Abstract
The processing of recycled paper into packaging materials is becoming one of the most important activities of paper mills. However, the use of recycled paper as a raw material causes an important increase of dissolved colloidal substances in industrial waters, known as anionic trash, which greatly increases water conductivity and cationic demand disturbing the function of commonly used retention agents (cationic starch, cationic polyacrylamides). On the other hand, several investigators showed that lignocellulosic nanofibers (LCNF) can be used as reinforcement in papermaking, but their retention can be affected by anionic trash. This work aims to study the technical viability of the application of triticale straw lignocellulose nanofibers in recycled fiber suspensions at industrial scale. For this purpose, a complex retention system of LCNF was proposed to improve the reinforcement efficiency of LCNF. Results show that, with the addition of only 1.5% (w/w) of LCNF, it is possible to fulfill the physical–mechanical requirements of the commercial test liner, and the addition of 4.5% of LCNF would allow the reduction of basis weight and additives or the development of applications with higher mechanical requirements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recycling paper is a great solution for the reduction of virgin fiber use for packaging papers production like test liners. The use of recycled paper as a raw material for packaging papers presents several advantages in terms of environmental, economic, and social aspects (Hubbe 2014). However, the use of this kind of raw material also has a series of disadvantages. The most important of these disadvantages is the introduction in the system of a variety of contaminants in large amounts (Garver et al. 1997). On the other hand, the high demand of recovered papers, actually 54% of worldwide fibers used (Pöyry 2011); contributes to an important decrease in fiber properties. This reduction in the bonding capacity of fibers is produced during the recycling process due to the hornification phenomena. Usually, this reduction in fiber properties is compensated by the addition of virgin fibers and by mechanical refining (Hubbe et al. 2007).
Recently, several authors studied new strategies for the improvement of mechanical properties without producing morphological fiber damage. Some strategies to reduce or replace mechanical refining include the use of virgin fibers from agroforestry waste (Hurter 2002a, 2002b), enzymatic refining (Delgado-Aguilar et al. 2015a, b, c; González et al. 2012), new strategies of chemical-based bonding (Hubbe 2014) and the addition of cellulose nanofibers (Boufi et al. 2016; Tarrés et al. 2017). Cellulose nanofibers are one of the most studied alternatives in terms of publications and patents (Afra et al. 2013; Hietala et al. 2015; Sehaqui et al. 2013). However, it is known that the addition of cellulose nanofibers in papermaking requires a retention system for its correct operation (Tarrés et al. 2017). It is expected that the presence of colloidal dissolved matter (named as anionic trash) will present interactions with the commonly used retention agents with opposite charge (cationic starch, cationic polyacrylamides). To avoid this effect, the so-called “anionic trash catchers” are usually added to neutralize the negative dissolved and colloidal substances in the slurries. Usually, these substances can be subdivided into inorganic and low molecular weight organic salts, dissolved anionic organic compounds with medium molecular weight and anionic colloidal materials. The presence of these electrolytes reduces the effectiveness of cationic polymers (cationic starch or cationic polyacrylamides) by the reduction of their electrostatic interaction with fibers. The salts increase the conductivity of white waters and act by shielding the electrostatic charges modifying polyelectrolytes conformation (Swerin and Ödberg 1996). These substances were introduced in white waters from wood as carbohydrates or from industrial processes in papermaking (coatings, reinforcement agents). Also it is widely known, that the use of mineral fillers in papermaking produces an important increase in some paper properties such as surface smoothness, opacity or light scattering. Nevertheless, the retention of mineral fillers is classically performed by polyelectrolytes. The not complete retention of these mineral fillers and polyelectrolytes (cationic or anionic polyacrylamide, cationic starch, alkenyl succinic anhydrous) cause the accumulation of dissolved and colloidal substances in the water system. In this sense, some studies present the possibility of the use cof ellulose nanofibers in the fillers retention in papermaking (Korhonen and Laine 2014; Lourenço et al. 2017).
