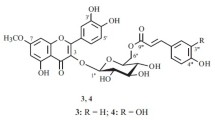
The chemical composition of two species in the family Boraginaceae, Nonea rossica Steven and Tournefortia sibirica L., in which alkaloids, hydroxycinnamates, and flavonoids, including two new acylglycosides 1 and 2 were observed, was studied. UV and NMR spectroscopy and mass spectrometry found that the new compounds were quercetin 3-O-(2′′-O-caffeoyl-6′′-O-acetyl)-β-D-glucopyranoside (noneaside, 1; from N. rossica) and kaempferol 3-O-(2′′-O-caffeoyl-6′′-O-acetyl)-β-D-glucopyranoside (tournefoside, 2; from T. sibirica). Both flavonoids possessed antiradical activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The family Boraginaceae is represented in Siberia by 28 genera including >100 species. Despite the broad distribution, the chemical compositions of most representatives have not been reported or are incomplete [1]. In continuation of research on this family [2, 3], two broadly distributed species in the region, Nonea rossica Steven [N. pulla subsp. pulla, N. pulla subsp. rossica (Steven) Soo] and Tournefortia sibirica L. [Arguzia rosmarinifolia Steven, Messerschmidia sibirica (L.) L.], were studied. The chemical composition of N. rossica is unknown while essential oil [4], flavones [5], the alkaloid tournesibirin [6], and cembrane diterpenoids [7] were observed in T. sibirica of Chinese origin. Both species were used in traditional Buryat medicine under the name gyer-shing-pa as antipyretic and antibacterial agents [8]. Herein, results of a chemical study of the aerial parts of N. rossica and T. sibirica growing in Baikal District are reported.
The EtOH extract of N. rossica was separated by column chromatography (CC) over polyamide, Al2O3, normal and reversed-phase silica gel, and Sephadex LH-20 and by preparative HPLC to afford 27 compounds including the alkaloids intermedine (3) [9], lycopsamine (4) [9], intermedine N-oxide (5) [9], and lycopsamine N-oxide (6) [9]; the flavonoids kaempferol 3-O-galactoside (7) [10], kaempferol 3-O-glucoside (8) [10], kaempferol 3-O-rutinoside (9) [10], kaempferol 3-O-neohesperidoside (10) [10], quercetin 3-O-glucoside (11) [10], quercetin 3-O-galactoside (12) [10], quercetin 3-O-rutinoside (13) [10], quercetin 3-O-neohesperidoside (14) [11], kaempferol 3-O-gentiobioside (15) [10], quercetin 3-O-gentiobioside (16) [10], quercetin 3-O-(2′′-O-acetyl)-glucoside (22) [12], quercetin 3-O-(6′′-O-acetyl)-glucoside (23) [12], and quercetin 3-O-(2′′,6′′-di-O-acetyl)-glucoside (24) [12]; hydroxycinnamates 2-O-caffeoylthreonic acid (17) [13], 3-O-caffeoylthreonic acid (18) [13], 2-O-caffeoylglyceric acid (19) [13], 3-O-caffeoylglyceric acid (20) [13], globoidnan B (21) [13], rosmarinic acid (25) [13], and salvianolic acids B (26) [13] and L (27) [14]; in addition to new compound 1.
The molecular formula C32H28O16 for 1 was determined using mass spectrometry (HR-ESI-MS, m/z 669.4083 [M + H]+, calcd for C32H29O16, 669.5174) and 13C NMR spectroscopy. The shape of the absorption spectrum of 1 was characteristic of flavonoids acylated by a caffeic acid moiety, which was observed after hydrolysis of 1 with trifluoroacetic acid (TFA, 2 M) together with quercetin and D-glucose. Daughter ions of the protonated ion in the mass spectrum appeared at m/z 507, 465, and 303, indicating loss of fragments with masses of 162 (caffeoyl), 42 (acetyl), and 162 amu (glucose), respectively.
