Abstract
Crop wastes are renewable and abundant lignocellulosic resource, while their effective utilization is limited due to the recalcitrance of plant cell wall. In this study, by using a facile mechanochemical method, reed straw fiber was simultaneously cationized and defibrillated to obtain cationic lignocellulose nanofibers (LCNFs) without organic solvent. The obtained cationic LCNFs were 2–4 nm wide and several micrometers long with excellent re-dispersibility in water arising from the high zeta potential of + 40 mV. As a paper-reinforcement agent, cationic LCNFs could give coated paper good oil resistance with the maximum Kit rating of 12/12. Meanwhile, the mechanical properties of the coated paper were also remarkably enhanced with the tensile strength and Young’s modulus increased by 144% and 124%, respectively. They also gave the paper antibacterial properties because of the presence of quaternary ammonium groups. Overall, this study provides an efficient utilization option for crop wastes as well as a value-added lignocellulosic product.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crop wastes, including various straw, rice husk, corn cob, bagasse, etc., are rich biomass resources, which mainly consist of cellulose, lignin, and hemicellulose. They are potential substitutes for fossil energy because of their renewability, abundance, less pollution and low price (Yan et al. 2020; Zhang et al. 2019a, b). In China, a large amount of crop straw is produced annually, but only a small amount is used effectively because of their recalcitrance. Common reed (Phragmites australis), as one kind of crop wastes, is widespread with annual production reaching millions of tons in Chinese wetlands (Ye et al. 2016). In addition to applications for pulp, animal feed, and weaving, reed straw is always burned to generate low-value heat or discarded in nature, which not only causes a waste of resources, but also further aggravates environmental pollution (Zhang et al. 2016). In order to alleviate the energy crisis and reduce environmental pollution, the exploitation and utilization of biomass resources have attracted people’s attention. The three main components (cellulose, lignin, and hemicellulose ) of biomass are used to prepare target chemicals and fuels by the process of oxidation, hydrogenation, dehydration, pyrolysis, catalytic polymerization, dissolution and biorefining (Cheng et al. 2021; Jiang et al. 2021; Padilla et al. 2021). Nevertheless, these steps are so complicated and the yields of the product are not always satisfactory (Fahmy et al. 2020; McClelland et al. 2017; Pourkarimi et al. 2019; Yang et al. 2019).
In recent years, to achieve innovative valorization of crop wastes, many efforts have been focused on preparing cellulose nanomaterials (CNM) (Priyadarshana et al. 2022; Rajinipriya et al. 2018; Ramadhani et al. 2022; Ventura-Cruz et al. 2021). For instance, the crop wastes of orange bagasse, corn husks, sugarcane straw produced in Brazil, were used to prepare cellulose nanofibers by alkali treatment, bleaching with sodium chlorite and extraction with oxalic acid, followed by sonication. This approach could add new value to crop wastes and might bring great economical valorization to crops production (Marino et al. 2021). Cellulose nanowhiskers were also successfully obtained from agricultural wastes and isolated with a 33% average yield by mild acid treatment (Moreno et al. 2018).
In addition, several studies prepared functional CNM from crop wastes, especially the cationic CNM, such as cationic nanocrystalline cellulose and cellulose nanoparticles (Arnata et al. 2020; Gu et al. 2020). Cationic CNM have received extensive attention recently and were used as antibacterial agent, biological flocculant, adsorbent and emulsion stabilizer for wastewater treatment, papermaking, composite and other fields (Lai et al. 2021; Zhang et al. 2021; Morantes et al. 2019; Sehaqui et al. 2016; Silva et al. 2020). The techniques of preparing cationic CNM mainly rely on a two-step method. For one, the raw material was reacted with cationic agent following by mechanically treating to obtain cationic CNFs (Rol et al. 2019; Ru et al. 2019). For another, the CNM were fabricated firstly, which were then reacted with cationic agent (Wei et al. 2021). Aulin et al. (2010) were the first to obtained cationic CNFs by reacting with 2,3-epoxypropyl trimethylammonium chloride (EPTAC) in water, isopropanol and sodium hydroxide, following with microfluidization. After that, many different preparation processes of cationic CNM were reported and the cationic agents used were 2,3-epoxypropyl trimethylammonium chloride (EPTAC), 3-chloro-2-hydroxypropyltrimethylammonium chloride (CHPTAC), 3-chloro-2-hydroxy-propyl dodecyl dimethyl ammonium, N,N-dimethyl-1-octadecylamine, Girard’s reagent T ((2-hydrazinyl-2-oxoethyl) trimethylazanium chloride, GT), cetyltrimethyl ammonium bromide (CTAB), etc. (Arnata et al. 2020; Keyvani et al. 2021; Lu et al. 2020; Rol et al. 2019). Among these, EPTAC has the highest reaction efficiency and is commonly used in industry (Prado et al. 2014; Zaman et al. 2012).
