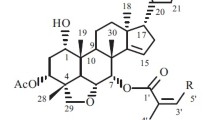
A new isoflavane, 6,2′-dihydroxy-5,7-dimethoxyisoavanone (1), was isolated from the herb Suaeda glauca (Bunge) Bunge (Chenopodiaceae), in addition to six known compounds (2–7). The structures of the compounds were determined using chemical and spectral methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Suaeda glauca (Bunge) Bunge belongs to the genus Suaeda Forsk. ex Scop. of the family Chenopodiaceae, which is a wild resource in seashore salt marsh and inland saline soil and has invaluable ecological, economic, and social benefits [1]. Research into Suaeda glauca has been carried out in China and other countries but has mostly focused on planting and cultivation, with little basic or industrial research reported. Therefore, basic research into Suaeda glauca is needed, including its chemical components and pharmacological activities, to guarantee its all-round industrial development. Our investigation of the chloroform extract of Suaeda glauca resulted in the isolation of a new isoflavane, suaeglaucin C (1), and six known compounds, namely, octacosanol (2), dotriacontane (3), β-sitosterol (4), β-daucosterol (5), stigmasterol (6), and spinasterol (7). The compound structures were established using chemical and spectral methods.
Compound 1 was a colorless oil (CH2Cl2). TLC analysis of 1 showed a dark spot under 254 nm UV light, while spraying with 10% H2SO4–EtOH reagent followed by heating at 120°C showed a pink spot that gradually became bright yellow. The FeCl3 reaction of 1 was positive, while Molisch’s test was negative, indicating that the compound could be a flavonoid. The molecular formula of 1 was determined to be C17H16O6 from its 1H NMR, 13C NMR, DEPT, and HR-ESI-MS spectra (positive mode), which displayed a quasimolecular ion peak at m/z 317.1027 [M + H]+ (calcd for C17H17O6, 317.0980).
The 1H NMR (300 MHz, CDCl3, δ, ppm, J/Hz) spectrum of 1 (Table 1) indicated the presence of two phenolic hydroxyl groups at δ 8.40 (1H, br.s) and 6.41 (1H, br.s), and two methoxyl groups at δ 3.88 (6H, s). Furthermore, the aromatic region of the spectrum showed four protons at δ 6.96 (1H, d, J = 7.9 Hz), 7.18 (1H, dd, J = 7.9, 7.6 Hz), 6.89 (1H, dd, J = 7.6, 7.5 Hz), and 7.49 (1H, d, J = 7.5 Hz), one aromatic proton signal at δ 6.36 (1H, s), an OCH2 group at δ 4.89 (1H, dd, J = 11.9, 3.5 Hz) and 4.73 (1H, dd, J = 11.9, 4.5 Hz), and one aliphatic CH signal at 3.97 (1H, dd, J = 4.5, 3.5 Hz).
In appropriate 13C NMR (75 MHz, CDCl3, δ, ppm) and DEPT spectra (Table 1), 17 carbon signals were observed, including six CH signals at δ 129.3, 126.9, 120.9, 118.0, 98.9, and 46.9, seven quaternary carbon signals at δ 160.2, 157.0, 155.5, 155.5, 135.5, 123.3, and 107.3, one C=O signal at δ 191.9, two CH3 signals at δ 61.5 and 61.3, and a CH2 signal at δ 69.2.
The 1H and 13C NMR signals of compound 1 were fully assigned with the assistance of spectral analysis of HSQC, HMBC, 1H–1H COSY, and ROESY experiments. Compound 1 is a new isoflavane, named suaeglaucin C. The 1H and 13C NMR, 1H–1H COSY, and HMBC (Fig.1) data are shown in Table 1.
Experimental
General Comments. TLC was carried out on silica gel 60 F254 (Merck) plates, and spots were visualized by spraying with 10% H2SO4–EtOH reagent followed by heating at 120°C. Column chromatography was performed on silica gel (200–300 mesh; Qingdao Haiyang Chemical Co., Ltd.) and Sephadex LH-20 (GE Healthcare, Sweden). Melting points were measured on a XT4 Boetius melting point apparatus and were uncorrected. 1D and 2D NMR spectra were recorded using a Bruker-ACF-300 with CDCl3 as solvent and SiMe4 as the internal standard (δ, J in Hz). Mass spectra were recorded on an Agilent 6520 UPLC/Q-TOF/MS spectrometer (in m/z).