Work up of 1 with NaOH solution (0.5%) formed quercetin-3-O-glucoside (11) [10], which indicated acyl fragments were present in the carbohydrate part of the compound. A comparative analysis of 1H NMR spectra of 1 and 11 revealed weak-field shifts of resonances in 1 for C-2′′ (δC 73.4→74.9) and C-6′′ (δC 61.4→65.3) (Table 1). The HMBC spectrum showed correlations between resonances of H-2′′ and the carbonyl C atom of caffeic acid (δH/δC 5.14/168.1) and H-6′′ and the carbonyl C atom of acetyl (δH/δC 4.29, 4.54/169.3). As a result, compound 1 was elucidated as quercetin 3-O-(2′′-O-caffeoyl-6′′-O-acetyl)-β-Dglucopyranoside, which we called noneaside.
Chromatographic separation of the EtOH extract of T. sibirica isolated 1, 4, 6, 8, 9, 11, 13, 23–25, 7-O-acetyllycopsamine (28) [15], 7-O-acetyllycopsamine N-oxide (29) [15], piperonal (30) [16], lithospermic acid (31) [13], rosmarinic acid 9-O-methyl ester (32) [17], kaempferol 3-O-(6′′-O-acetyl)-glucoside (33) [10], and a new flavonoid 2. All compounds were observed in T. sibirica for the first time.
Compound 2 (C32H28O15, HR-ESI-MS, m/z 653.3004 [M + H]+, calcd for C32H29O15, 653.5184) gave kaempferol, D-glucose, and caffeic acid after hydrolysis by TFA (2 M). Work up with NaOH solution (0.5%) gave kaempferol-3-O-glucoside (8) [10]. UV and NMR spectroscopic (Table 1) and mass spectrometric data indicated that 2 was an analog of 1 containing kaempferol as the aglycon or kaempferol 3-O-(2′′-O-caffeoyl-6′′-O-acetyl)-β-D-glucopyranoside, which was named tournefoside.

A study of the antiradical activity against DPPH radical showed the greatest activity (IC50 7.43 μM) for 1, which exceeded those of the reference compound Trolox (IC50 30.08 μM) and 2 (IC50 35.71 μM).
According to the results, N. rossica and T. sibirica contained dehydropyrrolizidine alkaloids, caffeic acid esters, rosmarinic acid and its derivatives, and quercetin and kaempferol O-glycosides. These compound groups were encountered in other Boraginaceae species [18, 19]. However, flavonoid acylglycosides were found for the first time in the family.
Experimental
The aerial part of the flowering plants was collected in the Republic of Buryatia and air-dried in the shade (humidity < 5%): N. rossica, near Mukhorshibir (Mukhorshibirsky District, Jun. 20, 2020; 51°01′58.9′′ N, 107°49′15.1′′ E, 650 m above sea level); T. sibirica, near Gusinozersk (Selenginsky District, Jul. 15, 2019; 51°12′39.2′′ N, 106°31′47.3′′ E, 420 m above sea level). The species were determined by Dr. N. K. Chirikova. Specimens of raw material are preserved in the herbarium of the IGEB, SB, RAS (No. BU/BOR-0620/51-062, BU/BOR-0719/92-114). Column chromatography (CC) used polyamide, Al2O3, normal (SiO2) and reversed-phase silica gel (RP-SiO2), and Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO, USA). Spectrophotometric studies used an SF-2000 spectrophotometer (OKB Spectr, St. Petersburg, Russia). Mass spectra were recorded in an LCMS-8050 TQ-mass spectrometer (Shimadzu, Columbia, MD, USA) [20]. NMR spectra were taken on a VXR 500S spectrometer (Varian, Palo Alto, CA, USA). Preparative HPLC used an LC-20 Prominence liquid chromatograph (Shimadzu) equipped with a Shim-pak PREP-ODS column (20 × 250 mm, d 15 μm) and an SPD-M30A diode array detector (Shimadzu) using flow rate 1.0 mL/min and column temperature 20°C. Antiradical activity of the compounds against 2,2-diphenyl-1-picrylhydrazyl radicals (DPPH, Sigma-Aldrich) was determined by a microplate spectrophotometric method [21].
