Chemical investigation of the dichloromethane extracts of the flowers of Dendranthema grandiflora cv. Yoko Ono afforded a new sesquiterpene lactone, grandiflorolide (1), 1,2-dilinoleoyl-3-linolenoylglycerol (2), a mixture of pseudotaraxasterol (3a), taraxasterol (3b), β-amyrin (3c), α-amyrin (3d), and lupeol (3e) in about 2:1:1:0.5:0.5 ratio, and another mixture of β-sitosterol (4a) and stigmasterol (4b) in about 2:1 ratio. The structure of 1 was elucidated by extensive 1D and 2D NMR spectroscopy, while those of 2–4b were identified by comparison of their NMR data with literature data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dendranthema grandiflora Tzvelev (syn. Chrysanthemum morifolium Ramat.) consists of about 7000 cultivars [1] and is one of the most popular commercial flowers in the world. Dendranthema grandiflora cv. Yoko Ono originated from a cross of D. grandiflora (K.O.96.925.1) as the female or seed parent with D. grandiflora (K.O.96.768.1) as the male or pollen parent. It is a herbaceous pompon-type cut Chrysanthemum [2].
D. grandiflora flowers have previously yielded flavonoids [3,4,5,6,7,8,9,10,11,12], anthocyanins, and carotenoids [13,14,15,16,17]. A recent study reported that 21 compounds were isolated and identified as octacosyl alcohol, β-sitosterol, lupeol, α-amyrin, daucosterol, ineupatorolide B, syringin, chlorogenic acid, petasiphenol, physcion, acacetin, eupatilin, quercetin, diosmetin, luteolin, apigenin, apigenin-7-O-β-D-glucopyranoside, quercetin-3-O-β-D-glucopyranoside, luteolin-7-O-β-D-glucopyranoside, apigenin-7-O-β-D-neospheroside, and acacetin-7-O-β-D-glucoside [18].
We earlier reported the isolation of fatty acid esters of maniladiol, heliantriol C, faradiol, and arnidiol from C. morifolium [19]. We report herein the isolation of a new sesquiterpene lactone, grandiflorolide (1), together with the known compounds 1,2-dilinoleoyl-3-linolenoylglycerol (2), a mixture of pseudotaraxasterol (3a), taraxasterol (3b), β-amyrin (3c), α-amyrin (3d), and lupeol (3e), and a mixture of β-sitosterol (4a) and stigmasterol (4b). The structure of 1 was elucidated by extensive 1D and 2D NMR spectroscopy, while those of 2–4b were identified by comparison of their NMR data with reported data. To the best of our knowledge, this is the first report on the isolation of 1–3e from D. grandiflora.
Silica gel chromatography of the dichloromethane extract of D. grandiflora flowers afforded a new sesquiterpene lactone, grandiflorolide (1). The structure of 1 was elucidated by extensive 1D and 2D NMR spectroscopy as follows.
The 1H NMR spectrum of 1 indicated resonances for exocyclic methylene protons at δ 5.48 and 6.18, olefinic protons at δ 6.22 and 6.32, oxymethine protons at δ 5.10 (lactone) and 3.76 (hydroxyl), an allylic methyl at δ 1.71, a methyl singlet at δ 1.36, an acetoxy methyl at δ 2.15, allylic methines at δ 3.74 and 2.74, and methylene protons at δ 2.10.
The 13C NMR spectrum gave resonances for six olefinic carbons at δ 93.6, 99.2, 121.8, 133.5, 137.3, and 137.5; a lactone carbonyl at δ 169.0; an acetate carbonyl at δ 170.5; two oxygenated methine carbons at δ 71.6 and 75.8 and a nonprotonated oxygenated carbon at δ 70.8; two methine carbons at δ 46.4 and 69.1; a methylene carbon at δ 41.5; and three methyl carbons at δ 13.7, 21.3, and 27.5.
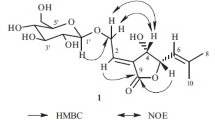
Correlation spectroscopy (COSY) analysis (Fig. 1) indicated two isolated spin systems as follows: coupled olefinic protons (H-2 and H-3); and a methine proton (H-5) coupled to an oxymethine proton (H-6), which was coupled to another methine proton (H-7), which was in turn coupled to exocyclic methylene protons (H2-13) and a lactonic proton (H-8), which was finally coupled to methylene protons (H2-9).
