Abstract
Three new flavonoids; kaempferol-4′-phenoxy-3,3′,5′-trimethylether (3), rhamnocitrin-4′-(4-hydroxy-3-methoxy)phenoxy-3-methyl ether (4), and rhamnocitrin-3-O-neohesperoside-4′-O-rhamnoside (6), along with three known compounds; 4-methoxy-benzyldehyde (1), kaempferol-3-methylether (2), and stachydrine (5) were isolated from the aerial parts of Cadaba glandulosa Forssk. Their chemical structures were established by physical, chemical, and spectral methods, as well as comparison with literature data. The antioxidant and anti-inflammatory activities of the isolated compounds were determined. Compounds 2–4, and 6 exhibited potent anti-inflammatory activity comparable with indomethacin and moderate antioxidant activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadaba farinosa Forssk. (family Capparidaceae) is a highly viscid low shrub with small inconspicuous flowers, strongly furrowed stem, and closely packed glandular-hispid, round leaves (Gohar 2002). It is widely spread in the Kingdom of Saudi Arabia on the Red Sea coastal region, Abha, Bisha, Nagran, and South Jedda to Madina area as well as between Dahna and Arabian Gulf (Gohar 2002). The leaves are used for the treatment of haemorrhoids and urinary tract infections (Al-Fatimi et al. 2007). Cadaba species were reported to contain alkaloids, terpenes, and flavonoids (Viqar Uddin et al. 1990, 1985, 1987; Yousif et al. 1984; Al-Musayeib et al. 2013; Gohar 2002). Previous phytochemical studies on Chamaecrista glandulosa resulted in the isolation of flavonoids, alkaloids, and sterols (Gohar 2002; Attia 2002). Herein, we report the isolation and identification of three new flavonoids (3, 4, and 6), along with three known compounds (1, 2, and 5) from the aerial parts of C. glandulosa. In addition, the antioxidant and anti-inflammatory activities of the isolated flavonoids as well as the total MeOH extract (TME) were evaluated. The isolation of phenoxyflavonoids might be useful as chemotaxonomic markers for the genus Cadaba.
Materials and methods
General
Melting point was carried out in Electrothermal 9100 Digital Melting Point (England), IR on Schimadzu Infrared-400 spectrophotometer (Japan), UV spectra on Perkin-Elmer Lambda 25 UV/VIS spectrophotometer, low resolution mass spectra on a Finnigan MAT TSQ 7000 mass spectrometer, HRESIMS spectra with a Micromass Qtof 2 mass spectrometer, and NMR spectra on Bruker DRX 500 spectrometer (Bruker, Rheinstetten, Germany). Vacuum liquid chromatography (VLC) was carried out on silica gel 60 (0.04–0.063 mm, Merck). Column chromatographic (CC) separations were performed over silica gel 60 (0.04–0.063 mm, Merck) and Sephadex LH-20 (0.25–0.1 mm Merck). TLC analyses were carried out on pre-coated silica gel F254 aluminium sheets and RP-18 F254s glass plates (Merck) using developing systems: CHCl3:MeOH (95:5, S1), CHCl3:MeOH (90:10, S2), CHCl3:MeOH (85:15, S3), and n-BuOH:HOAc:H2O (4:1:2, S4). Compounds were detected by UV at wavelength 255 and 366 nm and visualized with: AlCl3 (flavonoids), FeCl3 (phenolics), p-anisaldehyde/H2SO4 (triterpenes), and Dragendorff`s (alkaloids) (Jork et al. 1990). Authentic flavonoids and sugars were obtained from the Department of Pharmacognosy, Faculty of Pharmacy, Assiut University. Reference compounds: 2,2-diphenyl-1-picrylhydrazyl (DPPH), propyl gallate (PG), carrageenin, and indomethacin were purchased from Sigma Chemical Co. (Germany).
Plant material
The plant C. glandulosa Forssk. was collected in March 2011 from Abha, Saudi Arabia and identified by Dr. A. A. Fayed, Prof. of Plant Taxonomy, Faculty of Science, Assiut University, Assiut, Egypt. A voucher specimen (registration number CG-6-2009) has been deposited at the Herbarium of the research center for medicinal aromatic and poisonous plants, Saudi Arabia.