However, the above method of preparing CNM or cationic CNM always contained delignification and bleaching processes and only cellulose was utilized, which caused a waste of other components. Actually, the lignin component can endow CNM with advanced properties, such as hydrophobicity, UV absorption ability and thermal stability (Bai et al. 2021; Pylypchuk et al. 2021; Shao et al. 2021). Therefore, the lignin-containing cellulose nanomaterials (LCNM) obtained from crop wastes were studied and various techniques have been applied to prepare LCNM based on mechanical methods, chemical treatments and their combination (Liu et al. 2021; Zhou et al. 2023). Among these, commonly used mechanical methods were high pressure homogenization (Tarres et al. 2020; Zhang et al. 2019a, b), ball-milling (Ewulonu et al. 2019) and ultrasonication. For improving the efficiency of mechanical processing, chemical methods were used for pretreatment, such as traditional pulping process, alkali treatment, acid hydrolysis, TEMPO oxidation and enzymatic hydrolysis (Ehman et al. 2016). Other treatment was also designed for preparing LCNM such as pretreating thermomechanical pulp and bagasse in deep eutectic solvent (Jiang et al. 2020; Zou et al. 2022). However, few studies about functional LCNM prepared from crop wastes were reported as far as we know except one report about cationic LCNFs prepared by sugarcane bagasse reacting with glycidyltrimethylammonium chloride and following by high pressure homogenization. There are hardly any researches on one-step preparation of functional LCNM from crop wastes.
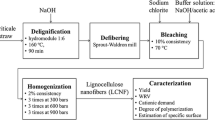
Here, we developed a one-pot method for the preparation of cationic LCNFs from reed straw, which was achieved simply by ball milling reed flour with EPTAC in mild alkaline condition. During ball milling process, the straw fiber was defibrillated and cationized simultaneously. Long nanofibers were obtained only by ball milling for 2 h. The composition, morphology, chemical and crystalline structures of the obtained cationic LCNFs were systematically characterized, and the zeta potential of the cationic LCNF suspension was also tested. Furthermore, the barrier and mechanical properties of paper coated by cationic LCNFs were analyzed.
Experimental
Materials
Reed (Phragmites australis) straw was collected at Baiyang Lake in Hebei Province, China. The root of the straw was removed and the stem was dried at 60 °C and cut into approx. 50 mm-long pieces after being rinsed with water. The straw was pulverized by a waring blender and 80-mesh-pass fraction was collected as flour (Fig. S1). 2,3-epoxypropyl trimethyl ammonium chloride (EPTAC, 95%) was purchased from Innochem (Yinuokai Technology Co., Beijing, China). Deionized water was used in all experiments while Milli-Q water was used for dialysis.
Preparation of cationic LCNFs
Dried reed flour (0.5 g), EPTAC and 10 mL of NaOH solution (3.7 wt%) were added to a 45 mL zirconia pot containing seven zirconia balls (d = 10 mm) and milled by a planetary ball mill (Pulverisette 7, Fritsch, Germany). Control experiment was also performed without EPTAC. Ball-milling was carried out with punctuated operation (working time 20 min and interval of 2 min) with a rotation speed of 300 rpm at room temperature. The milling time was varied from 1 to 12 h. The milled mixture was neutralized to pH = 7 with dilute HCl (3 wt%) and washed with water several times until no free chloride detected in the supernatant. Finally, the product was purified by dialyzing against Milli-Q water for 3 days.
aAmount of hydroxyl group was calculated by 3 m/162 (assuming all solid was cellulose).
Redispersion of dried cationic LCNFs
About 0.3 g freeze-dried cationic LCNFs (1-M4) were dispersed in 100 mL of water by sonication for 2, 4, 10 min in an ice bath by intermittent operation (2 s run-2 s pause at the power of 180 W (Scientz-IID, Ningbo Scientz Biotechnology)) and the cationic LCNF dispersion was obtained. The dispersion was centrifuged at 2000 rpm for 5 min. The sediment and the turbid supernatant were separated by pipetting and dried following by weighing. The yield of LCNFs was calculated according to Eq. (1) as follows:
where ms is the solid weight of supernatant, and M is the total dry weight of redispersed cationic LCNFs.
Compositional analysis
The compositional analysis of raw reed flour and obtained samples were analyzed according to laboratory procedures of Determination of Structural Carbohydrates and Lignin in Biomass by the National Renewable Energy Laboratory (NREL) (Sluiter et al. 2011). Briefly, cellulose and hemicellulose were hydrolyzed into monosaccharides by sulfuric acid, and then the concentration of monosaccharides was detected by external standard method using HPLC to calculated the content of cellulose and hemicellulose. The total content of lignin included acid-soluble lignin and acid-insoluble lignin and the later was calculated by measuring the absorbance of the sample at 320 nm on a UV–visible spectrophotometer.