Protons attached to carbons were assigned from heteronuclear single quantum coherence (HSQC) 2D NMR data, and the structure of 1 was elucidated by analysis of heteronuclear multiple bond coherence (HMBC) 2D NMR data: key HMBC correlations are shown in Fig. 1. The hydroxyl was located at C-6 on the basis of long-range correlations between the oxymethine proton (H-6) and the methine carbons (C-5 and C-7). The exocyclic methylene protons were attached to C-13 due to long-range correlations between these protons (H2-13) and C-7, C-11, and C-12. The allylic methyl was assigned to C-15 since long-range correlations were observed between this methyl (H3-15) and the olefinic carbons (C-3 and C-4) and themethine carbon (C-5). The methyl singlet was attributed to C-14 based on long-range correlations between this methyl (H3-14) and C-1, C-9, and C-10. All long-range correlations observed are consistent with the structure of 1.
The relative configuration of 1 was deduced from nuclear Overhauser effect spectroscopy (NOESY) analysis. The methyl singlet (H3-14) was close in space to the olefinic proton (H-2), while the lactonic proton (H-8) was close to the oxymethine proton (H-6). On the opposite face of 1, the methine protons (H-5 and H-7) were close to each other. All NOESY correlations were consistent with the relative configuration of 1.
The structure of 1 was confirmed by HR-ESI-MS analysis, which revealed a molecular ion of m/z 304.1312 [M]+, corresponding to a molecular formula of C17H20O5. Literature search revealed that 1 is a new compound. The trivial name grandiflorolide is proposed for 1.
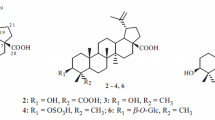
In addition to the above sesquiterpene lactone isolated from Dendranthema grandiflora flowers, several other constituents (2–4d) were isolated from this plant. The NMR data of 2 are in accordance with data reported in the literature for 1,2-dilinoleoyl-3-linolenoylglycerol [20]; 3a for pseudotaraxasterol [21, 22]; 3b for taraxasterol [22, 23, 25]; 3c for β-amyrin [26,27,28]; 3d for α-amyrin [27,28,29]; 3e for lupeol [29,30,31]; 4a for β-sitosterol [32, 33]; and 4b for stigmasterol [34, 35].
The presence of α-linolenic acid in the triacylglycerol (2) was deduced from the methyl triplet at δ 0.96 (t, J = 7.8 Hz), the double allylic methylenes at δ 2.78, and the olefinic protons at δ 5.34 (m) [34]. The presence of linoleic acid was deduced from the methyl triplet at δ 0.86 (t, J = 6.6 Hz), the double allylic methylene at δ 2.80, and the olefinic protons at δ 5.34 (m) [34]. Based on integrations of the triacylglycerol methyls at δ 0.96 (t, J = 7.8 Hz) and 0.86 (t, J = 6.6 Hz), the ratio of linolenic acid and linoleic acid in the triglycerides is about 1:2 [34].
The ratio of about 2:1:1:0.5:0.5 for the mixture of 3a, 3b, 3c, 3d, and 3e was deduced from integrations and relative intensities of the 1H NMR resonances for the olefinic protons of 3a at δ 5.24 (br.d, J = 7.2 Hz), 3b at δ 4.60 (d, J = 2.4 Hz) and 4.58 (d, J = 2.4 Hz), 3c at δ 5.16 (t, J = 3.6 Hz), 3d at δ 5.10 (t, J = 3.6 Hz), and 3e at δ 4.55 (d, J = 2.4 Hz) and 4.66 (d, J = 2.4 Hz).
The ratio of about 2:1 for the mixture of 4a and 4b was deduced from integrations and relative intensities of the 1H NMR resonances for the olefinic protons of 4a at δ 5.33 (dd, J = 1.8, 3 Hz, H-5) and 4b at δ 5.33 (dd, J = 1.8, 3 Hz, H-5), 5.13 (dd, J = 9, 15 Hz, H-22), and 5.01 (dd, J = 8.4, 15 Hz, H-23) [33].
EXPERIMENTAL
General Experimental Procedures. NMR spectra were recorded on a Varian VNMRS spectrometer in CDCl3 at 600 MHz for 1H NMR and 150 MHz for 13C NMR spectra. HR-ESI-MS was obtained on a Thermo Scientific Q exactive focus orbitrap instrument. Column chromatography was performed with silica gel 60 (70–230 mesh). Thin-layer chromatography was performed with plastic backed plates coated with silica gel F254, and plates were visualized by spraying with vanillin/H2SO4 solution followed by warming.
