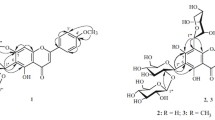
The aerial part of Melandrium divaricatum Fenzl (Caryophyllaceae) afforded 11 glycosylflavones including two new compounds that were characterized using UV, IR, and NMR spectroscopy and mass spectrometry as apigenin-6-C-(2″ -O-α-L-rhamnopyranosyl)-β-D-glucopyranoside-7-O-(6″″ -O-feruloyl)-β-Dglucopyranoside (divarioside A, 1) and apigenin-6-C-(2″-O-D-glucopyranosyl)- β-D-glucopyranoside-7-O-(6″″-O-feruloyl)- β-D-glucopyranoside (divarioside B, 2).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Melandrium species (Caryophyllaceae) are capable of accumulating apigenin and luteolin derivatives including their C-, O-, and C,O-glycosides, which have been observed in M. album (Mill.) Garcke (Silene latifolia Poir.) [1]; M. dioicum (L.) Coss. & Germ. [S. dioica (L.) Clariv.] [2]; and M. vespertinum Fr. [S. latifolia subsp. alba (Mill.) Greuter et Burdet] [3]. The biennial species M. divaricatum Fenzl (M. balansae Boiss., M. pratense Roehl.) occurs broadly in thinned forests of Turkey and the western Caucasus [4]. However, the chemical composition of this species is unreported. We studied the flavonoid composition of M. divaricatum growing in Georgia and characterized two new compounds.
Chromatographic separation of the EtOAc and BuOH fractions from M. divaricatum herb afforded 11 glycosylflavones that were identified using UV, IR, and NMR spectroscopy and mass spectrometry as saponarin-2″-O-glucoside (3) [5], saponarin-2″-O-rhamnoside (4) [6], saponarin (5) [7], isovitexin-2″-O-glucoside (6) [8], isovitexin-2″-O-rhamnoside (7) [9], isovitexin (8) [7], saponarin 6″′-O-ferulate (9) [7], cosmosiin (10) [10], cosmosiin 6″-O-ferulate (11) [10], and two new compounds 1 and 2.

Compound 1 was a pale-yellow powder with mp 192−194°C. Its solutions had negative optical rotation (\( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) –92.4°). Its UV spectrum showed bands at 271 and 333 nm that were characteristic of flavones [11]. Its IR spectrum exhibited peaks at 1646 and 1726 cm–1 that were indicative of esterified carboxyl [12]. Acid hydrolysis of 1 produced isovitexin (8), D-glucose, L-rhamnose, and ferulic acid. Alkaline hydrolysis of 1 produced saponarin-2″-O-rhamnoside (4) [6] and ferulic acid.
The HR-ESI-MS spectrum of 1 contained a peak for deprotonated [M – H]– with m/z 915.826 (calcd for C43H47O22, 915.811), which indicated the molecular formula was C43H48O22. ESI-MS2 spectra of the fragment with m/z 915 [M – H]– gave daughter ions resulting from loss of O-bound rhamnose (C6H10O4), glucose (C6H10O5), and ferulic acid (C10H8O3) with m/z 769 [(M – H) – C6H10O4]–; 739 [(M – H) – C10H8O3]–; 593 [(M – H) – C6H10O4 – C10H8O3]–; 577 [(M – H) – C6H10O5 – C10H8O3]–; and 431 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3]– [13]. Further fragmentation of the ion with m/z 431 was characteristic of flavone-C-glycosides and formed ions with m/z 341 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3 – C3H6O3]–, 313 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3 – C3H6O3 – CO]–, 311 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3 –C4H8O4]–, and 283 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3 – C4H8O4 – CO]– [14].