Morphology analysis
The morphology of the cationic LCNFs was observed by atomic force microscopy (AFM, Bruker Multimode 8, Germany) in ScanAsyst mode to evaluate the extent of defibrillation of the fibers. 5 µL of diluted LCNF suspension (0.01%) was deposited on freshly cleaved mica after sonicating for 2 min, and it was completely dried at room temperature before testing.
The surface morphology of paper coated by cationic LCNFs was observed using Scanning electron microscopy (SEM, Hitachi S-4800, Japan) at 5 kV acceleration voltage. The SEM samples were pre-coated with gold for 60 s using a vacuum-ion sputter-coater (Hitachi MC1000).
Chemical structure analysis
The cationization of the reed flour was characterized by Fourier transform infrared spectroscopy (Varian 3100) with the KBr disc method (1:100 dilution by KBr) for 400–4000 cm− 1 with 64 scans in absorption mode.
X-ray photoelectron spectroscopy (XPS) spectra were obtained with an ESCALAB220i-XL Photoelectron Spectrometer (VG Scientific). A Gaussian curve fitting program was used to analysis the signal of C1s, O1s, N1s and the following binding energies.
Crystalline structures analysis
X-ray diffraction (XRD) patterns of the products were recorded using an X’Pert PRO X-ray diffractometer with Cu Kα radiation (λ = 0.154184 nm) in the 2θ range of 5–50° with increment step of 0.02°. Then, the crystallinity index (CrI) of samples was calculated according to the empirical Eq. (2) (Segal et al. 1959):
where I200 is the maximum peak intensity of the crystalline region of cellulose (2θ = 22.3°), and Iam is the intensity of the amorphous phase (2θ = 18.6°).
Quaternary ammonium group content determination
The content of trimethylammonium chloride groups in cationic LCNFs was measured by elemental analysis of N. Elemental analysis was performed using Vario MACRO cube (Elementar, Germany). The content of quaternary ammonium group in cationic LCNFs was determined by the following Eq. (3):
where Nc is the content of quaternary ammonium group, and the x is the weight% of N element in the cationic LCNFs (x wt%).
Zeta potential
Zeta potential of LCNFs was measured using Zetasizer Nano ZS (Malvern) at the concentration of 0.1 wt%. Each sample was tested three times at 25 °C and the results were averaged.
Application of cationic LCNFs to paper
The cationic LCNF (1-M4) dispersion was coated on the paper (grammage 80 g/m2) surface by vacuum filtration. The coating level was controlled by changing the concentration of LCNF suspensions.
Grease resistance of coated paper
Grease resistance of the paper coated with cationic LCNFs was tested according to the TAPPI T 559 cm-12 standard. The solution containing different ratios of castor oil/heptane/toluene ranging from No. 1 to 12 was dropped on the paper surface and wiped out after 15 s. The results were reported as Kit rating number, where a higher number indicates a better oil resistance performance.
Mechanical properties of coated paper
The mechanical properties of paper with the cationic LCNF coating were measured by an MTS Sintech tensile tester (MTS Sintech, Beijing) with a strain rate of 5 mm/min. Specimen strip was 5 mm wide and 60 mm long with a gauge span of 30 mm. At least five strips were measured and the results were averaged.
Results and discussion
Cationization and defibrillation of reed flour and chemical compositional analysis
Cationic lignocellulose nanofibers (LCNFs) were prepared by a mechanochemical method. During the ball milling process, the lignocellulose fiber would be swelled by NaOH solution and defibrillated under the action of mechanical force, exposing more hydroxyl groups. Meanwhile, hydroxyl groups reacted with EPTAC to increase the electrostatic repulsion between fibers, which in turn could further promote defibrillation of fibers (Fig. 1). As a result, LCNFs were obtained and the compositional analysis of the reed flour and LCNFs was performed (Fig. 2). In reed flour, the content of cellulose, hemicellulose, and lignin was 42%, 19%, and 26%, respectively. With the ball milling time increasing, the content of hemicellulose and lignin was decreased. The hemicellulose content was reduced by 71.4% after ball milling 12 h, since the hemicellulose could be dissolved and removed during milling. In addition, lignin-hemicellulose ester bonds could also be hydrolyzed by the alkaline treatment, which also resulted in the hemicellulose and lignin dissolution (Liu et al. 2019; Ru et al. 2019).