The aim of this study was to demonstrate the technical viability of the application of triticale straw LCNF in recycled fiber suspensions for the manufacture of test liner paper on an industrial scale. For this purpose, low-cost nanofibers have been selected and recycled fibers have been applied to an industrial-grade suspension with a reasonable anionic trash content. The presence of anionic trash decreases the bonding capacity of nanofibers. Therefore, a dual system for anionic trash management compatible with the retention of the nanofibres in the paper sheet has been designed.
Materials and methods
Materials
Triticale straw was obtained from the Fundació Mas Badia S.A, La Tallada d’empordà, Girona, Spain. Papelera de la Alquería S.A, Girona, Spain supplied the test liner recycled paper and the test liner recycled pulp. The pulp sample and process water were obtained from the machine chest at the time of paper manufacture. Process waters were characterized by pH, conductivity and cationic demand.
Cationic starch, colloidal silica, aluminum polychloride, anionic polyacrylamide and poly DADMAC were provided by LC Paper S.A., Besalú, Spain and characterized by charge density, equivalent molecular mass (MME) and density of ionic groups (Dm).
Triticale LCNF preparation and characterization
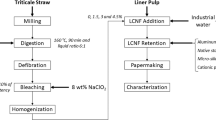
Lignocellulose nanofibers from triticale straw were prepared as described by Boufi and Gandini 2015. The best results obtained in a previous work were used to produce triticale straw LCNF (Tarrés et al. 2017). Fibers were cooked in the presence of 7% of NaOH at 160 °C for 90 min, with a liquid/solid ratio of 6 followed by a step of defibration in a Sprout-Waldron mill and a delignification stage in presence of 8% of NaClO2 for 1 h at 10 wt. % of consistency and 70 ◦C under constant stirring. The fibers were then destructured by passing through a high-pressure homogenizer at a consistency of 2% using a cycle of three steps at 300 bar, three steps at 600 bars and three steps at 900 bars. The characterization of LCNF followed the protocol explained in Espinosa et al. (Espinosa et al. 2016).
Cationic demand (µeq g/g) of the LCNF was obtained by polyDADMAC titration, carboxyl group content (µeq g/g) by conductimetric titration, and yield (%) by centrifugation. Transmittance (%) of LCNF suspensions was determined using a UV–Vis Shimadzu spectrophotometer UV-160A at 12 kV at 800 nm of wavelength. A detailed description of the technics can be found in Espinosa et al. (Espinosa et al. 2016).
The content of carboxylic groups and the cationic demand of the LCNF were used to calculate the average surface area of nanofibrillated pulp and the average diameter of nanofibers. A detailed description of the methodology of LCNF preparation and characterization can be found in a previous work (Tarrés et al. 2017).
A methodological scheme of lignocellulose nanofibers preparation and characterization is shown in Fig. 1.
Retention agents characterization
The charge density of the retention agents was calculated by charge titration with a Mütek PCD 04 particle charge detector (BTG Instruments, Germany). Retention agent solution was driven to 0.01 wt.% of concentration for its titration. Then, 10 ml of diluted solution was titrated with the corresponding standard polymer solution, poly-dadmac 0.001 N for anionic agents and Pes-Na 0.001 N in cationic agents cases (Carrasco et al. 1998). The equivalent molecular weight (MME) was calculated using the values obtained for charge density titration by the following equation:
where C is retention agent concentration (expressed in g/L), V 1 is the volume of retention agent titrated (L), N is the standard polymer concentration (eq g/L), V 2 is the required volume in the titration (L) and f is the activity factor of the standard polymer.
The ionic group’s density (Dm) is defined as the ionic group’s number for one gram of sample. Dm was calculated by the following equation:
where N A is the Avogadro number.
Lignocellulose nanofibers incorporation into a test-liner pulp with the classic and complex retention system
Classic retention system
The retention system consisted of the addition of cationic starch and colloidal silica together. Test-liner pulp was dispersed in water at 1.5% of consistency during 20 min at 3000 rpm in a laboratory pulper. Then, 3% of LCNF was added into the pulper and dispersed during 1 h at 3000 rpm in order to ensure the correct LCNF dispersion in the pulp suspension. After dispersion, pulp containing LCNF was stirred for 30 min at 500 rpm in the presence of 0.5 w/w % cationic starch and 0.8 w/w % of colloidal silica.