A comparison of PMR and 13C NMR spectra of 1 and saponarin-2″-O-rhamnoside (4) showed that they were similar except for additional resonances in spectra of 1 for an acyl substituent, i.e., ferulic acid (Tables 1 and 2). Weak-field shifts of the resonances for H-6″″ of 7-O-glucopyranose in 1 relative to those in 4 (δH 3.71, 3.94→4.10, 4.37) and a shift of the C-6″″ resonance (δC 60.0→64.6) indicated that the substituent was on this atom. This was confirmed by HMBC spectra in which correlations were observed between resonances for H-6″″ of 7-O-glucopyranose (δH 4.10, 4.37) and ferulic acid carbonyl C-9″″′ (δC 166.9) (Table 3).
Enzymatic hydrolysis of 1 by β-glucosidase produced isovitexin-2″-O-rhamnoside (7) [9] and 6-O-feruloylglucose [15]. Treatment of 1 with α-rhamnosidase gave a hydrolysate in which saponarin-6″′-O-ferulate (9) [7] was identified. This was also indicative of a feruloyl fragment on C-6″″ of 7-O-glucopyranose.
Thus, the studies determined the structure of 1 as apigenin-6-C-(2″-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside-7-O-(6″″-O-feruloyl)-β-D-glucopyranoside (saponarin-2″-O-rhamnoside-6″″-O-ferulate), which we called divarioside A.
Compound 2 was a pale-yellow powder with mp 184−186°C and optical rotation \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) –33.0°. Its UV spectrum contained bands at 270 and 333 nm. Its IR spectrum showed peaks at 1650 and 1718 cm–1 (esterified carboxyl). The acid-hydrolysis products of 2 were isovitexin (8), D-glucose, and ferulic acid. The last was cleaved during alkaline hydrolysis to give saponarin-2″-O-glucoside (3) [5]. HR-ESI-MS gave a molecular formula of C43H48O23 (m/z 931.789 [M – H]–; calcd for C43H47O23, 931.810). ESI-MS spectra of daughter fragments taken in negative-ion mode exhibited peaks due to cleavage of O-bound glucose and ferulic acid with m/z 769, 755, 593, and 431 and peaks with m/z 341, 313, 311, and 283 that were typical of apigenin C-glycosides. PMR and 13C NMR spectra of 2 differed from those of 1 by resonances due to 2″-O-β-D-glucopyranose and were similar to spectra of saponarin-2″-O-glucoside (3) (Tables 1 and 2).
HMBC spectroscopy (Table 3) and enzymatic hydrolysis by β-glucosidase indicated that the feruloyl moiety was positioned on C-6″″ of the 7-O-glucopyranose. The results indicated that 2 was apigenin-6-C-(2″-O-β-D-glucopyranosyl)-β-D-glucopyranoside-7-O-(6″″-O-feruloyl)-β-D-glucopyranoside (saponarin-2″-O-glucoside-6″″-O-ferulate), which we called divarioside B.
Previously, isovitexin derivatives acylated by ferulic acid were isolated from Cerastium arvense L. (Caryophyllaceae), isovitexin-2″-O-ferulate [16]; M. vespertinum Fr. (Caryophyllaceae), saponarin 6″′-O-ferulate (9) [17]; Gentiana punctata L. (Gentianaceae), isosaponarin-2″-O-ferulate [18]; and Cucumis sativus L. (Cucurbitaceae), isovitexin-2″-O-glucoside-6″′-Oferulate and isovitexin-4′,2″-di-O-glucoside-6″′-O-ferulate [19]. Isovitexin O-glycosides 3−9 were found in other Melandrium species, including M. album and M. vespertinum [3], and were probably a chemical signature of the genus.
Experimental
General comments have been published [20, 21]. The aerial part of M. divaricatum was collected during flowering in the vicinity of Lebarde resort (Samegrelo-Verkhnyaya Svaneti, Georgia; May 10, 2015; 42°44′36.14″ N, 42°29′15.99″ E; 1767 m above sea level). A specimen of the raw material is preserved in the Herbarium of the IGEB, SB, RAS (No. Ca/ae-15/05-07/2217). The species was determined by Dr. T. A. Aseeva (IGEB, SB, RAS). Raw material was dried in a convection oven (45°C) to <5% moisture.
