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. Compounds 1 and 2 were evaluated for their antibacterial and antioxidant activities. The results showed that compounds 1 and 2 exhibited good anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity with IZD of 22.4 ± 2.4 and 24.6 ± 2.2 mm, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endophytic fungi are a kind of microorganism that live in the organs of plants, insects, and animals, and they can bring biologically beneficial substances to hosts without causing any diseases [1]. An increasing number of endophytic fungi have arisen wide attention in recent years due to their secondary metabolites with structural complex skeletons and diverse biological activities [2,3,4]. Among them, the Aspergillus is an important phytopathogenic genus [5, 6], and they produce a number of secondary metabolites with various biological activities including antimicrobial, antioxidant, antiviral, antitumor compounds, and the like [7, 8].
Aspergillus oryzae is an aerobic filamentous fungus and belongs to the Aspergillus subgenus Circumdati section Flavi, and it has been safely used in various East Asian cuisines for centuries to ferment soybeans, potatoes, rice, and other grains in the making of alcoholic beverages such as huangjiu, sake, makgeolli, and so on [9]. In addition, A. oryzae has also been considered an important source of natural products. Some bioactive metabolites, such as isocoumarins [10], sesquiterpenes [11, 12], diterpenoid [13, 14], alkaloids [15,16,17], and the like, had been isolated from this fungus. In this study, our efforts on the endophytic fungus A. oryzae, isolated from the leaves of cigar tobacco, led to the isolation and identification of two new indole alkaloids (1 and 2) and four known ones (3–6). Herein, we report on the isolation and structure elucidation of new compounds and their antibacterial activity.
A 95% aq. ethanol extract prepared from the fermented substrate was partitioned between EtOAc and 3% tartaric acid. The aqueous layer was adjusted to pH 9.0 with saturated Na2CO3 aq. and extracted with EtOAc again. The EtOAc-soluble alkaloid materials were subjected repeatedly to column chromatography and preparative HPLC to afford two new indole alkaloids, 6-(4-methoxyphenoxy)-4-methoxy-2-methyl-1H-indole (1) and 6-(3,5-dimethoxyphenoxy)-4-methoxy-2-methyl-1H-indole (2), together with four known indole alkaloid derivatives (3–6). The NMR data of 1 and 2 were listed in Table 1. The known compounds, cimitrypazepine (3) [18], cephalandoles B (4) [19], bruceollines L (5) [20], and 3-prenylindole-5-carbaldehyde (6) [21], were identified by comparison of their spectroscopic data with the literature.

Compound 1 was isolated as a pale-yellow gum. HR-ESI-MS analysis gave a quasi-molecular ion at m/z 306.1113 [M + Na]+, consistent with a molecular formula of C17H17NO3. The UV spectrum of 1 exhibited absorption bands at 304, 246, and 215 nm, highly suggesting the existence of an aromatic chromophore. Strong absorption bands accounting for amino (3392 cm–1) and aromatic groups (1618, 1546, and 1348 cm–1) could also be observed in its IR spectrum. The 1H and 13C NMR spectrum of 1 (Table 1) showed the presence of one 1,2,3,5-tetrasubstituted benzene ring (C-4–C-9, H-5 and H-7), one 4-methoxyphenoxy group (CH3O-C6H4-O-, C-1′∼C-6′, H2-2′, 6′, H2-3′, 5′, and CH3O-4′) [22], one methyl group (H3-10), one methoxy group (δC 56.3 q and δH 3.83 s), and a –NH-C=CH- moiety (C-2, C-3, and H-3). According to the preceding NMR signals, the –NH-C=CH- moiety should be incorporated with a benzene ring to form an indole core [23] to support the molecular 10 degrees of unsaturation. In addition, the existence of the indole core was also supported by the HMBC correlations (Fig. 1) from H-3 to C-4, C-8, and C-9, from H-7 to C-8 and C-9, and from NH to C-7, C-8, and C-9.
Since the indole skeleton was determined, the positions of substituents (methyl, methoxy, and methoxyphenoxy groups) can also be determined by further analysis of its HMBC data (Fig. 1). The HMBC correlations from the H3-10 to C-2 and C-3, from H-3 to C-10 established that the methyl group was located at C-2. The methoxy group located at C-4 was clearly indicated by the HMBC correlations from methoxy proton (δ 3.83 s) to C-4. Furthermore, the methoxyphenoxy group should be linked to C-6 to support the typical 1H NMR signals (H-5, 6.32 d, J = 1.8 Hz; H-7, 6.42 d, J = 1.8 Hz) of the protons on the benzene ring. On the basis of the aforementioned evidence, the structure of 1 was established as shown and given the systematic name of 6-(4-methoxyphenoxy)-4-methoxy-2-methyl-1H-indole.