Complex retention system
The complex retention system, based on the combination of cationic and anionic polymers, was added following the methodology kindly supplied by LC Paper S.A (Besalú, Spain). After LCNF dispersion on pulp suspension, 6.7 w/w % of Poli-Dadmac, 0.5 w/w % of cationic starch, and 0.5 w/w % of aluminum polychloride were added, stirring for 20 min at 500 rpm. Then, 10.5 w/w % of anionic polyacrylamide and 0.8 w/w % of colloidal silica were added under stirring 10 min at 500 rpm.
In both cases, laboratory sheets were formed in a Rapid-Köthen sheet former (ISP mod. 786 FH) following the standard method (ISO 2008b).
Paper and pulps characterization
Pulp drainage was evaluated by the Schopper Riegler degree (ISO 1999).
Paper sheets were conditioned at 23 °C and 50% moisture (ISO 1990). The thickness of the sheets was determined using a deadweight micrometer (ISO 2011). The percentage of void volume was calculated as: \( Porosity \left( \% \right) = 100x(1 - \rho_{sample} /\rho_{cellulose} \)); where “ρsample” is the density of the paper hand sheet (calculated from basis weight, thickness, and area) and “ρcellulose” is the density of cellulose, assumed to be 1.5 g/cm3. Gurley air flow resistance (ISO 2013) was also determined. Tensile index and breaking length (ISO 2008a) were determined using an Instron universal testing machine provided with 2.5 kN load cell and is reported as “Tensile Load” (σtP), i.e., the ratio of the maximum tensile force applied to the width of the test piece. Modulus of elasticity (Young’s Modulus, EtP) is also reported. The internal bond was determined by a Scott Bond tester (model IBT 10A IDM) (TAPPI 2014), Burst index by a Mullen Tester (mod. EM-50IDM) (ISO 2014), and Tear Index by an Elmendorf Tearing Tester (mod. F53.98401 Frank PTI) (ISO 2012).
Results and discussion
Lignocellulose nanofibers in the laboratory system
Lignocellulose nanofibers (LCNF) from triticale straw were produced as described in previous work (Tarrés et al. 2017). Triticale LCNF with different chemical compositions, LCNF-0, LCNF-1, LCNF-2 and LCNF-3 with 8.42, 5.74, 1.23 and 0.54% of lignin, respectively, were tested in the laboratory produced liner paper. Drainage rate of test liner suspension in tap water was 31 ºSR and the breaking length of isotropic sheets was 3552 m. The obtained results with 3% addition of each LCNF using cationic starch and silica as retention agents are shown in Fig. 2.
Triticale LCNF-1 has shown the highest enhancement of the mechanical properties of paper. LCNF-1 characteristics were: 5.74% of lignin and 12.78% of hemicelluloses, a theoretical diameter of 34 nm, a nanofibrillation yield of 21.3%, and a transmittance at 800 nm of 32.2% (Tarrés et al. 2017). The use of 3% of LCNF-1 in the test liner suspension increased breaking length in 54%. The increase of mechanical properties is inferior to that obtained when 3% of LCNF-1 was added to a bleached kraft eucalyptus pulp (98.7% of increase), as observed in previous works (Delgado-Aguilar et al. 2015a, b, c; Espinosa et al. 2016). Recycled pulp always has less strength than virgin pulp because the damage on fiber properties caused during the recycling process, mechanical refining, and hornification reduces the bonding capacity of fibers (Fernandes Diniz et al. 2004; Hubbe et al. 2007). The presence of high amounts of fines also reduces the reinforcement capacity of cellulose nanofibers. It was reported that the presence of high amounts of fines in pulp suspensions interferes with the beneficial effect of CNF addition on the breaking length of the resulting papers (Delgado-Aguilar et al. 2016). Results also show that there is a linear relationship between the cationic demand of LCNF and the breaking length of test liner when 3% of each LCNF is added as a dry strength additive (Fig. 2). This effect is directly related to the bonding capacity of lignocellulose nanofibers since cationic demand depends on their specific surface (198 µeq g/g and 73.44 m2/g, respectively, in the case of LCNF-1) (Espinosa et al. 2016; Rouger and Mutje 1984). LCNF-1 was used in the following sections.