Extraction and isolation
The air-dried powdered aerial parts (490 g) were extracted with MeOH (3 L × 4) at room temperature. The combined extracts were concentrated to afford a dark residue (16.5 g) which was suspended in 200 mL distilled water and partitioned between n-hexane (500 mL × 5), EtOAc (500 mL × 5), and n-BuOH (500 mL × 5), successively. Each fraction was concentrated to give n-hexane (5.1 g), EtOAc (2.8 g), n-BuOH (1.7 g), and aqueous (4.1 g) residues. The EtOAc residue was subjected to VLC using CHCl3:MeOH gradients. Four fractions were collected: CE-1 (0.65 g, 100 % CHCl3), CE-2 (0.48 g, 75:25 CHCl3:MeOH), CE-3 (0.70 g, 50:50 CHCl3:MeOH), and CE-4 (0.77 g, 100 % MeOH). Fraction CE-2 (0.48 g) was subjected to CC (120 g silica gel × 50 × 3 cm) using n-hexane:EtOAc gradient to afford compound 1 (13 mg, white needles). Fraction CE-3 (0.70 g) was subjected to CC (120 g silica gel × 50 × 3 cm) using n-hexane:EtOAc gradient to give 2 (21 mg, yellow crystals) and 3 (15 mg, yellow crystals). Fraction CE-4 (0.77 g) was chromatographed over Sephadex LH-20 column (150 g × 100 × 5 cm) using eluent CHCl3:MeOH (90:10) to afford three main sub-fractions CE-4-A to CE-4-C. Sub-fractions CE-4-B (0.19 g) and CE-4-C (0.23 g) were separately subjected to silica gel CC (80 g × 50 × 3 cm) using CHCl3:MeOH gradients followed by purification on RP-18 column (0.04–0.063 mm; 100 g × 50 × 2 cm) using MeOH:H2O gradients to give 4 (19 mg, yellow crystals) and 5 (24 mg, colourless oil). The n-BuOH (1.7 g) fraction was chromatographed over Sephadex LH-20 column (150 g × 100 × 5 cm) using MeOH as an eluent, to furnish two sub-fractions CB-1 and CB-2. Sub-fraction CB-1 (0.49 g) was subjected to RP-18 column (0.04–0.063 mm; 100 g × 50 × 2 cm) using MeOH:H2O gradients to give 6 (20 mg, yellow amorphous powder).
Acid hydrolysis of 6
Compound 6 (3 mg) was refluxed with 10 mL of 1 N HCl for 4 h. The aglycone was extracted with CHCl3. The sugars in the aqueous layer were identified by co-paper chromatography (PC) with authentic materials using solvent system (S4).
Antioxidant activity
The antioxidant activity was determined as previously outlined ((Al-Musayeib et al. 2013; El-Kumarasamy et al. 2004) by the decrease in the absorption of each of the isolated compounds (20 μM) or TME (50, 100, and 150 μg/mL) in DPPH solution (4 mg were dissolved in 50 mL), monitored at 517 nm using a spectrophotometer. The absorbance of DPPH in MeOH (with or without compounds) was measured after 2 min. The antioxidant activity of each compound was measured in relation to reference propyl gallate set as 100 % antioxidant activity. Determinations were performed in triplicate. The antioxidant activity was calculated:
Carrageenin-induced rat paw edema
The anti-inflammatory activity was evaluated as previously described (Aguilar et al. 2002). Hind paw edema (skin edema) was induced by subplanter administration of 0.1 mL carrageenin (1 % w/v) in normal saline in the right hand paw of the rats. The inflamed animals were divided randomly into twelve groups (6 for each); inflamed control group, inflamed treated with indomethacin (at a dose of 10 mg/kg subcutaneously), eight groups of inflamed animals were treated with the tested compounds individually (at doses of 5 and 10 mg/kg subcutaneously), and two groups were treated with TME at doses of 50 and 100 mg/kg subcutaneously (the plant extract was dissolved in sterile distilled water). The change in paw thickness in all tested animals was measured with Plethysmometer 7150 (UGO, Basil, Italy) at 0, 1, 2, 4, and 6 h after carrageenin solution injection.
Animals
Adult male Sprague–Dawley rats weighing 100–120 g were obtained from the animal facility of Faculty of Pharmacy, King Saud University, Riyadh, KSA. Animals were housed at a temperature of 23 ± 2 °C with free access to water and standard food pellets. Rats were left to acclimatize in the animal facility for 1 week prior to experimentation. All animal procedures were conducted in accordance with the guidelines of the Institutional Animal Ethical Committee of King Saud University (approval number KSU/IAEC/1432/12) (El-Badry 2011).