Compound 2 was also obtained as a pale-yellow gum. The molecular formula was established as C18H19NO4 by the [M + Na]+ ion at m/z 336.1208 (calcd for C18H19NNaO4, 336.1212) in HR-ESI-MS. The 1H and 13C NMR data (Table 1) of 1 and 2 showed high similarity in C-2–C-9. The obvious chemical shift differences resulting from the methoxyphenoxy group was replaced by a 3,5-dimethoxyphenoxy group ((CH3O)2C6H3-O-, C-1′∼C-6′, H2-2′, 6′, H-4′, CH3O-3′, and CH3O-5′) [24] in 2. In addition, the position of the methyl group can also be ascertained by further analysis of its HMBC correlations; thus, the structure of 6-(3,5-dimethoxyphenoxy)-4-methoxy-2-methyl-1H-indole (2) was determined as shown.
Since a certain amount of the indole alkaloids from fungi Aspergillus exhibit potential antibacterial activity [25,26,27], compounds 1 and 2 were evaluated for their antibacterial activity. The anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity was screened according to arbitrary criterion [28] using the inhibition zone diameter (IZD) as follows: very weak inhibition (with IZD of 6–8 mm), weak inhibition (with IZD of 8–12 mm), good inhibition (with IZD of 12–16 mm), and strong inhibition (with IZD of > 16 mm) activities, respectively. The IZD of the positive control was 32 mm and the negative control was about zero. The results revealed that compounds 1 and 2 showed good inhibition with IZD of 22.4 ± 2.4 and 24.6 ± 2.2 mm, respectively.
Experimental
General Methods. UV spectra were obtained using a Shimadzu UV-1900 spectrophotometer. A Bio-Rad FTS185 spectrophotometer was used for scanning IR spectra. 1H, 13C, and 2D NMR spectroscopic data were recorded on a DRX-500 NMR spectrometer with TMS as the internal standard. ESI-MS and HR-ESI-MS analyses were measured on Agilent 1290UPLC/6540 Q-TOF mass spectrometer. Chemical shifts (δ) are expressed in ppm with reference to the TMS signal. Semipreparative HPLC was performed on an Agilent 1260 preparative liquid chromatograph with Zorbax PrepHT GF (2.12 mm × 25 cm) or Venusil MP C18 (2.0 mm × 25 cm) columns. Column chromatography was performed using silica gel (200–300 mesh, Qingdao Marine Chemical, Inc., Qingdao, China), Lichroprep RP-18 gel (40–63 μm, Merck, Darmstadt, Germany), Sephadex LH-20 (Sigma-Aldrich, Inc., USA), or MCI gel (75–150 μm, Mitsubishi Chemical Corporation, Tokyo, Japan). Column fractions were monitored by TLC and visualized by spraying with 5% H2SO4 in ethanol and heating.
Fungal Material. The culture of Aspergillus oryzae YNCA-1220 was isolated from the leaves of cigar tobacco, collected from Gengma County, Lincang Prefecture, Yunnan Province, in 2019. The strain was identified by one of the authors (Dr. Qi-Li Mi) based on the analysis of the ITS sequence. It was cultivated at room temperature for 7 days on potato dextrose agar at 28°C. Agar plugs were inoculated into 250-mL Erlenmeyer flasks each containing 100-mL potato dextrose broth and cultured at 28°C on a rotary shaker at 180 rpm for 5 days. The large-scale fermentation was carried out in 20 Fernbach flasks (5.0 L) each containing 1.0 kg of gelatinized cornstarch and 120 mL of 1.2% urea solution. Each flask was inoculated with 50 mL of cultured broth and incubated at 30°C for 20 days.
Extraction and Isolation. The fermented substrate was extracted four times with ethanol (4 × 20 L) at room temperature and filtered. The filtered solution was concentrated under reduced pressure to yield a crude extract, which was suspended in 3% tartaric acid and partitioned between EtOAc. The aqueous layer was adjusted to pH 9 with saturated Na2CO3 aq., and extracted with EtOAc again. The EtOAc-soluble alkaloid materials were applied to silica gel (200–300 mesh) column chromatography, eluting with a CHCl3–MeOH (20:1, 9:1, 8:2, 7:3, 6:4, 5:5), to give six fractions (A–F). The further separation of fraction B (9:1, 28.5 g) by silica gel column chromatography using CHCl3–(Me)2CO (9:1, 8:2, 7:3, 6:4, 1:1) eluent afforded five subfractions (B1–B5). The further separation of subfraction B2 (8:2) by silica gel column chromatography using petroleum ether–EtOAc, and preparative HPLC (with 58% aqueous MeOH as mobile phase, flow rate 20 mL/min) afforded 1 (16.4 mg), 2 (14.8 mg), and 6 (18.4 mg). The further separation of subfraction B3 (7:3) by silica gel column, eluted with petroleum ether–EtOAc, and preparative HPLC (with 50% aqueous MeOH as mobile phase, flow rate 20 mL/min) afforded 3 (23.6 mg), 4 (15.2 mg), and 5 (18.8 mg).