Extraction and Fractionation. Milled raw material (720 g) was extracted (3.) with EtOH (70%) at 70°C for 2 h in an ultrasonic bath (100 W, 35 kHz). The EtOH extracts were combined and evaporated to dryness under vacuum. The dry residue was suspended in H2O (2 L) and extracted with hexane, EtOAc, and n-BuOH. Concentration of the EtOAc fraction produced a precipitate that was purified by column chromatography (CC) over Sephadex LH-20 (2 . 50 cm, eluent EtOH−H2O, 80:20→30:70) to give 1 (620 mg). The EtOAc fraction after removal of 1 (46 g) was chromatographed over polyamide (CC, 5 . 40 cm, eluent H2O−EtOH, 100:0→90:10). Fractions eluted by 30−50% EtOH were separated again by prep. HPLC [LiChrospher RP-18 column (250 . 10 mm, ∅10 μm; Supelco, Bellefonte, PA, USA); mobile phase H2O (A) and MeCN (B); flow rate (ν) 1 mL/min; column temperature 30°C; gradient mode (%B): 0−60 min, 10−40%, 60−80 min, 40−60%] to afford 1 (62 mg; tR prep. HPLC, 30−34 min) and cosmosiin (apigenin-7-O-β-D-glucopyranoside, 12 mg, 10; tR prep. HPLC, 36−38 min) [10]. Fractions eluted by 80−90% EtOH were separated by prep. HPLC [gradient mode (%B): 0−60 min, 50−80%] to give cosmosiin 6″-O-ferulate (18 mg) [apigenin-7-O-(6″-O-feruloyl)-β-D-glucopyranoside, 11; tR prep. HPLC, 40−42 min] [10]. The BuOH fraction (108 g) was separated using CC over polyamide (6 . 50 cm, eluent H2O−EtOH, 100:0→90:10). Fractions eluted by 10−20% EtOH were separated by prep. HPLC [gradient mode (%B): 0−60 min, 0−30%, 60−80 min, 30−40%] to produce saponarin-2″-O-glucoside [apigenin-6-C-(2″-O-β-D-glucopyranosyl)-β-D-glucopyranoside-7-O-β-D-glucopyranoside, 27 mg, 3; tR prep. HPLC, 20−22 min] [5]; saponarin-2″-O-rhamnoside [apigenin-6-C-(2″-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside-7-O-β-D-glucopyranoside, 16 mg, 4; tR prep. HPLC, 23−24 min] [6]; and saponarin (apigenin-6-C-β-D-glucopyranoside-7-O-β-D-glucopyranoside, 22 mg, 5; tR prep. HPLC, 24−25 min) [7]. The fraction eluted by 30% EtOH was recrystallized and rechromatographed over Sephadex LH-20 (CC, 2 . 70 cm, eluent EtOH−H2O, 80:20→30:70) to give isovitexin-2″-O-glucoside (37 mg) [apigenin-6-C-(2″-O-β-D-glucopyranosyl)-β-Dglucopyranoside, 6] [8]. Fractions eluted by 50−70% EtOH were separated using prep. HPLC [gradient mode (%B): 0−60 min, 10−40%, 60−100 min, 40−80%] and CC over Sephadex LH-20 (1 . 50 cm, eluent EtOH−H2O, 90:10→30:70) to isolate 2 (31 mg; tR prep. HPLC, 30−32 min), isovitexin-2″-O-rhamnoside [apigenin-6-C-(2″-O-α-L-rhamnopyranosyl)-β-Dglucopyranoside, 11 mg, 7; tR prep. HPLC, 28−29 min] [9], isovitexin (apigenin-6-C-β-D-glucopyranoside, 14 mg, 8; tR prep. HPLC, 29−30 min) [7]; and saponarin 6″′-O-ferulate [apigenin-6-C-β-D-glucopyranoside-7-O-(6″′-O-feruloyl)-Dglucopyranoside, 18 mg, 9; tR prep. HPLC, 44−48 min] [7].