Statistical analysis
All data were expressed as mean ± standard error of mean using the student t test. The statistical significance was evaluated by one-way analysis of variance (ANOVA). The values were considered to be significantly different when p values were less than 0.05 (p < 0.05).
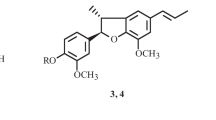
Kaempferol-4′-phenoxy-3,3′,5′-trimethylether (3)
Yellow crystals (EtOH). R f 0.81 (S1). M.p. 254–256 °C. UV λmax (MeOH): 267, 354; +NaOMe: 269, 368; +AlCl3: 274, 388; +AlCl3/HCl: 271, 385; +NaOAc: 284, 355; +NaOAc/H3BO3: 269, 357 nm. IR (KBr): ν max cm−1 = 3410, 2925, 1665, 1615, 1570. HRESIMS: m/z 437.1162 [M+H]+, (calcd. for C24H21O8, 437.1158). NMR data see Table 2.
Rhamnocitrin-4′-(4-hydroxy-3-methoxy)phenoxy-3-methyl ether (4)
Yellow crystals (EtOH). R f 0.76 (S1). M.p. 276–278 °C. UV λmax (MeOH): 228 sh, 268, 351; +NaOMe: 274, 331, 374; +AlCl3: 279, 354, 391; +AlCl3/HCl: 278, 352, 388; +NaOAc: 268, 354; +NaOAc/H3BO3: 269, 352 nm. IR (KBr) ν max cm−1 = 3365, 2942, 1646, 1613, 1569. HRESIMS m/z 437.1159 [M+H]+ (calcd for C24H21O8, 437.1158). NMR data see Table 2.
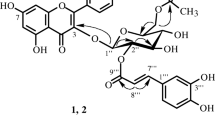
Rhamnocitrin-3-O-neohesperoside-4′-O-rhamnoside (6)
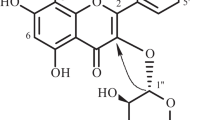
Yellow amorphous powder. R f 0.53 (S3). UV λmax (MeOH): 212 sh, 266, 345; +NaOMe: 271, 325, 351; +AlCl3: 257 sh, 274, 304, 350, 385; +AlCl3/HCl: 256 sh, 275, 303, 349, 381; +NaOAc: 269, 315, 349; +NaOAc/H3BO3: 265, 278 sh, 351 nm. IR (KBr) ν max cm−1 = 3397, 2930, 1656, 1608, 1573, 1517. HRESIMS m/z 755.2317 [M+H]+ (calcd for C34H43O19, 755.2320). NMR data see Table 1 (Fig. 1).
Results and discussion
Compound 6 was obtained as yellow amorphous powder and gave positive tests for flavonoids (Mabry et al. 1970). The molecular formula was determined as C34H42O19, on the basis of the pseudo-molecular ion peak at m/z 755.2317 [M+H]+ in HRESIMS. The UV spectrum bands at 212, 266, and 345 nm, as well as reaction with diagnostic shift reagents suggested 6 to be 3,7,4′-tri-substituted flavonol glycoside with free hydroxyl group at C-5 (Mabry et al. 1970). The IR spectrum indicated the presence of OH group at 3,397 cm−1, α,β-unsaturated CO group at 1,656, and aromatic ring at 1,608 cm−1 (Silverstein and Webster 1998). The 1H NMR spectrum showed resonances for two meta-coupled protons at δH 6.39 (1H, d, J = 2.2 Hz) and 6.75 (1H, d, J = 2.2 Hz). The HSQC spectrum correlated these signals to the carbons resonating at δC 97.9 and 93.1, respectively characterizing the presence of tetra-substituted ring A (Williams 1994; Agrawal 1992). Two pairs of ortho-coupled protons at δH 8.09 (2H, d, J = 7.5 Hz, H-2′,6′) and 6.92 (2H, d, J = 7.5 Hz, H-3′,5′). They correlated with carbons at δC 130.8 and 115.1, respectively in the HSQC spectrum, suggesting the presence of AA′BB′ system due to a 1,4-di-substituted ring B (Table 1). In addition, a singlet signal for methoxy group at δH 3.87 correlated with the carbon at δC 56.1 in HSQC and 165.1 (C-7) in the HMBC indicated its attachment at C-7 of ring A. The hydroxyl group at δH 12.64 was located at C-5 