Extraction and Isolation of 1 and 3–27 from N. rossica. Ground raw material (3.5 kg) was extracted with EtOH [70%, 1:10, 50°C, 3 ×, ultrasound (US) bath]. The EtOH extract was concentrated to dryness. The dry solid was exhaustively extracted with hexane and water-saturated BuOH. The BuOH fraction (630 g) was separated by CC (1:10) over polyamide with elution by H2O (fraction A), EtOH (60%, faction B), and NH3 (0.5% in 95% EtOH, fraction C). Fraction A (320 g) was separated by CC over Al2O3 (eluent CHCl3–EtOH–NH3, 99:0:1→59:40:1) and RP-SiO2 (1 × 30 cm, eluent H2O–MeCN, 90:10→50:50) and by preparative HPLC (eluent A, 0.2% HCOOH in H2O; eluent B, 0.2% HCOOH in MeCN; isocratic mode, 15% B) to isolate intermedine (10 mg, 3) [9], lycopsamine (5 mg, 4) [9], intermedine N-oxide (35 mg, 5) [9], and lycopsamine N-oxide (30 mg, 6) [9].
Fraction B (80 g) was chromatographed over SiO2 (3 × 40 cm, eluent hexane–EtOAc, 100:0→70:30, EtOAc–Me2CO, 100:0→80:20), Sephadex LH-20 (2 × 80 cm, eluent EtOH–H2O, 90:10→50:50), and RP-SiO2 (1 × 30 cm, eluent H2O–MeCN, 95:5→20:80) and by preparative HPLC (eluent A, H2O; eluent B, MeCN; gradient mode, %B: 0–60 min, 5–50%, 60–80 min, 50–60%) to afford kaempferol 3-O-galactoside (trifolin, 10 mg, 7) [10], kaempferol 3-O-glucoside (astragalin, 50 mg, 8) [10], kaempferol 3-O-rutinoside (nicotiflorin, 60 mg, 9) [10], kaempferol 3-O-neohesperidoside (30 mg, 10) [10], quercetin 3-O-glucoside (isoquercitrin, 25 mg, 11) [10], quercetin 3-O-galactoside (hyperoside, 20 mg, 12) [10], quercetin 3-O-rutinoside (rutin, 15 mg, 13) [10], quercetin 3-O-neoherperidoside (calendoflavobioside, 8 mg, 14) [11], kaempferol 3-O-gentiobioside (5 mg, 15) [10], and quercetin 3-O-gentiobioside (9 mg, 16) [10]. Fraction C (200 g) was separated over Sephadex LH-20 (2 × 80 cm, eluent EtOH–H2O, 50:50→0:100) and RP-SiO2 (1 × 30 cm, eluent H2O–MeCN, 95:5→50:50) and by preparative HPLC (eluent A, 0.5% AcOH in H2O; eluent B, 0.5% AcOH in MeCN; gradient mode, %B: 0–20 min, 5–10%, 20–90 min, 10–40%, 90–150 min, 40–50%) to isolate 12 compounds including 1 (30 mg), 2-O-caffeoylthreonic acid (20 mg, 17) [13], 3-O-caffeoylthreonic acid (25 mg, 18) [13], 2-O-caffeoylglyceric acid (55 mg, 19) [13], 3-O-caffeoylglyceric acid (70 mg, 20) [13], globoidnan B (25 mg, 21) [13], quercetin 3-O-(2′′-O-acetyl)-glucoside (30 mg, 22) [12], quercetin 3-O-(6′′-O-acetyl)-glucoside (40 mg, 23) [12], quercetin 3-O-(2′′,6′′-di-O-acetyl)-glucoside (65 mg, 24) [12], rosmarinic acid (1.4 g, 25) [13], salvianolic acid B (30 mg, 26) [13], and salvianolic acid L (45 mg, 27) [14].