Divarioside A (1). C43H48O22, mp 192–194°C, \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) –92.4° (c 0.11, MeOH). UV spectrum (MeOH, λmax, nm): 271, 333. IR spectrum (ν, cm–1): 1646, 1726. HR-ESI-MS, m/z 915.826 [M – H]– (calcd 915.811 for C43H47O22). ESI-MS, m/z: 915 [M – H]–; MS2 [915]: 769 [(M – H) – C6H10O4]–, 739 [(M – H) – C10H8O3]–, 593 [(M – H) – C6H10O4 – C10H8O3]–, 577 [(M – H) – C6H10O5 – C10H8O3]–, 431 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3]–; MS3 [431]: 341 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3 – C3H6O3]–, 313 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3 – C3H6O3 – CO]–, 311 [(M – H) –C6H10O4 – C6H10O5 – C10H8O3 – C4H8O4]–, 283 [(M – H) – C6H10O4 – C6H10O5 – C10H8O3 – C4H8O4 – CO]–. Table 1 lists the PMR spectrum (500 MHz, MeOH-d4, δ, ppm). Table 2 lists the 13C NMR spectrum (125 MHz, MeOH-d4, δ, ppm).
Divarioside B (2). C43H48O23, mp 184–186°C, \( {\left[\upalpha \right]}_{\mathrm{D}}^{20} \) –33.0° (c 0.10, MeOH). UV spectrum (MeOH, λmax, nm): 270, 333. IR spectrum (ν, cm–1): 1650, 1718. HR-ESI-MS, m/z 931.789 [M – H]– (calcd 931.810 for C43H47O23). ESI-MS, m/z: 931 [M – H]–; MS2 [931]: 769 [(M – H) – C6H10O5]–, 755 [(M – H) – C10H8O3]–, 593 [(M – H) – C6H10O5 – C10H8O3]–, 431 [(M – H) – 2 . C6H10O5 – C10H8O3]–; MS3 [431]: 341 [(M – H) – 2 . C6H10O5 – C10H8O3 – C3H6O3]–, 313 [(M – H) –2 . C6H10O5 – C10H8O3 – C3H6O3 – CO]–, 311 [(M – H) – 2 . C6H10O5 – C10H8O3 – C4H8O4]–, 283 [(M – H) – 2 . C6H10O5 – C10H8O3 – C4H8O4 – CO]–. Table 1 lists the PMR spectrum (500 MHz, MeOH-d4, δ, ppm). Table 2 lists the 13C NMR spectrum (125 MHz, MeOH-d4, δ, ppm).
Acid Hydrolysis of 1 and 2. A weighed portion (7 mg) was heated with TFA (2 M, 4 mL) at 120°C for 2 h. The hydrolysate was evaporated with MeOH to dryness under vacuum. The dry residue was dissolved in EtOH (50%, 2 mL) and chromatographed over polyamide (3 g) with elution sequentially by H2O (50 mL, eluate I) and EtOH (70%, 100 mL, eluate II). Monosaccharides were isolated by derivatizing a portion of eluate I with 3-methyl-1-phenyl-2-pyrazolin-5-one as before [22] and analyzing by analytical HPLC (conditions 1). Monosaccharides in eluate I were assigned as D- and L-isomers using reductive amination with L-tryptophan [23] followed by analytical HPLC (conditions 2). Eluate II was analyzed using 13C NMR spectroscopy and mass spectrometry. The hydrolysate of 1 contained isovitexin (8), D-glucose, L-rhamnose, and ferulic acid; of 2, 8, D-glucose, and ferulic acid.
Alkaline Hydrolysis of 1 and 2. A weighed portion (5 mg) was dissolved in MeOH (2 mL); treated with NaOH (2 M, 1 mL); incubated at 30°C for 40 min; neutralized with HCl (2 M); placed on an RP-SiO2 cartridge (5 g) that was preconditioned with H2O; and eluted with H2O until the eluate was neutral, MeCN (40%, 50 mL eluate I), and MeCN (90%, eluate II). The eluates were analyzed by HPLC (conditions 3) and mass spectrometry. The hydrolysate of 1 contained saponarin-2″-Orhamnoside (4, eluate I) [6] and ferulic acid (eluate II); of 2, saponarin-2″-O-glucoside (3, eluate I) [5] and ferulic acid (eluate II).
