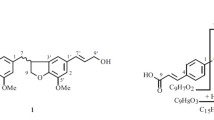
A new neolignan glucoside has been isolated from Chrysanthemum morifolium ‘Baekma’ cultivar through repeated silica gel and octadecyl silica gel (ODS) column chromatographies. On the basis of spectroscopic data including NMR, MS, and IR, the chemical structure of the new neolignan glucoside was determined to be (7S,8S)-7-O-[1′-(4′,5′-dihydroxy-3′-methoxyphenyl)propanol]guaiacylglycerol 4-O-β-D-glucopyranoside (1), named baekmaoside A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chrysanthemum morifolium (Compositae) is a herbaceous perennial plant distributed widely in Korea, China, and Japan. C. morifolium has mainly been used for ornamental purposes but also as tea or for fragrance in East Asian cultures [1]. C. morifolium is classified into standard mums, spray mums, and pollen mums. More than 700 kinds of C. morifolium cultivars have been developed in Korea, and standard mums occupy an overwhelming 80% share of the Korean market [2]. The Horticultural Research Institute of the Korea Rural Development Administration recently bred a new cultivar called “Baekma” from the standard type C. morifolium [3]. The distribution of C. morifolium for ornamental purposes yield a large quantity of by-products, the majority of which are the stems of C. morifolium. Alternative uses of by-products from C. morifolium lead to cost reduction and increased income for farmers. Also, C. morifolium stems have been reported to have antioxidant [4] and antithrombogenic [5] effects. Despite several reports on the pharmacological activities of C. morifolium stems, only one phytochemical study has been reported, which listed four phenolic compounds and one lignan as the components of C. morifolium stems [6]. This study was carried out to isolate physiologically active substances from the Korean domestic C. morifolium cultivar, “Baekma”.
The stems of C. morifolium were extracted with aqueous MeOH, and the concentrated extract was fractionated into EtOAc, n-BuOH, and water fractions. From the n-BuOH fraction, a new neolignan glucoside was isolated through repeated SiO2 and octadecyl silica gel (ODS) column chromatography.
Compound 1, a brown amorphous powder, showed UV absorption characteristics at 365 and 254 nm and a black color on TLC by spraying with 10% H2SO4 and heating. The molecular formula was determined to be C26H36O13 from the molecular ion peak [M + H]+m/z 557.2230 (calcd for C26H37O13, 557.2234) in positive-mode HR-FAB-MS. The IR spectrum suggested the presence of a hydroxyl group (3375 cm–1) and an aromatic double bond (1604, 1580 cm–1).
The 1H NMR spectrum (Table 1) showed three aromatic methine signals [δ 7.13 (1H, d, J = 8.4 Hz, H-5), 7.02 (1H, d, J = 2.0 Hz, H-2), 6.92 (1H, dd, J = 8.4, 2.0 Hz, H-6)] due to a 1,2,4-trisubstituted benzene ring and two aromatic methine signals [δ 6.72 (1H, br.s, H-2′), 6.70 (1H, br.s, H-6′)] due to a 1,2,3,5-tetrasubstituted benzene ring. In the oxygen region, two oxymethines [δ 5.54 (1H, d, J = 6.0 Hz, H-7), 3.55 (1H, m, H-8)], two oxymethylenes [δ 3.29 (1H, overlapped, H-9), 3.27 (1H, overlapped, H-9′)], and two methoxyls [δ 3.84 (3H, s, 3′-OCH3), 3.81 (3H, s, 3-OCH3)] were observed. In the aliphatic region, two methylenes [δ 2.61 (2H, m, H-7′), 1.80 (2H, m, H-8′)] were observed. Therefore, the proton signals indicated the aglycon to be a lignan. The proton signals due to a hemiacetal at δ 4.87 (1H, d, J = 7.6 Hz, H-1′′), four oxymethines [δ 3.44 (1H, overlapped, H-5′′), 3.43 (1H, overlapped, H-3′′), 3.42 (1H, overlapped, H-2′′), 3.37 (1H, overlapped, H-4′′)], and one
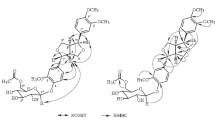
oxymethylene [δ 3.85 (1H, br.d, J = 13.6 Hz, H-6′′a), 3.73 (1H, dd, J = 13.6, 5.6 Hz, H-6′′b)] were interpreted as signaling a hexose moiety.
