Abstract
Stereotactic radiosurgery (SRS) is commonly used to treat brain metastases, particularly in the oligometastatic setting. This study analyses our initial experience in treating oligometastatic brain disease using Volumetric Modulated Arc Therapy (VMAT) to deliver hypofractionated stereotactic radiotherapy (HFSRT). Sixty-one patients were treated with HFSRT with a median dose of 24 Gy (range 22–40 Gy) in a median of three fractions (range 2–10 fractions). With a median follow-up of 23 months, the local control rate was 74 % for the entire cohort. Local control was 87 % for patients who had surgery with no radiological evidence of residual disease followed by HFSRT compared with 69 % in patients treated with HFSRT alone. The overall median time post radiotherapy to local failure was 8.6 months and to extracranial failure was 7.9 months. The mean time to distant brain failure was 9.9 months. Twenty-two patients (36 %) died during the study with median time to death of 4.4 months. Median overall survival (OS) from treatment was 21 months and 12 month OS was 60 %. Our experience with HFSRT using VMAT for oligometastatic brain metastases in the post-operative setting demonstrates comparable local control and survival rates compared with international published data. In the intact brain metastasis setting, local control using the dose levels and delivery in this cohort may be inferior to radio-surgical series. Local control is independent of histology. Careful selection of patients remains critical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are common in the cancer population, documented in 20–40 % of patients, contributing to significant morbidity and mortality [1–6]. Approximately 50 % of patients will present with a solitary brain metastasis [7]. Improved systemic therapies have lengthened survival, increasing the risk period for development of cerebral metastases and potential toxicity from treatment [3, 7–9]. Average OS in this cohort remains poor with most series suggesting OS of 6 months after diagnosis of brain metastases [3, 4, 6, 8, 10, 11].
Increasingly, SRS and HFSRT techniques have been utilised both in the intact and post-operative setting, providing improved local control and survival compared with whole brain radiotherapy (WBRT) alone [2, 4, 6]. For solitary brain metastatic disease, there is a survival advantage for radiosurgery with or without WBRT [4, 12–14]. For oligometastases (i.e. 1–3 metastases), there is evidence that SRS alone provides a local control and neuro-cognitive benefit [4, 13, 15]. It can maintain performance status with no demonstrated impact on survival, although there are more frequent intracranial relapses when WBRT is omitted [4, 14–16]. There is no randomised evidence for the use of SRS to the resection cavity but retrospective evidence exists that there is a local control benefit compared with no post-operative therapy [4, 6]. A phase III randomised study (RTOG1702/N107C) is currently accruing which is comparing WBRT and SRS for 1–4 brain metastases in the post-operative setting.
Hypofractionated SRS enables delivery of stereotactic doses in 2–10 fractions to maintain local control whilst decreasing the risk of late toxicity by utilising the radiobiological advantages of fractionation to increase the therapeutic range between tumour control and late effects. This enables treatment of larger lesions, close to critical structures or as a salvage treatment where WBRT has previously been utilised [2, 5, 8, 16–21]. Limiting dose to uninvolved areas mitigates toxicity risk and allows salvage treatment, but requires attentive surveillance for disease relapse [3, 8, 16, 22]. Radiosurgery is cost-effective [23], of short duration, decreasing time off systemic treatments which is particularly relevant in control of extracranial disease [9, 10, 17].
Volumetric Modulated Arc Therapy has emerged in recent years as a viable SRS and HFSRT treatment option for brain metastases in the intact and post-operative setting [24–27]. It allows highly conformal treatment (comparable to other stereotactic techniques) with decreased overall treatment time and with low toxicity profile [25]. There is a paucity of published data regarding local control rates and survival analysis, with a limited number of small retrospective series and comparative planning studies [24, 26–29]. Therefore, this study aims to examine our institution’s experience of HFSRT utilising VMAT and compare rates of local control, survival and patterns of failure with other SRS and HFSRT treatments to validate this technique as a viable stereotactic treatment option for brain oligometastases.