Chemical structure of cationic LCNFs
The reaction mechanism of lignocellulose and EPTAC under alkaline condition is that the alkali-activated hydroxyl groups of lignocellulose react with epoxy groups of EPTAC. Figure 3a, b show FT-IR spectra of reed flour and LCNFs. The chemical structure of the LCNFs is similar to that of the reed flour. The absorption peaks at 1600 cm−1, 1508 cm−1 and 1238 cm−1 are the characteristic peaks of the aromatic structure of lignin, which are the stretching vibration absorption peaks of the aromatic structure skeleton C=O and C=C (McClelland et al. 2017). The absorption peak at 1735 cm−1 represents the carbonyl groups, which are attributed to the hemicellulosic acetate and uronate ester groups, or ester groups of lignin/hemicellulose (Yang et al. 2018). Additionally, the absorption peak at 1735 cm−1 almost disappeared after ball milling, indicating that ester groups were hydrolyzed as well as hemicellulose was lost in alkaline condition evidenced by the hemicellulose content reduced during ball milling (Fig. 2). Appearance of new peak at 1489 cm−1 belongs to the stretching of methyl groups on the quaternary ammonium group (Zaman et al. 2012). The peak at 1418 cm−1 is ascribed to C–N stretching (Pal et al. 2005; Song et al. 2010). These are evidences of successful cationization of reed flour by EPTAC during ball milling.
Additional evidence of the cationization was obtained by X-ray photoelectron spectroscopy (XPS). Results of curve-fitting for the C1s, O1s and N1s region using a Gaussian function are shown in Fig. 4. Compared with the reed flour, a new peak of N1s appeared at 403 eV characteristic for C–N of the substituted ammonium. This further confirms that LCNFs are cationized by EPTAC. The content of quaternary ammonium groups on the fiber surface was tested as shown in Table 1. The content of quaternary ammonium groups on the fiber surface was increased with increasing milling time and the molar ratio of EPTAC/hydroxyl. However, milling more than 8 h and more EPTAC did not give more quaternary ammonium groups on the fiber surface. These could be resulted from the hydrolysis of EPTAC occurred in the reaction system, which would reduce reaction efficiency (Odabas et al. 2016; Zaman et al. 2012). Furthermore, the cationic LCNFs would also hydrolyze in alkaline condition (Moral et al. 2015).
Crystal morphology and crystallinity of cationic LCNFs
The crystal morphology and crystallinity of cationic LCNFs were characterized by X-ray diffraction (XRD) analysis (Fig. 5). It shows that the mechanochemical process did not change the crystal form of the cellulose and the cationic LCNFs maintained cellulose I crystallinity. The peak positions of samples before and after cationization were almost the same, appearing at 14.9°, 16.5° and 22.3° 2θ, respectively, which were corresponding to Miller indices [(1−10), (110) and (200)]. However, the degree of crystallinity decreased from 65.3 to 46.3% after 12 h milling at a constant molar ratio (EPTAC/hydroxyl) of 1. The degree of crystallinity was also decreased with increasing the molar ratio of EPTAC/hydroxyl and the molar ratio of 2 gave a lower crystallinity of 44.8%, which was likely to be associated with the increase of chemically modified cellulose chains on the fibril surfaces.
Morphology of cationic LCNFs
The morphology of prepared samples was examined by AFM (Fig. 6, Fig. S2). The fiber was partial defibrillated after 4 h ball-milling only under alkaline condition, which was same as our previous work (Liu et al. 2019). The addition of EPTAC to the milling system resulted in extensive individualization of the 2–4 nm-wide elementary fibrils. The diameter of lignocellulose fiber decreased with the milling time increasing. In fact, the nanofiber with diameter less than 10 nm was obtained only by milling for 2 h with the assistance of EPTAC. Besides, with the molar ratio of EPTAC/hydroxyl increasing, the diameter of lignocellulose fiber also decreased and became uniform. Since the content of quaternary ammonium groups on the fiber surface increased with the molar ratio of EPTAC/hydroxyl and milling time increasing, it can be concluded that the cationic groups introduced by EPTAC should be effective in separation of fibers by electrostatic mutual repulsion. In the AFM images, the minute particles attaching to LCNFs or distributing in the background were lignin particles (Fig. 6b, c) because alkali-soluble lignin could be precipitated and agglomerate into particles when neutralized.
Zeta potential of cationic LCNF suspensions
Figure 7a and b show the variation of the zeta potential of LCNFs with the milling time and molar ratio of the EPTAC/hydroxyl, respectively. It can be seen that the zeta potential of LCNFs is negative (−16 mV) after alkaline milling of the reed flour without EPTAC, which was consistent with our previous work (Liu et al. 2019). In contrast, the zeta potential of LCNFs became positive (+ 30∼ + 40 mV) by milling with EPTAC and the maximum of zeta potential reached at + 40.8 mV. Furthermore, the variation trend of the zeta potential was almost consistent with the variation trend of the quaternary ammonium group content, which further proved that the lignocellulose reacted with the EPTAC. Meanwhile, the introduced high electrostatic repulsion was beneficial to improving the stability of LCNF suspensions. Introduction of positive charge caused remarkable effect on re-dispersibility of LCNFs. Figure 7c–e show the AFM images of redispersed cationic LCNFs (1-M4) by sonication for different time. It was found cationic LCNFs could be quickly redispersed in water by short-time sonication (4 min, the yield reached 96%). The fibril width of redispersed LCNFs was around 4 nm, demonstrating excellent re-dispersibility and facilitating their storage and transportation.