based on its HMBC correlations with C-5, C-6, and C-10. The presence of 5-hydroxy-7-methoxy substituted ring A was confirmed by the fragment ion peak at m/z 167.3259 in HRESIMS (Crow et al. 1986). Thus, the aglycone part of 6 was assigned as rhamnocitrin and confirmed by the fragment ion peak at m/z 299.0176 (Gohar 2002). Moreover, two anomeric protons signal at δH 5.09 (2H, s, H-1′′′, 1′′′′) correlated with the carbon at δC 100.6, as well as, two doublet methyl signals at δH 0.78 (6H, d, J = 6.9 Hz, H-6′′′, 6′′′′) suggested the presence of two rhamnose moieties correlated with the carbons at δC 17.2 and 18.3. The signals at δH 5.68 (1H, d, J = 7.2 Hz, H-1″)/δC 98.3 indicated the presence of β-glucopyranose moiety (Agrawal 1992). These were confirmed by the fragment ion peaks at 608.5671 [M-Rh]+, 445.4259 [M–(Glu+Rh)]+, and 299.0176 [M–(Rh+Rh+Glu)]+. In the HMBC, the cross peak of H-1′′ to C-3 (δH 132.9) suggested the attachment of glucose moiety at C-3 of rhamnocitrin (Fig. 2). The cross peak observed between H-1′′′ and C-2′′ indicated the attachment of rhamnose at C-2′′ of glucose moiety at C-3 of ring C, suggested the presence of neohesperoside (Harborne 1988). This was confirmed by the downfield shift of C-2′′. The attachment of second rhamnose moiety at C-4′ was established based on the HMBC cross peak of H-1′′′′ with C-4′ (δC 156.4) (Fig. 2). Upon the hydrolysis of 6, rhamnocitrin, glucose, and rhamnose were identified by co-chromatography alongside with authentic samples. On the basis of the above evidences, the structure of 6 was determined as rhamnocitrin-3-O-neohesperoside-4′-O-rhamnoside.
Compound 4 was isolated as yellow crystals. The compound gave positive reaction for the flavonoids (Mabry et al. 1970). The HRESIMS spectrum showed a pseudo-molecular ion peak at m/z 437.1159 [M+H]+, which compatible with the molecular formula C24H20O8. The presence of 3,7,4′-tri-substituted flavonol moiety was deduced from the UV spectral data (Mabry et al. 1970). The NMR spectral data of 4 were similar to those of 6 except the signals for sugar moieties were not present (Table 2). Instead, new signals at δH/δC 7.43 (1H, brs, H-2′′)/105.6 (C-2′′), 7.53 (1H, d, J = 7.8 Hz, H-5′′)/128.6 (C-5′′), and 8.27 (1H, d, J = 7.8 Hz, H-6′′)/130.5, together with signals for methoxy (δH 3.99/δC 56.4) and hydroxyl (δH 9.87) groups, suggesting the presence of a 1,3,4-tri-substituted phenoxy moiety. This was confirmed by the observed HMBC correlations and fragment ion peak at m/z 313.2385 [M–C7H7O2]+ (Fig. 2). Furthermore, two methoxy groups signals at δH 3.99 (6H, s, 3,7-OCH3)/δC 60.4 (3-OCH3) and 56.4 (7-OCH3) were observed. Their locations at C-3 and C-7 of rhamnocitrin were confirmed by the HMBC cross peaks of 3-OCH3 to C-3 (δC 139.6) and 7-OCH3 to C-7 (δC 166.3). It was inferred that 4 was rhamnocitrin-3-methyl ether substituted at position 4 of ring B by a (4-hydroxy-3-methoxy)phenoxy moiety. Thus, the structure of 4 was unambiguously established and named rhamnocitrin-4′-(4-hydroxy-3-methoxy)phenoxy-3-methyl ether and considered as a new natural product.