Noneaside (1), C32H28O16. UV (MeOH, λmax, nm): 255, 267 sh., 300 sh., 341; +AlCl3 271, 296 sh., 348 sh., 422; +AlCl3 + HCl 265, 296 sh., 340, 400; +NaOAc 260, 296 sh., 388; +NaOAc + H3BO3 260, 360. HR-ESI-MS, m/z 669.4083 [M + H]+ (calcd for C32H29O16, 669.5174). ESI-MS, m/z (%): 669 [M + H]+ (100). ESI-MS2 [669]: 507 [(M + H) – 162]+ (73), 465 [(M + H) – 162 – 42]+ (14), 303 [(M + H) – 2 × 162 – 42]+ (100). 1H NMR (500 MHz, DMSO-d6, 298 K) and 13C NMR (125 MHz, DMSO-d6, 298 K), see Table 1.
Extraction and Isolation of 1, 2, 4, 6, 8, 9, 11, 13, 23–25, and 28–33 from T. sibirica. The above scheme was used to isolate from T. sibirica herb (1 kg) 1 (10 mg), 2 (35), 4 (15), 6 (40), 8 (10), 9 (25), 11 (20), 13 (10), 23 (140), 24 (35), 25 (220), 7-O-acetyllycopsamine (20 mg, 28) [15], 7-O-acetyllycopsamine N-oxide (5 mg, 29) [15], piperonal (heliotropin, 15 mg, 30) [16], lithospermic acid (40 mg, 31) [13], rosmarinic acid 9-O-methyl ester (35 mg, 32) [17], and kaempferol 3-O-(6′′-Oacetyl)-glucoside (25 mg, 33) [10].
Tournefoside (2). C32H28O15. UV (MeOH, λmax, nm): 270, 328; +AlCl3 272, 298, 340, 387 sh.; +AlCl3 + HCl 275, 298, 333, 388 sh.; +NaOAc 269, 332; +NaOAc + H3BO3 261, 350. HR-ESI-MS, m/z 653.3004 [M + H]+ (calcd for C32H29O15, 653.5184). ESI-MS, m/z (%): 653 [M + H]+ (100). ESI-MS2 [653]: 491 [(M + H) – 162]+ (70), 449 [(M + H) – 162 – 42]+ (10), 287 [(M + H) – 2 × 162 – 42]+ (100).1H NMR (500 MHz, DMSO-d6, 298 K) and 13Ñ NMR (125 MHz, DMSO-d6, 298 K), see Table 1.
Hydrolysis. Acid hydrolysis was performed in TFA (2 M) [22] followed by analysis of the monosaccharide composition (HPLC after derivatization with 3-methyl-1-phenyl-2-pyrazolin-5-one) [23], determination of the D/L-series type (HPLC after reductive amination with L-tryptophan) [24], and determination of the aglycons by HPLC-MS [25]. The hydrolysis products contained quercetin (1), kaempferol (2), D-glucose (1, 2), and caffeic acid (1, 2). Alkaline hydrolysis used NaOH solution (0.5%) as before [22]. The hydrolysis products of 1 and 2 were identified using UV, NMR, and mass spectrometry as quercetin 3-O-glucoside (11) [10] and kaempferol 3-O-glucoside (8) [10], respectively.