Cationic LCNFs applied to paper
One application of the cationic LCNFs is papermaking. When the cationic LCNFs were coated on the paper surface, the pores on the surface of paper were gradually covered with the increasing coating weight of the cationic LCNFs (Fig. S3). The fiber on the surface of paper was completely covered with cationic LCNFs and the fiber outline became blurred when the coating weight of the cationic LCNFs was 5 g/m2. When the coating weight was more than 3 g/m2, the cationic LCNFs formed a dense layer on the paper surface as Fig. S4 shows and the barrier property of paper was greatly improved. The air permeability was decreased significantly with the increasing coating weight of cationic LCNFs (Table S1) and the air permeability was lower than 0.01 μm/(Pa s) at the coating weight of 3 g/m2, which confirmed again that cationic LCNFs formed a barrier layer on the surface of the paper. When cationic LCNFs were applied to paper products, the lignin component was expected to improve the hydrophobicity of paper (contact angle reached 80°, Fig. S5).
The formed dense layer of cationic LCNFs can block oil penetration and the oil resistance of coated paper was characterized by Kit rating. The variation of the Kit rating with the coating weight was shown in Fig. 8a. There is a sharp increase in the Kit rating when the cationic LCNFs were coated on the paper surface and the Kit rating reached the maximum of 12/12 at the coating weight of 5 g/m2, giving the paper excellent oil-proof performance. The cationic LCNF coated paper-based container had the capability to hold rape oil for 5 days without leakage (Fig. S6). This mainly benefited from the dense layer formed by LCNFs and the positive charge on the LCNFs, which cannot only block the access of oil to penetrate the paper, but also generate electrostatic interactions with oleic acid molecules that prevented oil from permeating and transferring (Long et al. 2015).
The mechanical properties of the paper with and without cationic LCNF coating were tested as the Fig. 8b show. The tensile strength of the paper increased with the increasing coating weight. The tensile strength of paper with the coating weight of 9 g/m2 (22.5 ± 0.9 MPa) was far beyond that of paper without coated (9.6 ± 0.3 MPa). Meanwhile, the Young’s modulus of the coated paper also significantly increased with the increasing coating weight. The Young’s modulus of paper without coating was 0.8 ± 0.2 GPa while the paper with the coating weight of 9 g/m2 reached at 1.9 ± 0.1 GPa. It suggested that the cationic LCNFs can strengthen the interactions between cellulose fibers in paper. This was mainly due to the electrostatic interactions and hydrogen bonds formed between the paper fibers and LCNFs. The quaternary ammonium groups of LCNFs were expected to bind with negatively charged hydroxyl on the paper fiber surface through electrostatic interactions and hydrogen bonds can be formed between the unreacted hydroxyl groups of LCNFs and the hydroxyl groups on the paper fiber surface. The cationic LCNFs acted as a bridge in paper fibers through dual interactions, which leads to the mechanical properties of the coated paper significantly improved. Accordingly, the cationic LCNFs can be used as effective paper-strengthening agent. Moreover, the coated paper was also with good antibacterial property and the growth reduction rate against E. coli was 93% (Fig. S7), which was conducive to expanding the application of paper.
Conclusion
Cationic LCNFs were successfully prepared by the one-pot reaction of reed flour and EPTAC under mild alkaline condition. The obtained cationic LCNFs were 2–4 nm wide and several micrometers long. The suspension of cationic LCNFs with a high zeta potential of + 40 mV showed high dispersion stability and easy re-dispersibility. When cationic LCNFs were coated on paper surface, the hydrophobicity and oil resistance of paper were significantly improved. In addition, the tensile strength and Young’s modulus of the coated paper can be increased by 144% and 124%, respectively. The cationic LCNFs also endow paper with antibacterial property. This study provides a facile mechanochemical method to achieve value-added material from crop straw.
Data availability
All relevant data are included in this Article and its Supplementary Information.
Files.