Compound 3 was isolated as yellow crystals and gave positive reaction for flavonoids (Mabry et al. 1970). The molecular formula for 3 was C24H20O8 confirmed by HRESIMS. The UV absorption maxima at 254 and 354 nm and its reaction with diagnostic shift reagents suggested it to be a kaempferol derivative (Markham 1982; Harborne 1988). The bathochromic shift of the UV absorption bands I and II with AlCl3 and NaOAc suggested the presence of 5 and 7-OH groups, respectively. The absence of NaOCH3 band I shift indicated that the 4′-OH group was substituted (Mabry et al. 1970). The 1H NMR spectrum showed two singlet signals at δH 12.92 and 11.02 which were assigned to 5- and 7-OH groups, respectively (Table 2) (Harborne 1988; Agrawal 1992). The two meta-coupled protons at δH 6.32 (1H, d, J = 1.8 Hz) and 6.62 (1H, d, J = 1.8 Hz) were assigned to H-6 and H-8, respectively. The two protons signal at δH 7.50 (2H, brs, H-2′, 6′) indicated the presence of tetra-substituted ring B. 1H NMR spectrum of 3 displayed signals for mono-substituted phenoxy moiety at δH 7.69 (2H, d, J = 7.6 Hz, H-3′′, 5′′), 7.84 (1H, t, J = 7.6 Hz, H-4′′), and 8.20 (2H, d, J = 7.6 Hz, H-2′′, 6′′) and confirmed by COSY and HMBC correlations (Fig. 2). The connectivity of the phenoxy moiety at C-4′ of ring B was established on the basis of HMBC correlations of H-2′′ and H-6′′ with C-4′ (δC 139.3). Furthermore, two singlet signals at δH 3.92 (6H, s, 3′, 5′-OCH3) and 3.94 (3H, s, 3-OCH3) indicated the presence of three methoxy groups. The attachment of methoxy groups at C-3, C-3′, and C-5′ was established by their HMBC correlations (Fig. 2). On the basis of the previous findings, 3 was identified as kaempferol-4′-phenoxy-3,3′,5′-trimethylether and considered as a new natural product.
The known compounds were identified as 4-methoxybenzyldehyde (1) (El-Shanawany et al. 2012), kaempferol-3-methylether (2) (Harborne 1988), and stachydrine (5) (Al-Musayeib et al. 2013) by the analysis of the spectroscopic data (1D, 2D NMR, and MS) and comparison of their data with those in the literature.
The TME and isolated compounds (2–4, and 6) showed moderate concentration dependent scavenging activity by quenching DPPH radicals (Table 3), maximum inhibition (74.12 % for TME at 150 μg/mL and 61.49 for isolated compounds at 20 μM) of DPPH. This antioxidant capacity may be due to the presence of phenolics in C. glandulosa. Furthermore, the TME of C. glandulosa and compounds 2-4, and 6 were tested for their anti-inflammatory activity in carrageenin induced paw edema model. The TME (100 mg/kg) and 4 (10 mg/kg) exhibited highest anti-inflammatory activity compared with indomethacin (10 mg/kg). Also, 3, 2, and 6 showed potent activity at dose 10 mg/kg after 4 h (Table 4; Fig. 3). Thus, it was also observed that the presence of phenoxy group (compounds 3 and 4) and the free OH group (compound 2) at position 4′ may be essential for the anti-inflammatory activity. These results were also in accordance with previous studies that attributed the anti-inflammatory activity of flavonoids to the C-2,3 double bond, the presence of a methoxy group at C-3 and C-7, and the pyran ring (Ibrahim et al. 2012). The activity of TME may be due to the presence of terpenes, flavonoids, and alkaloids (Perez 2001). Flavonoids are known to inhibit prostaglandins synthesis enzymes, more specifically the endoperoxide. It was reported that prostaglandin like substances are released during the second phase of carrageenin induced edema (Alcaraz and Jimenez 1998).
Conclusions
This study reports the isolation and identification of six compounds from the aerial parts of C. glandulosa Forssk. three of them are new. The structures of the isolated compounds were established by spectroscopic data. The total MeOH extract as well as isolated flavonoids were evaluated for their antioxidant and anti-inflammatory activities. Phenoxyflavonoids (3 and 4) found in the present study might be useful as chemotaxonomic markers for cadaba species.
References
Agrawal, P.K. 1992. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 31: 3307–3330.
Aguilar, J.L., P. Rojas, A. Marcelo, A. Plaza, R. Bauer, E. Reininger, C.A. Klaas, and I. Merfort. 2002. Anti-inflammatory activity of two different extracts of Uncaria tomentosa (Rubiaceae). Journal of Ethnopharmacology 81: 271–276.
Al-Fatimi, M., M. Wurster, G. Schroder, and U. Lindequist. 2007. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. Journal of Ethnopharmacology 111: 657–666.