Hydrolysis of 1 and 2 byβ-Glucosidase. A weighed portion (5 mg) was dissolved in DMSO (100 μL), adjusted to 2 mL using MeOH (30%), and treated with β-glucosidase [3.2.1.21, 30 U/mg, Sigma-Aldrich; 2 U in 500 μL of phosphate buffer (100 mM, pH 5.0)]. The reaction mixture was incubated at 37°C for 10 h, heated to 95°C (15 min), and centrifuged (6,000 rpm, 15 min). The supernatant was chromatographed over polyamide (5 g) with elution by H2O (50 mL), EtOH (30%, 100 mL, eluate I), and EtOH (70%, 100 mL, eluate II). The eluates were analyzed by HPLC (conditions 3), 13C NMR spectroscopy, and mass spectrometry. The hydrolysate of 1 contained 6-O-feruloylglucose (eluate I), which was identified by comparison with an authentic sample (Synthose Inc., Concord, Ontario, Canada) and the literature [15], and isovitexin-2″-Orhamnoside (7, eluate II) [9]; of 2, 6-O-feruloylglucose (eluate I) and isovitexin-2″-O-glucoside (6, eluate II) [8].
Hydrolysis of 1 byα-Rhamnosidase. A weighed portion of 1 (5 mg) was dissolved in DMSO (100 μL), adjusted to 2 mL using MeOH (20%), treated with α-rhamnosidase [naringinase from Thermomicrobia sp., 3.2.1.40, 5 U/mg, Prokazyme, Vinlandsleid, Reykjavik, Iceland; 1 U in 300 μL of phosphate buffer (100 mM, pH 7.5)], incubated at 65°C for 2 h, heated to 95°C (15 min), and centrifuged (6,000 rpm, 15 min). The supernatant was chromatographed over polyamide (3 g) with elution by H2O (50 mL) and EtOH (60%, 100 mL). The EtOH eluate was analyzed by HPLC (conditions 3), 13C NMR spectroscopy, and mass spectrometry to identify saponarin 6″′-O-ferulate (9) [7].
Analytical HPLC. Conditions 1: Milichrom A-02 chromatograph (EcoNova, Novosibirsk, Russia) equipped with a ProntoSIL-120-5-C18 AQ column (2 . 75 mm, ∅ 5 μm; Metrohm AG, Herisau, Switzerland); mobile phase CH3COONH4 (100 mM, pH 4.5) (A) and MeCN (B); gradient mode (%B): 0−20 min, 20−26%; ν 150 μL/min; column temperature 35°C; UV detector at 250 nm. Retention times of monosaccharide derivatives with 3-methyl-1-phenyl-2-pyrazolin-5-one (tR, min): rhamnose 9.02; glucose 12.52, galactose 13.54, fucose 16.48. Conditions 2: Milichrom A-02 chromatograph; mobile phase NaH2PO4 (10 mM) and Na2B4O7 (50 mM), 1:1 (pH 9.6); isocratic mode; ν 200 μL/min; column temperature 35°C; UV detector at 220 nm. Retention times of monosaccharide derivatives with L-tryptophan (tR-, min): D-glucose 8.32, L-glucose 8.67, D-rhamnose 29.64, L-rhamnose 30.74. Conditions 3: LC-20 Prominence chromatograph (Shimadzu, Columbia, MD, USA) equipped with a GLC Mastro C18 column (2.1 . 150 mm, ∅ 3 μm; Shimadzu, Kyoto, Japan); mobile phase H2O (A) and MeCN (B); gradient mode (%B): 0−10 min, 10−20%, 10−30 min, 20–100%; ν 200 μL/min; column temperature 30°C; UV detector at 330 nm. Retention times of flavonoids (tR, min): 3 10.48, 4 12.04, 5 12.39, 6 13.14, 7 14.75, 2 14.86, 8 15.02, 1 15.54, 9 15.79, 10 16.02, 11 19.61, 6-O-feruloylglucose 21.34, ferulic acid 23.18, and apigenin 25.84.