From the above-mentioned 1H NMR data, compound 1 was expected to be a lignan monoglycoside with two methoxy groups [7, 8]. The 13C NMR spectrum showed 26 carbon signals including two methoxy groups [δ 56.7 (3′-OCH3), 56.6 (3-OCH3)], confirming compound 1 as composed of a lignan and a hexose moiety. In the downfield, five oxygenated olefine quaternary carbons [δ 150.9 (C-3), 147.5 (C-4), 147.4 (C-5′), 145.2 (C-3′), 137.0 (C-4′)], two olefine quaternary carbons [δ 138.3 (C-1′), 129.5 (C-1)], and five olefine methines [δ 119.3 (C-6), 117.9 (C-5), 117.8 (C-6′), 114.1 (C-2′), 111.1 (C-2)] were observed. In the oxygen region, two oxymethines [δ 88.4 (C-7), 75.1 (C-8)] and two oxymethylenes [δ 65.0 (C-9), 62.4 (C-9′)] were observed. In the aliphatic region, two methylenes [δ 35.8 (C-8′), 32.9 (C-7′)] were also observed. The carbon chemical shift of the hexose moiety, a hemiacetal [δ 102.7 (C-1′′)], four oxymethines [δ 78.2 (C-3′′), 77.8 (C-5′′), 74.8 (C-2′′),71.3 (C-4′′)], and one oxymethylene [δ 62.2 (C-6′′)] revealed the sugar to be a β-glucopyranose, and the coupling constant of the anomer proton signal (J = 8.0 Hz) confirmed the anomer hydroxyl as having a β-configuration. In the gHMBC spectrum (Fig. 1), the oxymethine proton signal (δ 5.54, H-7) showed a cross-peak with the oxygenated olefine quaternary carbon signal (δ 147.5, C-5′), and the anomer proton signal (δ 4.87, H-1′′) showed a cross-peak with the oxygenated olefine quaternary carbon signal (δ 147.5, C-4). Also, two methoxy proton signals [δ 3.84 (3H, s), 3.81 (3H, s)] showed cross peaks with their respective oxygenated olefine quaternary carbon signals [δ 145.2 (C-3′), 150.9 (C-3)]. Taken together, the planar structure of compound 1 was determined to be 7-O-[1′-(4′,5′-dihydroxy-3′-methoxyphenyl)propanol]guaiacylglycerol 4-O-β-Dglucopyranoside. The large coupling constant between H-7 and H-8 (J = 6.0 Hz) suggested the threo conformation of C-7/C-8 [9]. Because the CD spectrum of compound 1 showed a positive cotton effect at 297 nm and a negative cotton effect at 266 nm, the absolute configurations of chiral centers were assigned to be 7S and 8S [10]. Therefore, the chemical structure of compound 1 was determined to be (7S,8S)-7-O-[1′-(4′,5′-dihydroxy-3′-methoxyphenyl)propanol]guaiacylglycerol 4-O-β-D-glucopyranoside, which was revealed to be a new compound, named baekmaoside A.
Experimental
General Methods. The materials and methods used for this study were the same as those in the previous study [11, 12].
Plant Material. Stems of C. morifolium “Baekma” were purchased from Yangjae Flower Market Center, Seoul, Korea, in 2017 and identified by Prof. Ha-Seung Pak, Flower Research Institute, Chungcheongnam-do ARES, Yesan, Korea. Unlike other chrysanthemum varieties where the center of the flower is yellowish, “Baekma” has green centers, so it can be easily identified. A voucher specimen (NPCL-20170430) has been deposited at the Natural Products Chemistry Laboratory, Kyung Hee University, Yongin, Korea.