Methods
Patient characteristics
The cohort of patients comprised 61 consecutively treated patients with brain metastatic disease who received HFSRT between March 2012 and October 2014 at a single Australian tertiary referral centre. Where patients had multiple courses of HFSRT, the initial treatment was utilised in the series analysis. Patients who had previous intracranial radiotherapy were included in the analysis. The patient and treatment characteristics are outlined in Table 1. Institutional ethics approval was obtained for a retrospective analysis of patient data utilising medical and treatment records, and the Department’s radiotherapy planning system.
Simulation/planning
All patients were treated with a frameless linear accelerator based HFSRT technique. Patients were simulated supine using a Toshiba Aquilion LB CT Scanner (Toshiba America Medical Systems Inc., Tustin, CA, USA) with 2 mm slice thickness over the entire head region and immobilised with the Civco Type-S IMRT Reinforced Style 20 Mask (Civco Medical Solutions, Rotterdam, The Netherlands). Axial T1-weighted gadolinium (GD) contrast-enhanced MR sequence acquisitions with 1–2 mm slice thickness were obtained and these were co-registered with planning CT scans. Patients were inverse planned with volumetric arc radiotherapy (VMAT) either with the Pinnacle treatment planning system (TPS) versions 9.4 and 9.6 (Phillips, Amsterdam, The Netherlands) or the Monaco TPS versions 3.1 and 3.2 (Elekta, Stockholm, Sweden). The gross tumour volume (GTV) or high-risk target volume (HTV) for each metastasis was defined as the contrast enhanced lesion on the T1 + GD MRI with a 2–3 mm circumferential expansion cropped to anatomical boundaries to generate the clinical target volume (CTV). The planning target volume (PTV) was generated from a 2–3 mm expansion on the CTV to account for geometric uncertainties. The median covering isodose to the PTV was 24 Gy in three fractions which corresponds to the 80 % isodose compared to the point maximum which was located within the GTV/HTV.
Treatment
All treatments were delivered using a range of Elekta Linear Accelerators—Synergy head with 1 cm multileaf collimation (MLC), Agility head with 0.5 cm MLCs or Axesse with Beam Modulator head with 0.4 cm MLCs (Elekta, Stockholm, Sweden). Patient setup was performed using daily pre-treatment verification cone beam CTs (CBCT). The most common arc design was a full 360° axial co-planar arc with a sagittal (mohawk) non-coplanar arc. Treatment was delivered over consecutive days.
All patients received dexamethasone during and for a minimum of 3 days on completion of treatment, the dose determined by the pre-treatment requirements. Seizure prophylaxis was not routinely used.
Post-treatment surveillance and follow-up
Post-treatment, patients were followed with a combination of clinical review and serial MRI imaging approximately 4–6 weeks post treatment and then every 2–3 months to assess treatment response, acute and late toxicities and surveillance for new or recurrent disease. Local failure was defined as radiological evidence on MRI T1 weighted contrast enhanced images of increase in size of any abnormality in continuity to or immediately adjacent to the irradiated lesion. In cases of equivocal change, a repeat MRI was ordered at a 6 week interval and local failure recorded if there was evidence of progressive disease. Distant brain recurrence was defined as a new enhancing lesion on MRI consistent with metastases or leptomeningeal enhancement outside of the treated PTV. Extracranial progression was defined as development of new lesions, or growth of lesions noted at the time of HFSRT, on any imaging modality of extracranial sites following delivery of HFSRT.
Statistical analyses
The primary endpoint for this study was local control. Other endpoints assessed were distant brain failure and OS. Time to local failure or distant brain recurrence was calculated from the date of the first fraction of HFSRT to the date of imaging confirming recurrence. OS from the date of first HFSRT fraction, date of extracranial failure and modality of any salvage treatment were also recorded. Formal neuro-cognitive data was not collected.
Statistical analysis to test relationships between categorical variables such as histology, treatment in the intact brain metastasis and post-operative settings and patterns of failure was made using χ2 tests or Fisher’s exact tests when χ2 was not appropriate. Survival time to local brain failure, distant brain failure, extracranial failure and OS were estimated by using the Kaplan–Meier method.