References
R. A. Sharma, B. Singh, D. Singh, and P. Chandrawat, J. Med. Plants Res., 3, 1153 (2009).
D. N. Olennikov, Z. V. Daironas, and I. N. Zilfikarov, Chem. Nat. Compd., 53, 953 (2017).
D. N. Olennikov, D. S. Kruglov, Z. V. Daironas, and I. N. Zilfikarov, Chem. Nat. Compd., 56, 713 (2020).
K. Morteza-Semnani, M. Saeedi, and M. Akbarzadeh, J. Essent. Oil Res., 20, 207 (2008).
S. Diao, M. Jin, C. S. Jin, C.-X. Wei, J. Sun, W. Zhou, and G. Li, Nat. Prod. Res., 33, 3021 (2019).
H. Hu, A. Bao, S. Pan, J. Hao, and Y. Xin, Nat. Prod. Res., 36 (8), 2028 (2022).
S. Diao, M. Jin, J. Sun, C. Jin, R. Wang, W. Zhou, and G. Li, Tetrahedron Lett., 61 (4), 151413 (2020).
S. M. Batorova, G. P. Yakovlev, and T. A. Aseeva, Guide to Medicinal Plants of Traditional Tibetan Medicine [in Russian], Nauka, Novosibirsk, 2003, 291 pp.
S. M. Colegate, D. R. Gardner, J. M. Betz, and K. E. Panter, Phytochem. Anal., 25, 429 (2014).
S. S. Azimova and V. I. Vinogradova, Natural Compounds. Flavonoids: Plant Sources, Structure and Properties, Springer, New York, 2013.
N. F. Komissarenko, V. T. Chernobai, and A. I. Derkach, Chem. Nat. Compd., 24, 675 (1988).
Y. B. Wang, J. X. Pu, H. Y. Ren, J. F. Zhao, S. X. Mei, Z. Y. Li, H. B. Zhang, and L. Li, Chin, Chem. Lett., 14, 1268 (2003).
J. Krzyzanowska-Kowalczyk, L. Pecio, J. Moldoch, A. Ludwiczuk, and M. Kowalczyk, Molecules, 23, 2277 (2018).
Y. Lu and L.Y. Foo, Tetrahedron Lett., 42, 8223 (2001).
J. Roitman, Aust. J. Chem., 36, 769 (1983).
B. Meriga, B. Parim, V. R. Chunduri, R. R. Naik, H. Nemani, P. Suresh, S. Ganapathy, and V. V. S. Uddandrao, Nutr. Metab., 14, 72 (2017).
A. Abedini, V. Roumy, S. Mahieux, M. Biabiany, A. Standaert-Vitse, C. Riviere, S. Sahpaz, F. Bailleul, C. Neut, and T. Hennebelle, Evidence-Based Complementary Altern. Med., 2013, 604536 (2013).
E. Wollenweber, R. Wehde, M. Dorr, and J. F. Stevens, Z. Naturforsch., C: J. Biosci., 57, 445 (2002).
M. Petersen and M. S. J. Simmonds, Phytochemistry, 62, 121 (2003).
D. N. Olennikov, V. V. Chemposov, and N. K. Chirikova, Plants, 10, 2525 (2021).
D. N. Olennikov, C. S. Kirillina, and N. K. Chirikova, Antioxidants, 10, 1300 (2021).
D. N. Olennikov and N. K. Chirikova, Chem. Nat. Compd., 55, 1032 (2019).
D. N. Olennikov, N. K. Chirikova, N. I. Kashchenko, T. G. Gornostai, I. Y. Selyutina, and I. N. Zilfikarov, Int. J. Mol. Sci., 18, 2579 (2017).
M. Akabane, A. Yamamoto, S. Aizawa, A. Taga, and S. Kodama, Anal. Sci., 30, 739 (2014).
D. N. Olennikov, N. K. Chirikova, A. G. Vasilieva, and I. A. Fedorov, Antioxidants, 9, 526 (2020).
Acknowledgment
The research was supported by the Ministry of Education and Science of the Russian Federation (Project No. 121030100227-7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2022, pp. 859–862.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olennikov, D.N., Kartashova, M.E., Velichko, V.V. et al. New Flavonoids from Nonea rossica and Tournefortia sibirica. Chem Nat Compd 58, 1021–1025 (2022). https://doi.org/10.1007/s10600-022-03858-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03858-9