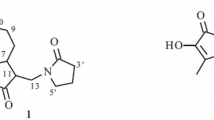
Cadabatone (1), a new eudesmanolide-type sesquiterpene lactone, has been isolated from the EtOAc soluble subfraction of the methanolic extract of Cadaba fruticosa (L.) Druce, along with known compounds, 3-epierivanin (2), 3,5,7,4-tetrahydroxyflavone (3), esculetin (4), rosmarinic acid (5), α-amyrin (6), and β-amyrin (7), isolated for the first time from this species. The structures of these compounds were elucidated by spectroscopic studies including MS, IR, 1D and 2D NMR spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Cadaba, belonging to the family Capparidaceae comprises 30 species of shrubs growing in Africa, Arabia and the Indo-Pakistan subcontinent. In Pakistan, the genus is represented by only two species, namely Cadaba fruticosa and Cadaba heterotricha. C. fruticosa (L.) Druce (syn. C. farinosa) is a multibranched shrub that is commonly found in Karachi and other parts of the Sindh province of Pakistan. Its leaves and roots have purgative, antihelmintic, antispasmodic, amenagogue, and aperient properties [1,2,3] and the fruits are commonly used to treat worm infection. The plant also possesses antimicrobial, antioxidant, antidiabetic, antipyretic, and anti-inflammatory activities [4]. A literature survey of the genus Cadaba revealed the presence of alkaloids [5,6,7], terpenoids [8, 9], and flavonoids [10,11,12]; however, only two compounds have so far been reported from C. fruticosa [13, 14]. The ethnopharmacological and chemotaxonomic importance of this genus prompted us to undertake further phytochemical studies on C. fruticosa. Herein we report the isolation and structural elucidation of a new sesquiterpene lactone, named cadabatone (1), along with known 3-epierivanin (2) [15], 3,5,7,4-tetrahydroxyflavone (3) [16], esculetin (4) [17], rosmarinic acid (5) [18], α-amyrin (6), and β-amyrin (7) [19], isolated from the species for the first time.
Cadabatone (1) was obtained as a colorless gummy solid. The HR-EI-MS showed [M]+ peak at m/z 384.1936 corresponding to the molecular formula C23H28O5. The IR spectrum showed absorption bands for hydroxyl groups (3422 cm–1), a γ-lactone moiety (1760 cm–1), an ester carbonyl (1718 cm–1), an aromatic ring (1620, 1508 cm–1), and an exocyclic double bond (1650, 865 cm–1).

The 13C NMR (BB and DEPT) spectra of 1 showed 21 signals comprising three methyls, four methylene, eight methine, and six quaternary carbons (Table 1). The downfield signals at δ 166.2 and 178.6 could respectively be assigned to the carbonyls of ester and γ-lactone moieties. The olefinic carbons resonated at δ 145.0 and 109.5, while the signals at δ 70.1, 81.9, and 78.5 could be ascribed to the oxymethine carbons, respectively. Additionally, an aromatic methyl resonated at δ 21.0 while the signal of another tertiary methyl was observed at δ 18.0. The signals ranging from δ 127.2–139.4 were due to aromatic carbons. The 1H NMR data showed the signals of a tertiary angular methyl and a secondary lactonic methyl at δ 1.19 (s, H3-14) and 1.26 (d, J = 6.5 Hz, H3-13), respectively, which are characteristic of eudesmanolides [20]. The exomethylene protons resonated as singlets at δ 5.19 and 4.97. Two oxymethine protons resonated at δ 3.79 (ddd, J = 9.5, 9.0, 3.5 Hz, H-2) and 3.85 (d, J = 9.5 Hz, H-3). The lactonic protons resonated at δ 3.98 (t, J = 11 Hz), 1.69 (m), and 2.32 (dq, J = 11.5, 6.5 Hz), being assigned to H-6, H-7, and H-11, respectively, which are consistent with the trans-fusion of the lactonic ring. The presence of 4-methylbenzoyl moiety was also inferred by the aromatic protons providing an AA′BB′ pattern with resonances at δ 8.18 (2H, d, J = 7.5 Hz) and 7.82 (2H, d, J = 7.5 Hz) and the singlet of aromatic methyl at δ 2.48. The presence of 4-methylbenzoyl moiety was also supported by the fragment ion peak in EI-MS at m/z 266 (C15H22O4). It further showed diagnostic fragments at m/z 222 [266 – CO2]+, 210 [266 – C3H4O]+, 193 [266 – C3H4O2]+, and 169 [266 – C5H5O2]+. These data indicated that compound 1 has eudesmanolide-type sesquiterpene lactone skeleton [15], including 4-methylbenzoyl moiety. The