Plant Material. Dried herb Suaeda glauca (Bunge) Bunge was purchased from Shouguang and Shandong Provinces, China in August 2013 and identified by Prof. Yuan Chang-Qi (Institute of Botany, Jiangsu Province and Chinese Academy of Sciences). A voucher specimen (IBJPCAS/SG/2013/08/10) has been deposited in the Herbarium of the Institute of Botany, Jiangsu Province and Chinese Academy of Sciences. The plant was air dried in the shade.
Extraction and Isolation. Air-dried herb Suaeda glauca (Bunge) Bunge (10 kg) was extracted with 80% EtOH–H2O (3 × 50 L, 2 h for each extraction) under reflux. The ethanolic extract was then filtered through absorbent gauze, and the combined filtrate was evaporated to dryness (1620 g) using a vacuum rotary evaporator (BUCHI R-210). The residue was suspended in water and partitioned with petroleum ether, chloroform, ethyl acetate, and n-butanol, successively. These four fractions were designated as PEF (102 g), CLF (76 g), EAF (140 g), and BUF (350 g), respectively.
The CLF fraction (76 g) was purified by silica gel column chromatography and eluted with petroleum ether–EtOAc (100:0→0:100) to afford five fractions (A–E). Fraction A afforded compounds 2 (3.0 g) and 3 (8.4 mg). Fraction B afforded compounds 4 (10.6 mg), 6 (7.7 mg), and 7 (7.4 mg) using silica gel column chromatography and eluted with petroleum ether–EtOAc (50:1→1:1). Fraction C underwent further silica gel column chromatography by step gradient elution with petroleum ether–EtOAc (1:1→0:100) and Sephadex LH-20 (MeOH) to yield compounds 1 (7.8 mg) and 5 (8.2 mg).
Suaeglaucin C (1). C17H16O6, colorless oil (CH2Cl2). A positive reaction was observed with FeCl3 reagent. ESI-MS m/z 317 [M + H]+; DEPT spectrum showed six CH, eight quaternary carbons, one CH2, one CO, and two CH3. Data from 1H NMR, 13C NMR, 1H–1H COSY, HMBC spectra are shown in Table 1.
The following compounds were identified by comparison with authentic compounds or published physical and spectral data.
Octacosanol (2). C28H58O, white amorphous powder (EtOAc), mp 86–87°C. 1H NMR (300 MHz, CDCl3, δ, ppm, J/Hz): 4.92 (1H, OH), 3.64 (2H, t, J = 6.6, CH2O), 1.56 (2H, m, CH2CH2O), 1.26–1.31 (50H, m), 0.87 (3H, t, J = 6.6, CH3). 13C NMR (75 MHz, CDCl3, δ, ppm): 63.1 (C-1), 32.8 (C-2), 31.9 (C-26), 29.3, 29.4, 29.6, 29.7 (C3–C25), 25.7 (C-3), 22.6 (C-27), 14.1 (C-28, CH3). Characterized as octacosanol by comparison with physical and spectral data in the literature [2].
Dotriacontane (3). C32H66, white amorphous powder (EtOAc), mp 84–85°C. 1H NMR (300 MHz, CDCl3, δ, ppm, J/Hz): 0.88 (6H, t, J = 6.8, 2 × CH3), 1.25 (30 × CH2). 13C NMR (75 MHz, CDCl3, δ, ppm): 32.0 (C-3, 30), 29.6 (C5–C28), 29.3 (C-4, 29), 22.7 (C-2, 31), 14.1 (C-1, 32). Characterized as dotriacontane by comparison with physical and spectral data in the literature [3].
β -Sitosterol (4). ESI-MS m/z 413 [M – H]– (calcd for C29H50O).
β_-Daucosterol (5). ESI-MS m/z 599 [M + Na]+ (calcd for C35H60O6).