References
N. Ya. Zykova and G. P. Pivenko, Chem. Nat. Compd., 11, 260 (1975).
O. Mastenbroek, J. J. Knorr, R. Kamps-Heinsbroek, J. W. Maas, J. M. Steyns, and J. Van Brederode, Z. Naturforsch., C: J. Biosci., 38c, 894 (1983).
O. Mastenbroek, H. C. Prentice, R. Kamps-Heinsbroek, J. Van Brederode, G. J. Niemann, and G. Van Nigtevecht, Plant Syst. Evol., 141, 257 (1983).
Flora of the USSR [in Russian], Vol. 6, AN SSSR, Moscow, Leningrad, 1936, 956 pp.
J. Van Brederode and G. Van Nigtevecht, Biochem. Genet., 11, 65 (1974).
J. Van Brederode and G. Van Nigtevecht, Phytochemistry, 13, 2763 (1974).
M. Ohkawa, J. Kinjo, Y. Hagiwara, H. Hagiwara, H. Ueyama, K. Nakamura, R. Ishikawa, M. Ono, and T. Nohara, Chem. Pharm. Bull., 46, 1887 (1998).
D. Brauch, A. Porzel, E. Schumann, K. Pillen, and H.-P. Mock, Phytochemistry, 148, 11 (2018).
L. M. de Magalhaes Camargo, J.-P. Ferezou, L. W. Tinoco, C. R. Kaiser, and S. S. Costa, Phytochem. Lett., 5, 427 (2012).
C. C. Chang, S. L. Ho, and S. S. Lee, Bioorg. Med. Chem., 23, 3388 (2015).
K. R. Markham, Techniques of Flavonoid Identification, Academic Press, London, New York, 1982, 113 pp.
V. P. Georgievskii, A. I. Rybachenko, and A. L. Kazakov, Physico-Chemical and Analytical Characteristics of Flavonoid Compounds [in Russian], Rostovskii Univ., Rostov, 1988, 143 pp.
K. Gluchoff-Fiasson, M. Jay, and M. R. Viricel, Phytochemistry, 28, 2471 (1989).
D. N. Olennikov, N. K. Chirikova, N. I. Kashchenko, V. M. Nikolaev, S.-W. Kim, and C. Vennos, Front. Pharmacol., 9, 756 (2018).
M. Bokern, S. Heuer, V. Wray, L. Witte, T. Macek, T. Vanek, and D. Strack, Phytochemistry, 30, 3261 (1991).
M.-A. Dubous, A. Zoll, and J. Chopin, Phytochemistry, 24, 1077 (1985).
G. J. Niemann, Acta Bot. Neerl., 30, 475 (1981).
M. D. Luong and A. Jacot-Guillarmod, Helv. Chim. Acta, 60, 2099 (1977).
M. M. Abou-Zaid, D. A. Lombardo, G. C. Kite, R. J. Grayer, and N. C. Veitch, Phytochemistry, 58, 167 (2001).
D. N. Olennikov, Chem. Nat. Compd., 55, 127 (2019).
D. N. Olennikov and N. K. Chirikova, Chem. Nat. Compd., 55, 552 (2019).
D. N. Olennikov, N. K. Chirikova, N. I. Kashchenko, T. G. Gornostai, I. Y. Selyutina, and I. N. Zilfikarov, Int. J. Mol. Sci., 18, 2579 (2017).
M. Akabane, A. Yamamoto, S. Aizawa, A. Taga, and S. Kodama, Anal. Sci., 30, 739 (2014).
Acknowledgment
The work was sponsored by the Ministry of Science and Higher Education of the Russian Federation (Project No. AAAA-A17-117011810037-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November−December, 2019, pp. 890−895.
Rights and permissions
About this article
Cite this article
Olennikov, D.N., Chirikova, N.K. New C,O-Glycosylflavones from Melandrium divaricatum. Chem Nat Compd 55, 1032–1038 (2019). https://doi.org/10.1007/s10600-019-02887-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02887-1