oxymethine proton at δ 3.85 showed 1H–1H-COSY correlation to another oxymethine proton at δ 3.79, which in turn showed further correlations with methylene protons at δ 1.30 and 2.44, permitting us to assign the position of the hydroxyl group at C-2. The larger coupling constant between H-2 and H-3 allowed us to assign the equatorial configuration to the hydroxyl moiety. It was further confirmed by HMBC correlations (Table 1); the oxymethine proton at δ 3.79 showed 2J correlations with C-1 (δ 48.2) and C-3 (δ 81.9) and 3J correlations with C-4 (δ 145.0) and C-10 (δ 40.8). The oxymethine proton at δ 3.85 showed 2J correlations with C-2 (δ 70.1) and C-4 (δ 145.0) as well as 3J correlations with C-1 (δ 48.2), C-5 (δ 45.1), C-15 (δ 109.5), and C-7′ (δ 166.2), confirming the position of 4-methylbenzoyl moiety and the exocyclic methylene group at C-3 and C-4, respectively. The 6,12-eudesmanolide moiety was further confirmed by 2J correlations of H-6 at δ 3.98 with C-5 (δ 45.1), C-7 (δ 52.7) as well as 3J correlations with C-4 (δ 145.0), C-8 (δ 24.0), and C-11 (δ 42.0). The close similarity of NMR chemical shifts to those of known eudesmanolides [15], allowed us to assign the same relative configuration of 1. The larger coupling constants of H-6 and H-7 as well as H-7 and H-11 suggested H-7 to be α-oriented which could further be authenticated by the NOESY spectrum. NOESY correlations (Fig. 1) were observed between β-oriented Me-14 with H-2 and H-6 as well as H-6 with H-11. Similarly, α-oriented H-5 showed correlations with H-3 and H-7 as well as H-7 with Me-13. All these pieces of evidence were in complete agreement with the assigned structure of 1 as 2α-hydroxy-3β-4- methylbenzoyl-5,7α,6,11β(H)-eudesm-4,15-en-6,12-olide.
EXPERIMENTAL
General Procedures. Optical rotations were measured on a JASCO DIP-360 polarimeter. UV spectra were recorded on a Hitachi UV-3200 spectrophotometer. IR spectra were recorded on a JASCO 302-A spectrophotometer in KBr, whereas NMR data were recorded on a Bruker AV-500 MHz spectrometer (500 MHz for 1H and 125 MHz for 13C) in CDCl3 with tetramethylsilane as an internal standard. EI- and HR-EI-MS were recorded on Finnigan MAT 12 and MAT 312 spectrometers Aluminum sheets precoated with silica-gel 60 F254 (E. Merck, Darmstadt, Germany) were used for thin-layer chromatography (TLC), while silica gel (250–400 mesh, E. Merck) was used for column chromatography.
Plant Material. The whole plants of C. fruticosa (L.) Druce were collected from Karachi, Sindh, Pakistan, in 2020 and identified by Prof. Dr. Beena Naqvi Plant Taxonomist, Pharmaceutical Research Centre, PCSIR Laboratories Complex Karachi, Pakistan, where a voucher specimen (No. CF-136/173-2021) was deposited in the Herbarium.
Extraction and Isolation. The whole plants of C. fruticosa (9 kg) were shade-dried, ground, and extracted with MeOH (3 × 25 L). The combined MeOH extract was evaporated under reduced pressure to yield the crude extract (200 g), which was divided into subfractions soluble in n-hexane (50 g), CHCl3 (18 g), EtOAc (25 g), n-BuOH (40 g), and H2O (38 g), respectively. The EtOAc-soluble fraction was subjected to column chromatography over silica gel, eluting with mixtures of n-hexane–EtOAc in increasing order of polarity to obtain six fractions F1–F6. Fraction F3, eluted with n-hexane–EtOAc (5.0:5.0), was chromatographed over silica gel and eluted with mixtures of n-hexane–EtOAc in increasing order of polarity. The subfractions, eluted with n-hexane–EtOAc (6.5:3.5), (6.0:4.0), and (4.5:5.5), furnished compounds 4 (18 mg), 6 (22 mg), and 7 (20 mg), respectively. Fraction F4, eluted with n-hexane–EtOAc (4.0:6.0), showed two major spots on TLC and was again chromatographed over silica gel using n-hexane–EtOAc (6.0:4.0) as an eluent to provide compounds 2 (15 mg) and cadabatone (1) (6 mg) from the top and tail fractions, respectively. Fraction F5, eluted with n-hexane–EtOAc (3.0:7.0), was further purified by column chromatography to afford two successive subfractions F5A and F5B, eluted with n-hexane–EtOAc (5.5:4.5) and (7.0:3.0) to afford compounds 3 (20 mg) and 5 (16 mg), respectively.