Stigmasterol (6). C29H48O, white needles (EtOAc), mp 166–168°C. Provided positive results in Molisch's test and the Liebermann–Burchard test. 1H NMR (300 MHz, CDCl3, δ, ppm): 5.72 (1H, m, H-6), 5.34 (1H, m, H-22), 5.29 (1H, m, H-23), 4.14 (br.s, OH), 3.64 (1H, m, H-3), 0.71–2.42 (43H, m). 13C NMR (75 MHz, CDCl3, δ, ppm): 37.5 (C-1), 30.2 (C-2), 71.9 (C-3), 42.6 (C-4), 141.5 (C-5), 121.5 (C-6), 32.0 (C-7), 32.2 (C-8), 50.6. (C-9), 36.9 (C-10), 21.5 (C-11), 40.2 (C-12), 42.5 (C-13), 56.8 (C-14), 24.8 (C-15), 29.3 (C-16), 57.0 (C-17), 12.5 (C-18), 19.8 (C-19), 40.8 (C-20), 21.2 (C-21), 138.5 (C-22), 129.0 (C-23), 52.1 (C-24), 32.4 (C-25), 20.9 (C-26), 21.5 (C-27), 25.6 (C-28), 12.7 (C-29). Characterized as stigmasterol by comparison with physical and spectral data in the literature [4].
Spinasterol (7). C29H48O, white crystals (EtOAc), mp 163–165°C. Provided a positive result in the Liebermann–Burchard test. TLC spraying with 10% H2SO4–EtOH reagent followed by heating at 120_C gave a blue color that did not fade. 1H NMR (300 MHz, CDCl3, δ, ppm, J/Hz): 0.55 (3H, s, CH3-18), 0.79 (3H, s, CH3-19), 0.80 (3H, s, CH3-26), 0.82 (3H, s, CH3-27), 0.86 (3H, d, J = 6.6, CH3-29), 1.03 (3H, d, J = 6.7, CH3-21), 3.59 (1H, m, H-3), 5.05 (1H, dd, J = 8.5, 8.7, H-22), 5.15 (1H, dd, J = 8.5, 8.7, H-23). 13C NMR (75 MHz, CDCl3, δ, ppm): 31.2 (C-1), 31.5 (C-2), 71.1 (C-3), 38.0 (C-4), 40.3 (C-5), 29.7 (C-6), 117.5 (C-7), 139.6 (C-8), 49.5 (C-9), 34.2 (C-10), 21.4 (C-11), 39.5 (C-12), 43.3 (C-13), 55.1 (C-14), 23.0 (C-15), 28.5 (C-16), 55.9 (C-17), 12.0 (C-18), 13.0 (C-19), 40.8 (C-20), 21.0 (C-21), 138.1 (C-22), 129.5 (C-23), 51.3 (C-24), 31.9 (C-25), 21.6 (C-26), 19.0 (C-27), 25.4 (C-28), 12.2 (C-29). Characterized as spinasterol by comparison with physical and spectral data in the literature [5].
References
Chinese Academy of Sciences Flora of China Editorial Committee, Flora of China (XXV: Volume II), Science Press, Beijing, 1979, pp. 115–135.
F. L. Zhan, Y. T. Lu, W. Kamil, and K. Ablajan, Chem. Nat. Compd., 51, 556 (2015).
Q. J. Zhang, X. S. Yang, H. Y. Zhu, and X. J. Hao, J. Guizhou Univ., 23, 270 (2006).
T. Yang, Q. Wu, S. Y. Li, Z. J. Lv, B. Hu, and B. J. Xie, Chem. Nat. Compd., 50, 1166 (2014).
Y. Y. Gu, P. F. Li, F. H. Lei, and Z. J. Huang, Chem. Nat. Compd., 52, 224 (2016).
Acknowledgment
This study was supported by the Capacity Building Foundation of Jiangsu Province (Grant No. BM2015019), the National Natural Science Foundation of China (Nos. 31570359, 31470425), the Industry Academic Joint Technological Innovations Foundation of Jiangsu Province (No. BY2015074-03), Jiangsu Key Laboratory for Bioresources of Saline Soils (No. JKLBS2013005), and Jiangsu Key Laboratory for the Research and Uti1ization of Plant Resources (SQ201402).
The authors are grateful to Prof. Chang-Qi Yuan (Nanjing Botanical Garden, Mem. Sun Yat-Sen) for helping with plant identification.
We thank Simon Partridge, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2018, pp. 35–37.
Rights and permissions
About this article
Cite this article
Wang, Q., Qiu, P., Guan, F. et al. A New Isoflavane from Suaeda glauca. Chem Nat Compd 54, 38–40 (2018). https://doi.org/10.1007/s10600-018-2254-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2254-x