References
Arnata IW, Suprihatin S, Fahma F, Richana N, Sunarti TC (2020) Cationic modification of nanocrystalline cellulose from sago fronds. Cellulose 27:3121–3141. https://doi.org/10.1007/s10570-019-02955-3
Aulin C, Johansson E, Wagberg L, Lindstrom T (2010) Self-organized films from cellulose I nanofibers using the layer-by-layer technique. Biomacromolecules 11:872–882. https://doi.org/10.1021/bm100075e
Bai FT, Dong TT, Chen W, Wang JL, Li XS (2021) Nanocellulose hybrid lignin complex reinforces cellulose to form a strong, water-stable lignin-cellulose composite usable as a plastic replacement. Nanomaterials 11:3426. https://doi.org/10.3390/nano11123426
Cheng J, Xie M, Xu L, Zhang L, Ren XH (2021) Chlorine release from co-pyrolysis of corn straw and lignite in nitrogen and oxidative pyrolysis. Energies 14:8827. https://doi.org/10.3390/en14248227
Ehman NV, Tarres Q, Delgado-Aguilar M, Vallejos ME, Felissia F, Area MC, Mutje P (2016) From pine sawdust to cellulose nanofibers. Cellul Chem Technol 50:361–367
Ewulonu CM, Liu XR, Wu M, Huang Y (2019) Ultrasound-assisted mild sulphuric acid ball milling preparation of lignocellulose nanofibers (LCNFs) from sunflower stalks (SFS). Cellulose 26:4371–4389. https://doi.org/10.1007/s10570-019-02382-4
Fahmy TYA, Fahmy Y, Mobarak F, El-Sakhawy M, Abou-Zeid RE (2020) Biomass pyrolysis: past, present, and future. Environ Dev Sustain 22:17–32. https://doi.org/10.1007/s10668-018-0200-5
Gu HM, Gao X, Zhang H, Chen KL, Peng LC (2020) Fabrication and characterization of cellulose nanoparticles from maize stalk pith via ultrasonic-mediated cationic etherification. Ultrason Sonochem 66:104932. https://doi.org/10.1016/j.ultsonch.2019.104932
Jiang J, Carrillo-Enriquez NC, Oguzlu H, Han XS, Bi R, Song MY, Saddler JN, Sun RC, Jiang F (2020) High production yield and more thermally stable lignin-containing cellulose nanocrystals isolated using a ternary acidic deep eutectic solvent. ACS Sustain Chem Eng 8:7182–7191. https://doi.org/10.1021/acssuschemeng.0c01724
Jiang HF, Lu R, Luo XL, Si XQ, Xu J, Lu F (2021) Molybdenum-catalyzed deoxygenation coupling of lignin-derived alcohols for functionalized bibenzyl chemicals. Chem Eur J 27:1292–1296. https://doi.org/10.1002/chem.202003776
Keyvani P, Nyamayaro K, Mehrkhodavandi P, Hatzikiriakos SG (2021) Cationic and anionic cellulose nanocrystalline (CNC) hydrogels: a rheological study. Phys Fluids 33:043102. https://doi.org/10.1063/5.0046291
Lai P, Yu S (2021) Cationic cellulose nanocrystals-based nanocomposite hydrogels: achieving 3D printable capacitive sensors with high transparency and mechanical strength. Polymers 13:688. https://doi.org/10.3390/polym13050688
Liu X, Li Y, Ewulonu CM, Ralph J, Xu F, Zhang Q, Wu M, Huang Y (2019) Mild alkaline pretreatment for isolation of native-like lignin and lignin-containing cellulose nanofibers (LCNFs) from crop waste. ACS Sustain Chem Eng 7:14135–14142. https://doi.org/10.1021/acssuschemeng.9b02800
Liu K, Du H, Zheng T, Liu W, Zhang M, Liu H, Zhang X, Si C (2021) Lignin-containing cellulose nanomaterials: preparation and applications. Green Chem 23:9723–9746. https://doi.org/10.1039/d1gc02841c
Long Z, Wu M, Peng H, Dai L, Zhang D, Wang J (2015) Preparation and oil-resistant mechanism of Chitosan/Cationic starch oil-proof paper. BioResources 10:7907–7920. https://doi.org/10.15376/biores.10.4.7907-7920
Lu Z, An X, Zhang H, Liu L, Dai H, Cao H, Lu B, Liu H (2020) Cationic cellulose nano-fibers (CCNF) as versatile flocculants of wood pulp for high wet web performance. Carbohydr Polym 229:115434. https://doi.org/10.1016/j.carbpol.2019.115434
Marino MA, Cypriano D, Tasic L (2021) Agroindustry residues as a source for cellulose nanofiibers production. J Braz Chem Soc 32:878–888. https://doi.org/10.21577/0103-5053.20200239
McClelland DJ, Motagamwala AH, Li Y, Rover MR, Wittrig AM, Wu C, Buchanan JS, Brown RC, Ralph J, Dumesic JA, Huber GW (2017) Functionality and molecular weight distribution of red oak lignin before and after pyrolysis and hydrogenation. Green Chem 19:1378–1389. https://doi.org/10.1039/c6gc03515a
