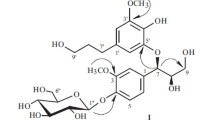
A new bisepoxylignan glucoside, named phillygenin 4-O-(6′′-O-acetyl)-β-D-glucopyranoside (1), along with two known compounds, 3β-acetoxyurs-11-en-28,13-olide (2) and epipinoresinol-4-O-β-D-glusoside (3), was isolated from the leaves of Forsythia suspensa. The structure was established on the basis of spectroscopic data and chemical evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Forsythia suspensa Vahl (Oleaceae) is a medicinal and ornamental plant widely distributed in Asia and Europe. Its leaves have been used as a health-promoting functional tea by Chinese folks for centuries [1]. In recent years, a broad spectrum of pharmacological activities of F. suspensa leaf extract, such as antioxidant, antiproliferative, hepatoprotective, hypolipidemic, hypoglycemic, cardioprotective, antiaging, antiobesity, antiallergy, and antifatigue effects, have been reported [2,3,4,5,6]. To date, 36 chemical constituents including triterpenoids, phenylethanoid glycosides, flavonoids, lignans, steroids, phenylpropionic acid esters, and phenolic acids have been isolated and identified from the leaves of F. suspensa by [7,8,9,10]. Further investigation on this plant part resulted in the discovery of a new compound, phillygenin 4-O-(6′′-O-acetyl)-β-D-glucopyranoside (1), along with two known compounds, 3β-acetoxyurs-11-en-28,13-olide (2) and epipinoresinol-4-O-β-D-glusoside (3). To the best of our knowledge, compound 1 is a new bisepoxylignan glucoside, 2 has not been isolated previously from the Forsythia genus, and 3 is reported in the leaves of this plant for the first time. Herein, details of the isolation and structure elucidation of the compounds are presented.
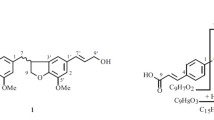
Compound 1 was assigned the molecular formula C29H36O12 from its [M – H]– ion peak at m/z 575.2126 in the HR-FAB-MS. The 1H NMR and 13C NMR spectra (Table 1) demonstrated signals of two sets of trisubstituted aromatic rings at δ 7.16 (1H, d, J = 8.2 Hz, H-5), 6.97 (1H, d, J = 1.8 Hz, H-2), and 6.88 (1H, dd, J = 8.2, 1.8 Hz, H-6) and 6.93 (1H, br.s, H-2′) and 6.86 (2H, br.s, H-5′, H-6′), and 12 aromatic carbons ranging from δ 109.0 to 150.8. Three methoxyl group protons at δ 3.91 (3H, s) and 3.89 (6H, s), and a glucopyranosyl anomeric proton at δ 4.64 (1H, d, J = 7.2 Hz, H-1′′) were also observed. The signals of four aliphatic methines at δ 87.3 (C-7), 54.6 (C-8), 82.0 (C-7′), and 50.2 (C-8′), and two aliphatic methylenes at δ 71.0 (C-9) and 69.8 (C-9′), as well as their corresponding proton signals at δ 4.48 (1H, d, J = 7.1 Hz, H-7), 2.90 (1H, q, J = 7.7 Hz, H-8), 4.15 (1H, d, J = 9.2 Hz, H-9a), 3.87 (1H, overlapped, H-9b) and 4.88 (1H, d, J = 5.4 Hz, H-7′), 3.35 (1H, m, H-8′), 3.85 (1H, overlapped, H-9′a), 3.34 (1H, overlapped, H-9′b) established the occurrence of a dioxabicyclo[3.3.0]octyl unit, which was also supported by analysis of the 1H–1H COSY, HMBC, and TOCSY spectra. The above NMR spectroscopic data of 1 suggested it to be a bisepoxylignan glucoside, and its structure was similar to forsythin isolated as one major active constituent from F. suspensa leaves [7], except for the presence of an additional acetyl group (δH 2.13; δC 171.8, 20.9). The HMBC (Fig. 1) correlations from H-6′′ (δ 4.52, 4.33) and a carbonyl carbon (δ 171.8) suggested that the acetyl group was attached to the oxygen at the C-6′′ hydroxyl group of the glucose unit. The presence of the strong NOESY correlation of H-7′/H-8 and the absence of a NOESY correlation of H-8′/H-7 indicated that compound 1 should have the same stereochemistry configuration as forsythin. According to the above evidence, the structure of 1 was proposed as phillygenin 4-O-(6′′-Oacetyl)-β-D-glucopyranoside.
Compound 2, obtained as white crystals (petroleum ether–EtOAc), was determined to be 3β-acetoxyurs-11-en-28,13-olide [11].
Compound 3, obtained as an amorphous powder (MeOH), was determined to be epipinoresinol-4-O-β-D-glucoside [12].
Experimental
General Procedures. NMR: Bruker Avance DRX 500 NMR spectrometer using TMS as internal standard. HR-FAB-MS: Bruker Apex II FI-ICR mass spectrometer. Optical rotations: Autopol IV polarimeter. Chromatography: silica gel 200–300 mesh (Qingdao Marine Chemical Factory, China), ODS-AA12S50 (YMC Co., Ltd, Japan).