Lignocellulose nanofibers addition when using industrial water
Lignocellulose nanofibers addition in industrial water was tested using a classic retention system. The industrial water characteristics were: pH of 6.43, high conductivity (4310 µS/cm), and high charge density (− 8580 µeq/L). The high conductivity and charge density of this kind of water are caused by the presence of high amounts of dissolved and colloidal substances. These substances, usually called “anionic trash”, can be divided into inorganic and organic salts, dissolved anionic organic compounds, and anionic colloidal material (Neimo 1999). The obtained results are shown in Tables 1 and 2.
The addition of 3% LCNF in the test liner pulp cause a considerable effect on physical properties of paper hand sheets. Sheets density (calculated as basis weight divided by thickness), increased 10.2% by the addition of 3% of lignocellulose nanofibers, which can be related to the high number of hydrogen bonds generated between fibers and nanofibers. Density increase implies a reduction of the porosity of hand sheets.
Table 2 shows that the use of 3% of LCNF-1 in industrial water produces an important decrease in the drainability of the pulp suspension (61 ºSR versus 44 ºSR). This reduction in drainage was related to the presence of LCNF in the pulp suspension (Delgado-Aguilar et al. 2015a, b, c), but also with the “anionic trash” in the industrial water (Miao et al. 2013). The anionic trash decreases the runnability of the paper machine and also has an adverse effect on the sheet formation (Zhang et al. 1999). The interference of the anionic trash also reduces breaking length at 10% compared with the value of test liner pulp 3552 m.
Even if the addition of 3% LCNF-1 improves all properties, the use of a classic retention system in industrial water produces an increase in mechanical properties of paper hand sheets lower than expected. The efficiency of the cationic starch added to retain LCNF resulted in less because industrial waters have negative electrostatic charged substances named “anionic trash”. This phenomenon was also observed in the paper industry when cationic chemicals are added to improve mechanical properties (Desharnais et al. 2002; Dunham et al. 2002; Whipple and Maltesh 2002).
Figure 3 shows a dual model of cationic starch and colloidal silica interaction with the anionic trash present in the pulp slurry. This interaction reduces dramatically LCNF retention, implying less reinforcement of the mechanical properties of paper (only 13.8% compared with 54% obtained in tap water (Quim Tarrés et al. 2017)).
Characterization of the agents used in the complex retention system
Colloidal agents can be classified by their nature in inorganic compounds, natural organic compounds, and synthetic organic compounds. Their most important characteristics are the equivalent molecular weight (ME), the sign of the charge and the density of the charge, so they can be classified in low molecular weight cationic, high molecular weight anionic, low molecular weight anionic and high molecular weight anionic agents. The characterization of the different agents involved in the selected complex retention system is shown in Table 3.
Poli-Dadmac presents a low equivalent molecular weight and a high cationic charge density, necessary to interact strongly with anionic trash. On the other hand, the efficiency of aluminum polychloride to deposit and fix the flocks is due to its low cationic charge density and high equivalent molecular weight. The ionic group’s density (Dm) of the cationic starch used for cellulose nanofiber retention was between the above-mentioned agents (7.36 × 1020). The anionic agents used in the complex retention system were polyacrylamide (PAA) and micro silica.
Lignocellulose nanofibers addition when using industrial water and a complex retention system
Different percentages of lignocellulose nanofibers were added to a test liner pulp in industrial water using the complex retention system. Results are shown in Tables 4 and 5.
Results in Table 4 show that when using the complex retention system, the thickness of hand sheets decreased when the addition of lignocellulose nanofibers increased. Paper density stabilized with the addition of 3% of LCNF (0.74 g/cm3 versus 0.65 g/cm3 obtained in Table 1 using a classic retention system). This increase in density was strongly related to the better retention of LCNF. Porosity (void volume/total volume) of paper and Gurley air flow resistance are also indications of the close paper structure generated by the lignocellulose nanofiber network. In this sense, the effect of cellulose nanofibers on physical properties of paper hand sheets is like refining in a typical papermaking process (Taipale et al. 2010).