Moral A, Aguado R, Ballesteros M, Tijero A (2015) Cationization of alpha-cellulose to develop new sustainable products. Int J Polym Sci 2015:380–388. https://doi.org/10.1155/2015/283963
Morantes D, Munoz E, Kam D, Shoseyov O (2019) Highly charged cellulose nanocrystals applied as a water treatment Flocculant. Nanomaterials 9:272. https://doi.org/10.3390/nano9020272
Moreno G, Ramirez K, Esquivel M, Jimenez G (2018) Isolation and characterization of nanocellulose obtained from industrial crop waste resources by using mild acid hydrolysis. J Renew Mater 6:362–369. https://doi.org/10.7569/jrm.2017.634167
Odabas N, Amer H, Bacher M, Henniges U, Potthast A, Rosenau T (2016) Properties of cellulosic material after cationization in different solvents. ACS Sustain Chem Eng 4:2295–2301. https://doi.org/10.1021/acssuschemeng.5b01752
Padilla R, Koranchalil S, Nielsen M (2021) Homogeneous catalyzed valorization of furanics: a sustainable bridge to fuels and chemicals. Catalysts 11:1371. https://doi.org/10.3390/catal11111371
Pal S, Mal D, Singh RP (2005) Cationic starch: an effective flocculating agent. Carbohydr Polym 59:417–423. https://doi.org/10.1016/j.carbpol.2004.06.047
Pourkarimi S, Hallajisani A, Alizadehdakhel A, Nouralishahi A (2019) Biofuel production through micro- and macroalgae pyrolysis - a review of pyrolysis methods and process parameters. J Anal Appl Pyrolysis 142:104599. https://doi.org/10.1016/j.jaap.2019.04.015
Prado HJ, Matulewicz MC (2014) Cationization of polysaccharides: a path to greener derivatives with many industrial applications. Eur Polym J 52:53–75. https://doi.org/10.1016/j.eurpolymj.2013.12.011
Priyadarshana R, Kaliyadasa PE, Ranawana S, Senarathna KGC (2022) Biowaste management: banana fiber utilization for product development. J Nat Fibers 19:1461–1471. https://doi.org/10.1080/15440478.2020.1776665
Pylypchuk I, Selyanchyn R, Budnyak T, Zhao Y, Lindstrom M, Fujikawa S, Sevastyanova O (2021) “Artificial wood” lignocellulosic membranes: influence of Kraft Lignin on the properties and gas transport in tunicate-based nanocellulose composites. Membranes 11:204. https://doi.org/10.3390/membranes11030204
Rajinipriya M, Nagalakshmaiah M, Robert M, Elkoun S (2018) Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustain Chem Eng 6:2807–2828. https://doi.org/10.1021/acssuschemeng.7b03437
Ramadhani DV, Holilah H, Bahruji H, Jadid N, Oetami TP, Jalil AA, Asranudin A, Ediati R, Masruchin N, Suryanegara L, Prasetyoko D (2022) Effect of lignocellulosic composition of Reutealis trisperma waste on nanocrystalline cellulose properties. Biocatal Agric Biotechnol 45:102516. https://doi.org/10.1016/j.bcab.2022.102516
Rol F, Saini S, Meyer V, Petit-Conil M, Bras J (2019) Production of cationic nanofibers of cellulose by twin-screw extrusion. Ind Crops Prod 137:81–88. https://doi.org/10.1016/j.indcrop.2019.04.031
Ru J, Tong C, Chen N, Shan P, Zhao X, Liu X, Chen J, Li Q, Liu X, Liu H, Zhao Y (2019) Morphological and property characteristics of surface-quaternized nanofiberlated cellulose derived from bamboo pulp. Cellulose 26:1683–1701. https://doi.org/10.1007/s10570-018-2146-z
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Sehaqui H, Mautner A, Perez de Larraya U, Pfenninger N, Tingaut P, Zimmermann T (2016) Cationic cellulose nanofibers from waste pulp residues and their nitrate, fluoride, sulphate and phosphate adsorption properties. Carbohydr Polym 135:334–340. https://doi.org/10.1016/j.carbpol.2015.08.091
Shao HJ, He L, Xiang L, Tang K, Li XZ, Qi JQ, Xie JL (2021) Transparent and UV-absorbing nanocellulose films prepared by directly dissolving microwave liquefied bamboo in TBAA/DMSO co-solvent system. Ind Crops Prod 171:113899. https://doi.org/10.1016/j.indcrop.2021.113899
Silva CEP, Tam KC, Bernardes JS, Loh W (2020) Double stabilization mechanism of O/W pickering emulsions using cationic nanofiberlated cellulose. J Colloid Interface Sci 574:207–216. https://doi.org/10.1016/j.jcis.2020.04.001
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass. NREL/TP-510-42618
Song Y, Zhang J, Gan W, Zhou J, Zhang L (2010) Flocculation properties and antimicrobial activities of quaternized celluloses synthesized in NaOH/urea aqueous solution. Ind Eng Chem Res 49:1242–1246. https://doi.org/10.1021/ie9015057