Al-Musayeib, N.M., G.A. Mohamed, S.R.M. Ibrahim, and S.A. Ross. 2013. Lupeol-3-O-decanoate, a new triterpene ester from Cadaba farinosa Forssk. growing in Saudi Arabia. Medicinal Chemistry Research 22: 5297–5302.
Alcaraz, M.J., and M.J. Jimenez. 1998. Flavonoids as anti-inflamatory agents. Fitoterapia 59: 25–38.
Attia, A.A. 2002. New quaternary alkaloid and other constituents and biological activities of Cadaba glandulosa F. Bulletin of Pharmaceutical Sciences, Assiut University 25: 115–124.
Crow, W.F., B.T. Tomer, H.J. Looker, and L.M. Gross. 1986. Fast atom bombardment and tandom mass spectrometry for structure determination of steroid and flavonoid glycosides. Analytical Biochemistry 155: 286–287.
El-Badry, M. 2011. Physicochemical characterization and dissolution properties of meloxicam-gelucire 50/13 binary systems. Scientia Pharmaceutica 79: 375–386.
El-Kumarasamy, Y., M. Byres, P.J. Cox, A. Delazar, M. Jaspars, L. Nahar, M. Shoeb, and S.D. Sarker. 2004. Isolation, structure elucidation, and biological activity of flavone 6-C-glycosides from Alliaria petiolata. Chemistry of Natural Compounds 40: 122–128.
El-Shanawany, M.A., H.M. Sayed, S.R.M. Ibrahim, M.A.A. Fayed, M.M. Radwan, and S.A. Ross. 2012. A new isoflavone from Blepharis ciliaris of an Egyptian origin. Medicinal Chemistry Research 19: 2346–2350.
Gohar, A.A. 2002. Flavonol glycosides from Cadaba glandulosa. Zeitschrift für Naturforschung 57C: 216–220.
Harborne, J.B. 1988. The flavonoids. Advances in Research since 1980. New York: Chapman & Hall.
Ibrahim, S.R., G.A. Mohamed, and N.M. Al-Musayeib. 2012. New constituents from the rhizomes of Egyptian Iris germanica L. Molecules 17: 2587–2598.
Jork, H., W. Funk, W. Fishcer, and H. Wimmer. 1990. Thin-layer chromatography reagents and detection methods: physical and chemical detection methods: Fundamentals, reagents I, vol. 1a. Weinheim: VHC.
Mabry, T.J., K.R. Markham, and M.B. Thomas. 1970. The systematic identification of flavonoids. New York: Springer.
Markham, K. 1982. Techniques of flavonoid identification. New York: Academic Press.
Perez, G.R.M. 2001. Anti-inflammatory activity of compounds isolated from plants. The Scientific World Journal 1: 713–784.
Silverstein, R.M., and F.X. Webster. 1998. Spectrometric identification of organic compounds. New York: Wiley.
Viqar Uddin, A., A. Azia-Ur-Rahman, F. Kaniz, and K. Arshad. 1990. Cadabicilone, a sesquiterpene lactone from Cadaba farinosa. Zeitschrift für Naturforschung 45b: 1100–1102.
Viqar Uddin, A., A. Aziz-Ur-Rahman, A.C. Shoib, H.M. Marie, and J. Cladry. 1985. Cadabicine, an alkaloid from Cadaba farinosa. Phytochemistry 24: 2709–2711.
Viqar Uddin, A., F. Kaniz, A. Azia-Ur-Rahman, and A. Shoib. 1987. Cadabacine and cadabacine diacetate from Crataeva nurvala and Cadaba farinosa. Journal of Natural Products 50: 1186.
Williams, C.A., and J.B. Harborne. 1994. The flavonoids. Advances in research since 1986. New York: Chapman & Hall.
Yousif, G., G.M. Iskander, and E.B. Eisa. 1984. Alkaloids of Cadaba farinosa and C. rotundifolia. Fitoterapia 55: 117–118.
Acknowledgments
The authors are grateful to the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University for the financial support and to Dr. Volker Brecht (Nuclear Magnetics Resonance, Institut fuer Pharmazeutische Wissenschaften, Albert-Ludwigs-Universität Freiburg, Germany) for HRESIMS measurements.
Conflict of interest
All the authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohamed, G.A., Ibrahim, S.R.M., Al-Musayeib, N.M. et al. New anti-inflammatory flavonoids from Cadaba glandulosa Forssk. Arch. Pharm. Res. 37, 459–466 (2014). https://doi.org/10.1007/s12272-013-0305-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0305-1