Cadabatone (1), colorless gummy solid, [α]D –26° (c 0.3, CHCl3). UV (MeOH, λmax nm) (log ε): 204 (3.78). IR (KBr, νmax, cm–1): 865, 1508, 1620, 1650, 1718, 1760, 3422. EI-MS (m/z, Irel., %): 384 (9), 369 (30), 366 (52), 266 (50), 222 (65), 210 (77), 193 (68), 169 (80), 119 (100), 97 (85), 73 (35), 55 (75), 44 (90). HR-EI-MS m/z 384.1936 (calcd for C23H28O5, 384.1940). For 1H, 13C NMR, and HMBC data, see Table 1.
The authors declare no conflict of interest.
References
S. M. H. Jafri, Flora of West Pakistan, S. I. Ali and E. Nasir, eds., Department of Botany, University of Karachi, Pakistan, 1973, No. 34, p. 35.
S. M. H. Jafri, Pak. J. For., 8, 204 (1958).
S. R. Baquer and M. Tasnif, Medicinal Plants of Southern West Pakistan, PCSIR Bulletin/Monograph, 1967, No. 3, p. 5.
S. Saboo, J. Pharmacogn. Phytochem., 9, 2331 (2020).
V. U. Ahmad, A. R. Amber, S. Arif, M. H. M. Chen, and J. Clardy, Phytochemistry, 24, 2709 (1985).
V. U. Ahmad, K. Fizza, A. R. Amber, and S. Arif, J. Nat. Prod., 50, 1186 (1987).
G. Yousif, G. M. Iskander, and E. B. Eisa, Fitoterapia, 55, 117 (1984).
V. U. Ahmad, A. R. Amber, K. Fizza, and A. Kamal, Z. Naturforsch., 45b, 1100 (1990).
N. M. Al-Musayeib, G. A. Mohamed, S. R. M. Ibrahim, and S. A. Ross, Med. Chem. Res., 22, 5297 (2013).
A. A. Gohar, Z. Naturforsch., 57C, 216 (2002).
G. A. Mohamed, S. R. M. Ibrahim, N. M. Al-Musayeib, and S. A. Ross, Arch. Pharm. Res., 37, 459 (2014).
G. A. Al-Hamoud, R. S. Orfali, S. Sugimoto, Y. Yamano, N. Alothyqi, A. M. Alzahrani, and K. Matsunami, Molecules, 24, 2167 (2019).
V. U. Ahmad, A. Basha, and Atta-ur-Rahman, Phytochemistry, 14, 292 (1975).
S. P. Garg, R. Bhushan, and R. C. Kapoor, Planta Med., 43, 293 (1981).
J. F. Sanz, E. Falco, and J. A. Marco, J. Nat. Prod., 53, 940 (1990).
G. R. Nagarajan and V. S. Parmar, Planta Med., 31, 146 (1977).
H. Tsukamoto, S. Hisada, S. Nishibe, D. G. Roux, and J. P. Rourke, Phytochemistry, 23, 699 (1984).
W. Eun-Rhan and S. P. Mei, Arch. Pharm. Res., 27, 173 (2004).
S. B. Mahato and A. P. Kundu, Phytochemistry, 37, 1517 (1994).
Atta-Ur-Rahman, Studies in Natural Products Chemistry, Elsevier Publishing Co., Amsterdam, 1990, p. 212.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2023, pp. 236–238
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jabbar, A.A., Khan, S., Kazmi, M.H. et al. Cadabatone, a New Sesquiterpene Lactone from Cadaba fruticosa. Chem Nat Compd 59, 278–281 (2023). https://doi.org/10.1007/s10600-023-03976-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-03976-y