Extraction ofC. morifolium“Baekma” Stems and Isolation of a Neolignan Glycoside. Three kilograms of C. morifolium “Baekma” stems were cut into pieces, dried, and extracted with 80% methanol (MeOH, 30 L × 3) for 24 h at room temperature. The concentrated methanol extract (177 g) was suspended in water (3.0 L) and then consecutively extracted with EtOAc (3.0 L × 3) and n-BuOH (2.4 L × 3). The fractions were concentrated in vacuo to produce EtOAc (CBAE, 19.3 g), n-BuOH (CBAB, 18.9 g), and H2O (CBAW, 138.8 g) residues. The CBAB fraction was subjected to silica gel (SiO2) column chromatography (CC) (5.5 cm × 17.0 cm) and eluted with CHCl3–MeOH (3:1, 2.0 L) and CHCl3–MeOH–H2O (7:3:1→65:35:10, 2.0 L of both). The eluting solutions were monitored by TLC to produce eight fractions (CBAB-1 to CBAB-8). Fraction CBAB-3 [1.4 g, elution volume/total volume (Ve/Vt) 0.312–0.454] was applied to an octadecyl silica gel (ODS) CC (3.5 cm × 8.0 cm) and eluted with MeOH–H2O (1:3→1:2, 1.3 L of both) to yield 12 fractions (CBAB-3-1 to CBAB-3-12). Fraction CBAB-3-11 (54.1 mg, Ve/Vt 0.840–0.880) was subjected to SiO2 CC (1.5 cm × 16 cm) and eluted with EtOAc–n-BuOH–H2O (25:3:1→16:3:1, 1.0 L of both) to produce eight fractions (CBAB-3-11-1 to CBAB-3-11-8) along with a purified compound 1 [CBAB-3-11-5, 19.6 mg, Ve/Vt 0.397–0.469, TLC (SiO2 F254) Rf 0.66, CHCl3–MeOH–H2O, 20:3:1].
Baekmaoside A (1), yellow amorphous powder (MeOH); \( {\left[\upalpha \right]}_{\mathrm{D}}^{23} \) +43.0° (c 0.10, MeOH). IR (CaF2 plate, cm–1): 3375, 1604, 1580. HR-FAB/MS m/z 557.2230 [M + H]+ (calcd for C26H37O13, 557.2234). 1H (400 MHz, CD3OD, δ, ppm) and 13C NMR (100 MHz, CD3OD, δ, ppm), see Table 1.
References
F. Wang, F. J. Zhang, F. D. Chen, W. M. Fang, and N. J. Teng, Scientific World J., 2014, 9 (2014).
Ministry of Agriculture, Food and Rural Affairs, 2016 Flower Cultivation Status [Korean translation], Saejong, Korea, 2017, p. 13.
Horticulture Industry Newspaper, in: Research Archievement in Division of Floriculture Research [in Korean], Horticulture Industry Newspaper, 2016, online article [http://www.wonyesanup.co.kr/news/articleView.html?idxno=33791]
L. X. Chen, D. J. Hu, S. C. Lam, L. Ge, D. Wu, J. Zhao, Z. R. Long, W. J. Yang, B. Fan, and S. P. Li, J. Chromatogr. A, 1428, 134 (2016).
L. Zhang, H. Fu, J. Tian, W. Wang, X. Zhai, R. Li, and W. Li, CN Pat. No. 2011-10184646, Shuomingsh, Gongkai, Faming Zhuanli Shenqing, 2011.
L. Qu, S. Bu, J. Li, L. Han, T. Wang, and Y. Zhang, Shenyang Yaoke Daxue, 33, 525 (2016).
J. S. Nam, S. Y. Park, H. L. Jang, and Y. H. Rhee, Appl. Biol. Chem., 60, 535 (2017).
C. H. Park, M. J. Ahn, G. S. Hwang, S. E. An, and W. K. Whang, Appl. Biol. Chem., 59, 485 (2016).
S. L. Spassov, Tetrahedron, 25, 3631 (1969).
C. Shi, M. J. Xu, M. Bayer, Z. W. Deng, M. H. G. Kubbutat, W. Waejen, P. Proksch, and W. H. Lin, Phytochemistry, 71, 435 (2010).
N. Nguyen Thi, H. S. Song, E. J. Oh, Y. G. Lee, J. H. Ko, J. E. Kwon, S. C. Kang, D. Y. Lee, I. H. Jung, and N. I. Baek, Appl. Biol. Chem., 60, 527 (2017).
J. Lee, J. P. Rodriguez, K. H. Lee, J. Y. Park, K. S. Kang, D. H. Hahm, C. K. Huh, S. C. Lee, and S. Lee, Appl. Biol. Chem., 60, 487 (2017).
Acknowledgment
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (317071-03-1-SB020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2019, pp. 527–529.
Rights and permissions
About this article
Cite this article
Kim, HG., Oh, HJ., Ko, JH. et al. A New Neolignan Glucoside from the Stems of “Baekma” Cultivar, Chrysanthemum morifolium. Chem Nat Compd 55, 610–613 (2019). https://doi.org/10.1007/s10600-019-02760-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02760-1