Plant Material. The flowers were collected from Baguio City, Philippines in September 2016. The sample was identified as Dendranthema grandiflora cultivar Yoko Ono by Virgilio Linis of the Biology Deapartment, De La Salle University-Manila, Philippines.
Extraction and Isolation. The D. grandiflora cv. Yoko Ono (147.7 g) was freeze-dried (18.5 g), then ground in a blender, soaked in CH2Cl2 for 3 days, and filtered. The filtrate was concentrated under vacuum to afford a crude extract (87.05 mg), which was chromatographed by gradient elution with petroleum ether, 5%, 10%, and 15% EtOAc in petroleum ether, CH2Cl2, CH3CN–Et2O–CH2Cl2 (0.5:0.5:9), CH3CN–Et2O–CH2Cl2 (1:1:8), and CH3CN–Et2O–CH2Cl2 (1.5:1.5:7). The 10% EtOAc in the petroleum ether fraction was rechromatographed using 2.5% EtOAc in petroleum ether to afford 2 (5 mg). The 15% EtOAc in the petroleum ether fraction was rechromatographed using 15% EtOAc in petroleum ether to yield a mixture of 3a–3e (2.8 mg) after washing with petroleum ether. The CH2Cl2 fraction was rechromatographed using 20% EtOAc in petroleum ether to provide a mixture of 4a and 4b (3.7 mg) after washing with petroleum ether. The CH3CN–Et2O–CH2Cl2 (1.5:1.5:7) fraction was rechromatographed using CH3CN–Et2O–CH2Cl2 (0.5:0.5:9) to afford 1 (2.4 mg) after washing with petroleum ether.
Grandiflorolide (1), colorless solid; \( {\left[\upalpha \right]}_{\mathrm{D}}^{25} \) + 108.3° (c 0.14, CH3OH); mp 102–104°C. 1H NMR (600 MHz, CDCl3, δ, ppm, J/Hz): 1.36 (s, H3-14), 1.71 (s, H3-15), 2.102 (d, J = 5.4, H-9b), 2.104 (d, J = 3.0, H-9a), 2.15 (s, OAc), 2.74 (d, J = 10.2, H-5), 3.74 (t, J = 10.2, H-6), 3.76 (tt, J = 3.0, 9.6, H-7), 5.10 (ddd, J = 3.0, 5.4, 9.6, H-8), 5.48 (d, J = 3.0, H-13a), 6.18 (d, J = 3.6, H-13b), 6.22 (d, J = 6, H-2), 6.32 (d, J = 6, H-3). 13C NMR (150 MHz, CDCl3, δ, ppm): 99.2 (C-1), 133.5 (C-2), 137.5 (C-3), 93.6 (C-4), 69.1 (C-5), 75.8 (C-6), 46.4 (C-7), 71.6 (C-8), 41.5 (C-9), 70.8 (C-10), 169.0 (C-11), 137.3 (C-12), 121.8 (C-13), 27.5 (C-14), 13.7 (C-15), 21.3, 170.5 (OAc). HR-ESI-MS m/z 304.1312 [M]+ (ñalcd for C17H20O5, 304.1311).