Results
Patient and treatment characteristics
A total of 107 metastases in 61 patients were treated. The median age was 63 years (range 24–87) and 61 % were male (Table 1). Forty-nine patients (80 %) had a solitary metastasis with mean tumour diameter of 24 mm (range 6–70). Thirty-three patients had “radio-resistant” histology of melanoma (n = 29) or renal cell carcinoma (n = 4) The most common histology was melanoma, followed by non-small cell lung cancer (n = 10). Forty-one patients (67 %) had extracranial disease present at the time of radiotherapy, with 32 (52 %) having chemotherapy at some point prior to HFSRT.
The primary therapy was HFSRT alone for 29 patients (48 %) and 32 (52 %) had resection of the brain metastasis immediately preceding HFSRT. Eleven had prior intracranial radiotherapy (Table 1). Of the previously irradiated patients, ten had WBRT and one had single fraction SRS. Of the 32 patients who had surgery, 29 patients had a solitary metastasis resected, while the remaining three patients had additional metastases left in situ. Nineteen patients (66 % of the surgical cohort) who underwent resection of a solitary metastasis had no residual disease on post-operative MRI.
Radiotherapy was delivered in a variety of schedules. The median dose was 24 Gy (range 22–40 Gy) in a median of three fractions (range 2–10). Larger lesions above 40 mm were treated with five or more fractions. Patients were followed for a median of 21 months (range 0.5–26) from the date of the first fraction.
Intracranial control
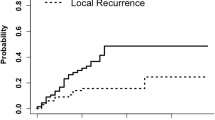
The local control rate was 74 % for the entire cohort (Table 2). Local control was 87 % for post-operative patients with no radiological evidence of residual disease followed by HFSRT compared with 69 % of patients treated with HFSRT alone. Fourteen patients (23 %) failed locally during the study with 43 % experiencing distant brain failure (Table 2). The overall median time post radiotherapy to local failure was 8.6 months (Fig. 1). Two patients had leptomeningeal failure. Both of these patients had surgical resection prior to leptomeningeal failure. The mean time to distant brain failure was 9.9 months (Fig. 2). Local control was independent of treatment program prescribed (p = 0.11) and of histology (p = 0.31). Distant brain relapse was significantly more likely for patients with a “radio-resistant” (melanoma and renal cell) histology compared with other tumour types (p = 0.04) but independent of treatment program prescribed (p = 0.69).
Extracranial control
Thirty patients (49 %) experienced extracranial failure. The median time to extracranial failure was 7.9 months (Fig. 3). There was a near significant (p = 0.051) relationship between the radiotherapy program prescribed and extracranial failure with those who had surgical resection prior to HFSRT having greater proportion of extracranial failure than those who had HFSRT alone.
Survival
Twenty-two patients (36 %) died with median time to death of 4.4 months. Median OS from treatment was 21 months with 1 year OS of 60 % (Fig. 4).
Retreatment
Of the 14 patients with local failure, nine (64 %) underwent salvage therapies including further HFSRT, WBRT and surgery (Table 2). Seventy-six percent of patients who had distant brain failure were suitable for salvage treatment that primarily consisted of further HFSRT (31 %) or WBRT (27 %).
Discussion
This study assessed experience at a single centre in treating intracranial metastases with HFSRT utilising VMAT. Our local control rate of 74 % for all lesions treated compares with other published SRS and HFSRT series, in which 12 month local control ranges from 70–90 % [6, 9, 11, 30]. Single fraction SRS 12 month control rates appear higher than those reported for HFSRT, in excess of 80 % and our data would support this finding [4, 6, 31]. Do et al. [6] and Broemme et al. [30] note that variation in local control rates may be at least partially attributable to discrepancies in the definition and determination of local failure, as difficulty remains in determining progression versus treatment effect or necrosis on imaging.
Our cohort of patients had improved median time to local failure of 8.6 months compared with comparable research which was less than 6 months [11], and near equivalent 12 month OS rate of 60 % [9]. This may reflect our definition of local failure, which required evidence of successive growth of the lesion. In the post-operative setting, our study demonstrates that HFSRT utilising VMAT provides an excellent method of ensuring local control, particularly for patients with no residual disease post-surgery, with a 87 % 12 month local control rate in this group. These results compare favourably to other studies of HFSRT to the surgical bed [9, 11]. Ahmed et al. [11] reported 12 month local control of 86 % for radio-resistant and 88 % for radiosensitive tumours, with no statistically significant difference between the two groups [11].