Tarres Q, Oliver-Ortega H, Boufi S, Pelach MA, Delgado-Aguilar M, Mutje P (2020) Evaluation of the fibrillation method on lignocellulosic nanofibers production from eucalyptus sawdust: a comparative study between high-pressure homogenization and grinding. Int J Biol Macromol 145:1199–1207. https://doi.org/10.1016/j.ijbiomac.2019.10.046
Ventura-Cruz S, Tecante A (2021) Nanocellulose and microcrystalline cellulose from agricultural waste: review on isolation and application as reinforcement in polymeric matrices. Food Hydrocolloids 118:106771. https://doi.org/10.1016/j.foodhyd.2021.106771
Wei P, Chen W, Song Q, Wu Y, Xu Y (2021) Superabsorbent hydrogels enhanced by quaternized tunicate cellulose nanocrystals with adjustable strength and swelling ratio. Cellulose 28:3723–3732. https://doi.org/10.1007/s10570-021-03776-z
Yan JP, Oyedeji O, Leal JH, Donohoe BS, Semelsberger TA, Li CL, Hoover AN, Webb E, Bose EA, Zeng YN, Williams CL, Schaller KD, Sun N, Ray AE, Tanjore D (2020) Characterizing variability in lignocellulosic biomass: a review. ACS Sustain Chem Eng 8:8059–8085. https://doi.org/10.1021/acssuschemeng.9b06263
Yang X, Berthold F, Berglund LA (2018) Preserving cellulose structure: delignified wood fibers for paper structures of high strength and transparency. Biomacromolecules 19:3020–3029. https://doi.org/10.1021/acs.biomac.8b00585
Yang WS, Du X, Liu W, Wang ZW, Dai HQ, Deng YL (2019) Direct valorization of lignocellulosic biomass into value-added chemicals by polyoxometalate catalyzed oxidation under mild conditions. Ind Eng Chem Res 58:22996–23004. https://doi.org/10.1021/acs.iecr.9b05311
Ye S, Laws EA, Costanza R, Brix H (2016) Ecosystem service value for the common reed wetlands in the Liaohe Delta, Northeast China. Open J Ecol 6:129–137. https://doi.org/10.4236/oje.2016.63013
Zaman M, Xiao H, Chibante F, Ni Y (2012) Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr Polym 89:163–170. https://doi.org/10.1016/j.carbpol.2012.02.066
Zhang L, Liu Y, Hao L (2016) Contributions of open crop straw burning emissions to PM2.5 concentrations in China. Environ Res Lett 11:014014. https://doi.org/10.1088/1748-9326/11/1/014014
Zhang N, Tao P, Lu YX, Nie SX (2019a) Effect of lignin on the thermal stability of cellulose nanofibers produced from bagasse pulp. Cellulose 26:7823–7835. https://doi.org/10.1007/s10570-019-02657-w
Zhang Y, He H, Liu Y, Wang Y, Huo F, Fan M, Adidharma H, Li X, Zhang S (2019b) Recent progress in theoretical and computational studies on the utilization of lignocellulosic materials. Green Chem 21:9–35. https://doi.org/10.1039/c8gc02059k
Zhang Q, Wang M, Mu G, Ren H, He C, Xie Q, Liu Q, Wang J, Cha R (2021) Adsorptivity of cationic cellulose nanocrystals for phosphate and its application in hyperphosphatemia therapy. Carbohydr Polym 255:117335. https://doi.org/10.1016/j.carbpol.2020.117335
Zhou L, Liu X, Jiang S, Wang X, Meng Z, Li X, Chen G, Wang S, Jiang Y (2023) In-situ microstructure regulation towards feasible production of self-reinforced lignocellulose nanopaper with multifunctionality. Ind Crops Prod 193:116229. https://doi.org/10.1016/j.indcrop.2022.116229
Zou X, Yao L, Zhou S, Chen G, Wang S, Liu X, Jiang Y (2022) Sulfated lignocellulose nanofiber based composite aerogel towards adsorption-photocatalytic removal of tetracycline. Carbohydr Polym 296:119970. https://doi.org/10.1016/j.carbpol.2022.119970
Funding
This study was supported by the National Natural Science Foundation of China (No. 52073291).
Author information
Authors and Affiliations
Contributions
JL: Investigation, Data curation, Formal analysis, Methodology, Writing-original draft. PX: Investigation and Methodology. SK: Conceptualization, Writing-review and editing. MW: Writing-review and editing, Supervision, Funding acquisition. YH: Supervision, Writing-review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Xiao, P., Kuga, S. et al. Preparation of cationic lignocellulose nanofibers from reed straw via mechanochemical method and its application. Cellulose 30, 7251–7264 (2023). https://doi.org/10.1007/s10570-023-05328-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05328-z