Anti-MRSA Agar Disc Diffusion Assay. The MRSA strain ZR11 was clinically isolated from infectious samples of critically ill patients in the Clinical Laboratory of the First People’s Hospital of Yunnan Province and confirmed by standard cefoxitin disk diffusion test following CLSI standard procedures [28]. The anti-MRSA activity of the compounds was evaluated via the disc diffusion method. The ZR11 strain was inoculated in Mueller–Hinton Broth and was incubated at 37°C for 24 h. The turbidity of bacterial suspension was adjusted to 0.5 McFarland standard, which equals 1.5 × 108 colony-forming units (CFU)/mL. Sterile filter paper discs (6 mm) were impregnated with 20 μL (50 μg) of each compound and placed on inoculated Mueller–Hinton agar containing bacterial suspension, which was adjusted to 0.5 McFarland standard. The commercially available discs containing 30-μg vancomycin were used as the positive control, whereas discs without samples (5% DMSO) acted as the negative control. The inhibition zones including the diameter of the disc (mm) were measured and compared after incubation at 37°C for 24 h. The tests were carried out in triplicate for each sample.
6-(4-Methoxyphenoxy)-4-methoxy-2-methyl-1 H -indole (1), C17H17NO3, obtained as pale-yellow gum. UV (MeOH, λmax, nm) (log ε): 304 (3.68), 246 (3.92), 215 (4.32). IR (KBr, νmax, cm–1): 3392, 3059, 2944, 1618, 1546, 1348, 1286, 1159, 1034, 786, and 732. 1H and 13C NMR data (CDCl3, 500 and 125 MHz, respectively), see Table 1. ESI-MS m/z 306; HR-ESI-MS m/z 306.1113 [M + Na]+ (calcd for C17H17NNaO3, 306.1106).
6-(3,5-Dimethoxyphenoxy)-4-methoxy-2-methyl-1 H -indole (2), C18H19NO4, obtained as pale-yellow gum. UV (MeOH, λmax, nm) (log ε): 306 (3.72), 248 (3.90), 215 (4.36). IR (KBr, νmax, cm–1): 3395, 3061, 2940, 1615, 1549, 1346, 1289, 1154, 1038, 792, and 730. 1H and 13C NMR data (CDCl3, 500 and 125 MHz, respectively), see Table 1. ESI-MS m/z 336; HR-ESI-MS m/z 336.1208 [M + Na]+ (calcd for C18H19NNaO4, 336.1212).
References
S. Gupta, P. Chaturvedi, M. G. Kulkarni, and J. Van Staden, Biotechnol. Adv., 39, 107462 (2019).
I. T. Malik and H. Brotz-Oesterhelt, Nat. Prod. Rep., 34, 815 (2017).
C. Schinke, T. Martins, S. C. N. Queiroz, I. S. Melo, and F. G. R. Reyes, J. Nat. Prod., 80, 121 (2017).
T. T. Liu, L. P. Wang, L. Zhang, H. Y. Jiang, Y. N. Zhang, and L. G. Mao, Nat. Prod. Res., 34, 1430 (2020).
F. Bongomin, C. R. Batac, M. D. Richardson, and D. W. Denning, Mycopathologia, 183, 485 (2018).
J. M. Restrepo-Florez, A. Bassi, and M. R. Thompson, Int. Biodeterior. Biodegrad., 88, 83 (2014).
S. Skanda and B. S. Vijayakumar, Curr. Microbiol., 78, 1317 (2021).
M. Y. Deng, X. Chen, Z. Y. Shi, and S. S. Xie, Fitoterapia, 151, 104882 (2021).
G. M. Daba, W. A. Elkhateeb, and F. A. Mostafa, Bioresour. Bioprocess., 8, 52 (2021).
M. Zhou, K. Zhou, P. He, K. M. Wang, R. Z. Zhu, Y. D. Wang, W. Dong, G. P. Li, H. Y. Yang, Y. Q. Ye, G. Du, X. M. Li, and Q. F. Hu, Planta. Med., 82, 414 (2016).