For intact treated brain metastases treated with HFSRT, similar total dose and fractionation schedules used by Minniti et al. [2] and Ogura et al. [5] reported 12 month local control rates of 88 % and 87 % respectively, with 12 month OS of 65 % in both series. These data are consistent with our findings.
Additionally, our series adds weight to the data that HFSRT offers the potential benefit of overcoming radio-resistance of traditionally resistant tumour types in both the intact and post-operative setting [2, 10, 11, 32]. Whereas other studies have reported that melanoma histology was predictive of local failure [2, 11], we found local failure was independent of histology. This series has the highest proportion of patients with melanoma histology of HFSRT papers in the current literature, which is reflective of high incidence in our region, and our facility being a referral centre for patients with advanced melanoma.
The rate of Leptomeningeal (LM) recurrence in our cohort is low (3.3 %) and comparable with other recent studies [33, 34], and not possible to predict. This rate of LM recurrence has not impacted on planning or target delineation at our centre.
There are a variety of HFSRT regimes in clinical practice and there is a need for prospective, randomised study to determine the optimal regime. Whilst there remains no consensus of optimal dose or fractionation for treatment of brain metastases, our data agrees with Wiggenraad et al. [35] who suggests that for a α/β value of 12 (BED12) for brain metastases, >40 Gy is necessary to achieve a 12 month local rate of greater than 70 % [2, 35]. Our standard fractionation of 24 Gy in three fractions delivers a BED10 of 43.2 Gy and BED12 of 42 Gy, and resulted in a 76 % 12 month local control rate. Märtens et al. [36] reported significant local control advantage with equivalent dose in 2 Gy fractions (EQD2) ≥ 35 Gy [36]. Our study concurs as our common fractionation of 24 Gy in three fractions is equivalent to EQD210 = 36 Gy and EQD212 = 34.3 Gy. Our study supports other retrospective research that have concluded that lesions larger than 25 mm can be safely treated with HFSRT (albeit with more prolonged fractionation) with promising local control rates [2].
Selection of patients has previously been demonstrated as critical as those with poor performance status or uncontrolled extracranial disease do not benefit significantly from radiosurgery to brain metastases [14]. The role of radiation and radiosurgery in the treatment of brain metastases have recently been formulated in an ASTRO evidence-based guideline [4]: In patients with good prognosis and single brain metastasis (<3 cm), either surgery or radiosurgery may be considered and selected patients with brain metastasis(es) may be treated with radiosurgery alone. Our work supports the omission of WBRT in carefully selected patients in the hope of decreasing potential toxicities such as neuro-cognitive dysfunction, somnolence, ataxia, steroid dependent oedema and pituitary dysfunction. However, close surveillance is required to enable early detection of intracranial progression or recurrence, enabling the option of salvage treatment. Whilst 43 % of our patients had distant brain failure, this represents 57 % who have not required any additional intracranial treatment outside of the initially treated metastases. Of those who failed distantly in the brain, 76 % were suitable for salvage treatment. Given the reasonable 12 month survival in our cohort and improving systemic therapies particularly for melanoma, there would be an expectation that more patients may require salvage treatments over time.
Treatment has been well tolerated in our cohort (Table 2). There were no cases of symptomatic radionecrosis. There were four cases of CTCAE v4 Grade 3 toxicity. One patient developed aphasia post-treatment and three patients required hospital admission and prolonged (>1 month) corticosteroid dependence.
We acknowledge the issue of paucity of randomised data regarding comparison of HFRT alone with either surgery or single fraction radiosurgery in the intact brain metastases or post-operative setting [1, 2]. There have historically been study accrual issues with physician and patient bias for choice of treatment modality. Our study, whilst having a comparable number of patients and metastases compared with other retrospective series, is not powered to determine small advantages between treatment regimes, and has the usual limitations of a retrospective study. It is also acknowledged that this study does not report on quality of life, neurological deficits or treatment-related death. However, given the range of patients’ age, gender, number of metastases and primary malignancies, we believe that our results have generalisability, and contributes to the body of knowledge supporting the application of HFSRT utilising VMAT as a useful treatment modality for oligometastatic brain metastases.