References
N. O. Anderson, Hort. Sci., 22, 313 (1987).
H. Niek Dekker (NL), US PP12,566 P2 (2002).
K. Hattori and Y. Futsuhara, J. Breed., 20, 261 (1970).
S. Asen, R. N. Stewart, and K. H. Norris, Phytochemistry, 14, 1443 (1975).
S. Sinha, R. K. Khanna, S. N. Srivastava, and A. Singh, Chem. Pharm. Bull., 3, 8 (1986).
N. Saito, K. Toki, T. Honda, and K. Kawase, Phytochemistry, 27, 2963 (1988).
K. Kawase, Y. Tsukamoto, N. Saitom, and Y. Osawa, Plant Cell Physiol., 11, 349 (1970).
M. Arisawa, Y. Ishiwari, T. Nakaoki, S. Sekino, and T. Takakuwa, Shoyakugaku Zasshi, 23, 49 (1969).
K. K. Hsu and W. H. Wang, Taiwan Ke Hsueh, 18, 102 (1964).
K. E. Schwinn, K. R. Markham, and N. K. Given, Phytochemistry,35, 145 (1994).
Y. J. Wang, X. W. Yang, and Q. S. Guo, Zhongguo Zhong Yao Za Zhi, 33, 526 (2008).
J. Q. Liu, Q. Q. Shen, J. S. Liu, and J. T. Wang, Zhongguo Zhong Yao Za Zhi, 26, 547 (2001).
N. O. Anderson, P. D. Ascher, and R. F. Widmer, Euphytica, 37, 229 (1988).
M. Kakehi, H. Yokota, and K. Yamauchi, J. Jpn. Soc. Hort. Sci., 34, 232 (1965).
K. Kawase and Y. Tsukamoto, J. Jpn. Soc. Hort. Sci., 43, 165 (1974).
K. Kawase and Y. Tsukamoto, J. Jpn. Soc. Hort. Sci., 45, 65 (1976).
M. Hodaei, M. Rahimmalek, and A. Arzani, Biochem. Syst. Ecol.,74, 1 (2017).
Q. Wei, X. Y. Ji, X. S. Long, Q. R. Li, and H. Yin, Zhong Yao Cai,38, 305 (2015).
C. Y. Ragasa, F. Tiu, and J. A. Rideout, ACGC Chem. Res. Commun.,18, 12 (2005).
C. Y. Ragasa, V. D. Ebajo Jr., M. M. De Los Reyes, E. H. Mandia, R. Brkljaca, and S. Urban, J. Appl. Pharm. Sci.,16, 5 (Suppl. 2), (2015).
A.-M. Yang, X. Liu, R.-H. Lu, and Y.-P. Shi, Pharmazie,61, 70 (2006).
X.-M. Ma, D.-L. Di, and Y.-P. Shi, Chem. Nat. Compd., 44, 399 (2008).
C. Y. Ragasa, V. D. Ebajo Jr., M. M. De Los Reyes, E. H. Mandia, R. Brkljaca, and S. Urban, Int. J. Pharmacogn. Phytochem. Res., 7, 718 (2015).
S. Ma, A. Yang, L. Yang, W. J. Guo, C. Li, and Q. Shang, Chem. Nat. Compd., 53, 586 (2017).
L. Wu, W. Xiong, J.-W. Hu, X.-H. Li, J.-P. Fu, C.-L. Si, and J. Wang, Chem. Nat. Compd., 54, 610 (2018).
C. Y. Ragasa, O. B. Torres, J. M. P. Gutierrez, H. P. B. C. Kristiansen, and C.-C. Shen, J. Appl. Pharm. Sci., 5, 94 (2015).
H. S. Al Hinai, W. M. Al-Subhi, F. R. S. Al-Rubaiai, S. I. Hassan, N. Sherwani, and M. O. Ftope, Chem. Nat. Compd., 54, 790 (2018).
L. Chen, J. Wang, and W. Kang, Chem. Nat. Compd., 52, 1141 (2016).
V. D. Ebajo Jr., C.-C. Shen, and C. Y. Ragasa, J. Appl. Pharm. Sci., 5, 33 (2015).
M. Pal, S. K. Tewari, X.-Q. Chen, and Q.-S. Zhao, Chem. Nat. Compd., 49, 190 (2013).
H.-S. Chang, Y.-S. Chen, M.-J. Cheng, H.-C. Wu, H.-Y. Chan, S.-Y. Hsieh, S.-S. Yang, I.- S. Chen, and I.-J. Chen, Chem. Nat. Compd., 54, 396 (2018).
C. Y. Ragasa, G. S. Lorena, E. H. Mandia, D. D. Raga, and C.-C. Shen, Am. J. Essent. Oils Nat. Prod., 1, 7 (2013).
C. Y. Ragasa, V. A. S. Ng, O. B. Torres, N. S. Y. Sevilla, K. V. M. Uy, M. C. S. Tan, M. G. Noel, and C.-C. Shen, J. Chem. Pharm. Res., 5, 1237 (2013).
Q. Wang, P. Qiu, F. Guan, Y. Shan, M. Yin, X. Feng, and F. Liu, Chem. Nat. Compd., 54, 38 (2018).
Acknowledgment
A research grant from the De La Salle University Science Foundation, through the University Research Coordination Office, De La Salle University, Manila, Philippines, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2020, pp. 378–380.
Rights and permissions
About this article
Cite this article
Ragasa, C.Y., Si, M., Tan, M.C.S. et al. A New Sesquiterpene from Dendranthema grandiflora Flowers. Chem Nat Compd 56, 436–439 (2020). https://doi.org/10.1007/s10600-020-03057-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03057-4