R. Orfali, S. Perveen, M. F. Khan, A. F. Ahmed, M. A. Wadaan, A. M. Al-Taweel, A. S. Alqahtani, F. A. Nasr, S. Tabassum, P. Luciano, G. Chianese, J. H. Sheu, and O. Taglialatela-Scafati, Mar. Drugs., 19, 333 (2021).
R. Ren, C. J. Chen, S. S. Hu, H. M. Ge, W. Y. Zhu, R. X. Tan, and R. H. Jiao, Chem. Biodiv., 12, 371 (2015).
W. G. Wang, L. Q. Du, S. L. Sheng, A. Li, Y. P. Li, G. G. Cheng, G. P. Li, G. L. Sun, Q. F. Hu, and Y. Matsuda, Org. Chem. Front., 6, 571 (2019).
C. W. Liu, A. Minami, T. Ozaki, T, J. Wu, H. Kawagishi, J. I. Maruyama, and H. Oikawa, J. Am. Chem. Soc., 141, 15519 (2019).
L. Liu, L. Bao, L. Wang, K. Ma, J. J. Han, Y. L. Yang, R. X. Liu, J. W. Ren, W. B. Yin, W. Z. Wang, and H. W. Liu, J. Org. Chem., 83, 812 (2018).
X. Hu, Q. W. Xia, Y. Y. Zhao, Q. H. Zheng, Q. Y. Liu, L. Chen, and Q. Q. Zhang, Chem. Pharm. Bull., 46, 942 (2015).
M. Shaaban, M. M. El-Metwally, and H. Nasr, Nat. Prod. Res., 28, 86 (2014).
D. Nikolic, T. Godecke, S. N. Chen, J. White, D. C. Lankin, G. F. Pauli, and R. B. van Breemen, Fitoterapia, 83, 441 (2012).
P. L. Wu, Y. L. Hsu, and C. W. Jao, J. Nat. Prod., 69, 1467 (2006).
H. Chen, J. Bai, Z. F. Fang, S. S. Yu, S. G. Ma, S. Xu, Y. Li, J. Qu, J. H. Ren, L. Li, Y. K. Si, and X. G. Chen, J. Nat. Prod., 74, 2438 (2011).
F. Tabassum, C. M. Hasan, M. M. Masud, S. Jamshidi, K. M. Rahman, and M. Ahsan, Phytochemistry, 186, 112744 (2021).
M. P. Epplin, A. Mohan, L. D. Harris, Z. J. Zhu, K. L. Strong, J. Bacsa, P. Le, D. S. Menaldino, S. F. Traynelis, and D. C. Liotta, J. Med. Chem., 63, 7569 (2020).
D. R. Beukes, M. T. Davies-Coleman, M. Kelly-Borges, M. K. Harper, and D. J. Faulkner, J. Nat. Prod., 61, 699 (1998).
H. Tanak, A. Agar, and M. Yavuz, J. Mol. Model., 16, 577 (2010).
F. D. Kong, S. L. Zhang, S. Q. Zhou, Q. Y. Ma, Q. Y. Xie, J. P. Chen, J. H. Li, L. M. Zhou, J. Z. Yuan, Z. Hu, H. F. Dai, X. L. Huang, and Y. X. Zhao, J. Nat. Prod., 82, 3456 (2019).
K. Xu, X. L. Yuan, C. Li, and X. D. Li, Mar. Drugs., 18, 54 (2020).
M. L. Wang, R. Chen, F. J. Sun, P. R. Cao, X. R. Chen, and M. H. Yang, Tetrahedron Lett., 68, 152914 (2021).
Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, Vol. 32, Clinical and Laboratory Standards Institute, Wayne, Pa, USA, 9th Edition, 2012.
Acknowledgment
This project was supported financially by the Foundation of Chemical and Biological Innovation Studio of Yunnan Industrial Co., Ltd., the Foundation of Yunnan Tobacco Industry Co., Ltd. (No. 2020JC02), the National Natural Science Foundation of China (No. 21967021), and the Foundation of Yunnan Innovative Research Team (2019HC020).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2022, pp. 920–923.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Mf., Xiao, D., Zhu, LC. et al. Indole Alkaloids from the Cigar Tobacco-Derived Endophytic Fungus Aspergillus oryzae and Their Antibacterial Activity. Chem Nat Compd 58, 1093–1097 (2022). https://doi.org/10.1007/s10600-022-03872-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03872-x