Conclusions
Hypofractionated stereotactic radiotherapy utilising VMAT technique demonstrates comparable results to other HFSRT modalities in treatment of brain metastases in terms of high rates of local control at 12 month. The fractionation also enables larger lesions to be treated safely and local control appears independent of histology. Careful selection of patients remains critical.
References
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary J-P (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363(9422):1665–1672
Minniti G, D’Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM, Trodella L (2014) Fractionated stereotactic radiosurgery for patients with brain metastases. J Neuro-Oncol 117(2):295–301
Jenkinson MD, Haylock B, Shenoy A, Husband D, Javadpour M (2011) Management of cerebral metastasis: evidence-based approach for surgery, stereotactic radiosurgery and radiotherapy. Eur J Cancer 47(5):649–655
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis (es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2(3):210–225
Ogura K, Mizowaki T, Ogura M, Sakanaka K, Arakawa Y, Miyamoto S, Hiraoka M (2012) Outcomes of hypofractionated stereotactic radiotherapy for metastatic brain tumors with high risk factors. J Neuro-Oncol 109(2):425–432
Do L, Pezner R, Radany E, Liu A, Staud C, Badie B (2009) Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol* Biol* Phys 73(2):486–491
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neuro-Oncol 75(1):5–14
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol 96(1):45–68
Connolly EP, Mathew M, Tam M, King JV, Kunnakkat SD, Parker EC, Golfinos JG, Gruber ML, Narayana A (2013) Involved field radiation therapy after surgical resection of solitary brain metastases—mature results. Neuro-oncology 328
Nieder C, Grosu AL, Gaspar LE (2014) Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol 9(155):717X–719
Ahmed KA, Freilich JM, Abuodeh Y, Figura N, Patel N, Sarangkasiri S, Chinnaiyan P, Yu H-HM, Etame AB, Rao NG (2014) Fractionated stereotactic radiotherapy to the post-operative cavity for radioresistant and radiosensitive brain metastases. J Neuro-Oncol 118(1):179–186
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol* Biol* Phys 37(4):745–751
Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann R-D, Carrie C (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29(2):134–141
Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, Mills M, Rogers CL, Souhami L (2005) The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol* Biol* Phys 63(1):37–46
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10(11):1037–1044
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H (2006) Stereotactic radiosurgery plus whole-brain radiation therapy versus stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295(21):2483–2491
Ammirati M, Kshettry VR, Lamki T, Wei L, Grecula JC (2014) A prospective phase II trial of fractionated stereotactic intensity modulated radiotherapy with or without surgery in the treatment of patients with 1 to 3 newly diagnosed symptomatic brain metastases. Neurosurgery 74(6):586–594
Rajakesari S, Arvold ND, Jimenez RB, Christianson LW, Horvath MC, Claus EB, Golby AJ, Johnson MD, Dunn IF, Lee EQ (2014) Local control after fractionated stereotactic radiation therapy for brain metastases. J Neuro-Oncol 120(2):339–346
Kilburn JM, Ellis TL, Lovato JF, Urbanic JJ, Bourland JD, Munley MT, McMullen KP, Shaw EG, Tatter SB, Chan MD (2014) Local control and toxicity outcomes in brainstem metastases treated with single fraction radiosurgery: is there a volume threshold for toxicity? J Neuro-Oncol 117(1):167–174
Leeman JE, Clump DA, Wegner RE, Heron DE, Burton SA, Mintz AH (2012) Prescription dose and fractionation predict improved survival after stereotactic radiotherapy for brainstem metastases. Radiat Oncol 7(1):107
Muacevic A, Kreth F, Mack A, Tonn J, Wowra B (2004) Stereotactic radiosurgery without radiation therapy providing high local tumor control of multiple brain metastases from renal cell carcinoma. Minim Invasive Neurosurg 47(4):203–208