Plant Material. The leaves of F. suspensa were collected from Taihang Mountain on May 19, 2009, Hebei Province, and identified by Prof. Jianhua Wang, Pharmacognosy Laboratory, School of Pharmaceutical Sciences, Hebei Medical University. A voucher specimen (NMC-2009-FSL-2) has been deposited in our Herbarium.
Extraction and Isolation. Dried F. suspensa leaves (4.4 kg) were extracted with 95% ethanol at room temperature. After evaporation of the solvent under reduced pressure, the residue was suspended in water and extracted with petroleum ether, CH2Cl2, EtOAc, and n-BuOH, successively. The EtOAc extract (115.4 g) was chromatographed on a silica gel column and eluted with CH2Cl2–MeOH (98:2→1:1) to furnish fractions E1–E6. Fraction E3 afforded compound 1 (5.1 mg) on an ODS column (MeOH–H2O, 25:75→100:0). Compound 3 (3.5 mg) was obtained by recrystallization from fraction E4. The petroleum ether extraction (194.1 g) was chromatographed on a silica gel column and eluted with CH2Cl2–EtOAc (17:1→1:1) to furnish fractions P1–P8. Fraction P2 was separated by preparative TLC (petroleum ether–EtOAc, 4:1) to yield compound 2 (4.2 mg).
Acid Hydrolysis. A 2 N HCl solution containing compound 1 (3.0 mg) was heated at 90°C for 2 h. After cooling, the reaction mixture was diluted with H2O and extracted with CDCl3. The CHCl3 layer was evaporated to dryness, and the residue was chromatographed on a silica gel column (petroleum ether–acetone, 4:1) to give its aglycone part, phillygenin, which was identified by comparison with 1H NMR, MS, and [α]D in the literature [13, 14].
Phillygenin 4- O -(6′′-O-Acetyl)-β-D-glucopyranoside (1). White crystals (CHCl3). \( {\left[\upalpha \right]}_{\mathrm{D}}^{25} \) +12.9° (c 0.12, CHCl3). HR-FAB-MS m/z 575.2126 [M – H]– (calcd for C29H35O12, 575.2129).
References
Q. Zhang, C. H. Jia, H. Y. Xu, Y. F. Wang, M. L. Zhang, C. H. Huo, Q. W. Shi, and S. H. Yu, Mini-Rev. Org. Chem., 9, 303 (2012).
W. Y. Kang, J. M. Wang, and L. Zhang, Zhongguo Zhongyao Zazhi, 35, 1156 (2010).
H. L. Xue and W. J. Wang, Shizhen Guoyiguoyao, 20, 1149 (2009).
G. X. Hou and J. X. Yang, J. Henan Univ. (Nat. Sci.), 40, 504 (2010).
Q. X. Lei, L. M. Zhao, X. Yan, Q. Zhang, B. Shan, Y. M. Gen, and B. E. Shan, Cancer Res. Prev. Treat., 39, 394 (2012).
J. Jiao, Q. Y. Gai, M. Luo, W. Wang, C. B. Gu, C. J. Zhao, Y. G. Zu, F. Y. Wei, and Y. J. Fu, Food Res. Inter., 53, 857 (2013).
Y. Ge, Y. Z. Wang, P. P. Chen, Y. F. Wang, C. C. Hou, Y. B. Wu, M. L. Zhang, L. G. Li, C. H. Huo, Q. W. Shi, and H. X. Gao, J. Agric. Food Chem., 64, 125 (2016).
Q. Zhang, Z. Lu, X. Li, Y. Zheng, D. G. Yao, Y. C. Gu, C. H. Huo, and B. Cong, Chem. Nat. Compd., 51, 178 (2015).
S. Kitagawa, S. Nishibe, R. Benecke, and H. Thieme, Chem. Pharm. Bull., 36, 3667 (1988).
S. Kitagawa, S. Hisada, and S. Nishibe, Phytochemistry, 23, 1635 (1988).
M. L. Tan, Y. Wang, L. G. Zhou, and W. B. Jiang, Nat. Prod. Res. Dev., 19, 232 (2007).
M. Chiba, S. Hisada, S. Nishibe, and H. Thieme, Phytochemistry, 19, 335 (1980).
F. Zhang, M. B. Zhao, J. Li, and P. F. Tu, Chin. Trad. Herb. Drugs, 44, 1529 (2013).
M. Chiba, S. Hisada, and S. Nishibe, Chem. Pharm. Bull., 25, 3435 (1977).
Acknowledgment
This work was supported by the Natural Science Foundation of Hebei Province, China (H2015206113) and Department of Education, Hebei Province (ZD2018088).
Author information
Authors and Affiliations
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2018, pp. 883–885.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhou, Q., Shi, N. et al. A New Bisepoxylignan Glucoside from the Leaves of Forsythia suspensa. Chem Nat Compd 54, 1038–1040 (2018). https://doi.org/10.1007/s10600-018-2549-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2549-y