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC (1999) Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol* Biol* Phys 45(2):427–434
Vuong DA, Rades D, van Eck AT, Horstmann GA, Busse R (2013) Comparing the cost-effectiveness of two brain metastasis treatment modalities from a payer’s perspective: stereotactic radiosurgery versus surgical resection. Clin Neurol Neurosurg 115(3):276–284
Mayo CS, Ding L, Addesa A, Kadish S, Fitzgerald T, Moser R (2010) Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. Int J Radiat Oncol* Biol* Phys 78(5):1457–1466
Andrevska A, Knight KA, Sale CA (2014) The feasibility and benefits of using volumetric arc therapy in patients with brain metastases: a systematic review. J Med Radiat Sci 61(4):267–276
Awad R, Fogarty G, Hong A, Kelly P, Ng D, Santos D, Haydu L (2013) Hippocampal avoidance with volumetric modulated arc therapy in melanoma brain metastases—the first Australian experience. Radiat Oncol (London, England) 8:62. doi:10.1186/1748-717X-8-62
Ma Y, Li M, Yin Y, Kong L, Sun X, Lin X, Yu J (2010) Hypofractionated stereotactic radiotherapy for brain metastases: a dosimetric and treatment efficiency comparison between volumetric modulated arc therapy and intensity modulated radiotherapy. Technol Cancer Res Treat 9(5):499–507
Ruschin M, Lee Y, Beachey D, Yeboah C, Wronski M, Babic S, Lochray F, Nico A, Khan L, Soliman H, Sahgal A (2015) Investigation of dose falloff for intact brain metastases and surgical cavities using hypofractionated volumetric modulated arc radiotherapy. Technol Cancer Res Treat. doi:10.1177/1533034614567277
Salkeld AL, Unicomb K, Hayden AJ, Van Tilburg K, Yau S, Tiver K (2014) Dosimetric comparison of volumetric modulated arc therapy and linear accelerator-based radiosurgery for the treatment of one to four brain metastases. J Med Imaging Radiat Oncol 58(6):722–728. doi:10.1111/1754-9485.12188
Broemme J, Abu-Isa J, Kottke R, Beck J, Wiest R, Malthaner M, Schmidhalter D, Raabe A, Aebersold D, Pica A (2013) Adjuvant therapy after resection of brain metastases. Strahlenther Onkol 189(9):765–770
Sanghavi SN, Miranpuri SS, Chappell R, Buatti JM, Sneed PK, Suh JH, Regine WF, Weltman E, King VJ, Goetsch SJ (2001) Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol* Biol* Phys 51(2):426–434
Rodrigues G, Zindler J, Warner A, Lagerwaard F (2013) Recursive partitioning analysis for the prediction of stereotactic radiosurgery brain metastases lesion control. Oncologist 18(3):330–335
Ling DC, Vargo JA, Wegner RE, Flickinger JC, Burton SA, Engh J, Amankulor N, Quinn AE, Ozhasoglu C, Heron DE (2015) Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases: clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neurosurgery 76(2):150–157
Strauss I, Corn BW, Krishna V, Shahar T, Matceyevsky D, Gez E, Shtraus N, Ram Z, Kanner AA (2015) Patterns of failure after stereotactic radiosurgery of the resection cavity following surgical removal of brain metastases. World Neurosurg. doi:10.1016/j.wneu.2015.07.073
Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H (2011) Dose–effect relation in stereotactic radiotherapy for brain metastases. Syst Rev. Radiother Oncol 98(3):292–297
Märtens B, Janssen S, Werner M, Frühauf J, Christiansen H, Bremer M, Steinmann D (2012) Hypofractionated stereotactic radiotherapy of limited brain metastases: a single-centre individualized treatment approach. BMC Cancer 12(1):497
Acknowledgments
Portions of this work were presented at the International Stereotactic Radiosurgery Congress, Yokohama, Japan, June 7-11, 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Croker, J., Chua, B., Bernard, A. et al. Treatment of brain oligometastases with hypofractionated stereotactic radiotherapy utilising volumetric modulated arc therapy. Clin Exp Metastasis 33, 125–132 (2016). https://doi.org/10.1007/s10585-015-9762-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9762-x