The high hydrophilicity of cellulose nanofibers and the increase of total pulp surface area produce an increase in Schopper Riegler degree. The good retention of lignocellulose nanofibers attained by the complex retention system drive an important increase in mechanical properties of paper hand sheets. The addition of 3% LCNF with the complex retention system provided an increase of 53.6% in breaking length, versus the 10% obtained with the classic retention system. The high amount of LCNF retained in the fiber matrix creates a nano-network inside the macroscopic fiber web (Salmi 2009) producing higher internal bonding when the complex retention system was used (601 J/m2 versus 314.62 J/m2 with the simple system). On the other hand, burst and tear were little affected by the increased retention of cellulose nanofibers. This fact could be explained because the fibers used in this study (recycled fibers) present a high degree of union between fibers, and the effect of LCNF addition is not really significant in burst and tear resistance.
The increase of breaking length with respect to LCNF content was linear. It means that the effect of the complex retention system was not influenced by the amount of LCNF. Moreover, the increase obtained with 3% LCNF by this system in industrial water (53.6%) resulted in a similarity to that obtained in tap water (54%), demonstrating the efficiency of this system to eliminate the effect of anionic trash. The proposed interaction of lignocellulose nanofibers and cellulose fibers in industrial water with the complex retention system is shown in Fig. 4.
The use of cationic starch as a cellulose nanofiber retention agent has been reported in many works (Delgado-Aguilar et al. 2015a, b, c; González et al. 2012; Tarrés et al. 2016). However, the addition of different fixatives is necessary when anionic trash is present in the suspension. The complex retention system can be understood in terms of two different stages.
In the first stage, the objective is the flocculation and fixation of anionic trash and the retention of lignocellulose nanofibers. The equivalent molecular mass and charge density of the different cationic agents produce a synergistic effect between the dissolved substances and the fixatives. Many authors reported theories about cationic polyelectrolytes interaction with anionic trash based on charge neutralization and flocculation (Hubbe et al. 2012; Shetty et al. 1994). The use of poly-diallyldimethyl ammonium chloride (Poli-Dadmac) with a high charge density becomes necessary to neutralize the anionic trash (Wang et al. 2014). The addition of aluminum polychloride contributes to fix the neutralized anionic trash by synergistic effects (Gill 1996). At the same time, the flocculation and fixation of anionic trash ensures the correct retention of lignocellulose nanofibers by the action of cationic starch. Due to the complexity of the system, the control of the flocculation/fixation process is necessary.
The second stage seems to involve the correct formation of paper hand sheets. Even if flocculation between anionic trash and polyelectrolytes is not reversible, the addition of anionic polyelectrolytes can drive the generation of small floccules (Bledzki and Gassan 1996), allowing their good distribution in paper hand sheets.
Conclusions
This work studied lignocellulose nanofiber addition under industrial water conditions. The main obtained conclusions are listed below:
-
The presence of anionic trash in industrial papermaking waters produce high conductivity and high charge density.
-
Physical and mechanical properties of paper hand sheets resulted in strongly being affected by anionic trash interactions when 3% of LCNF was added.
-
A complex retention system was designed to neutralize anionic trash and to retain LCNF, improving the reinforcement efficiency of LCNF.
-
Anionic trash interactions with LCNF when a classic retention system is used reduce the improvement in breaking length attained with tap water from 54% to only 10% when 3% of LCNF was added. However, with the complex retention system, the increase in breaking length with 3% of LCNF achieves 53.6%.
-
The breaking length of paper hand sheets with 4.5% of LCNF was 5261 m in isotropic conditions, overcoming test liner requirements (5534 m in the machine direction).
-
The proposed complex retention system allows the use of LCNF on industrial water and enhances mechanical properties of the test liner, which could drive the reduction of basis weight or the increase of mineral fillers in the end, maintaining the same requirements.
References
Afra E, Yousefi H, Hadilam MM, Nishino T (2013) Comparative effect of mechanical beating and nanofibrillation of cellulose on paper properties made from bagasse and softwood pulps. Carbohydr Polym 97(2):725–730. https://doi.org/10.1016/j.carbpol.2013.05.032
Bledzki AK, Gassan J (1996) Important properties of colloidal silica in microparticulate systems. Nord Pulp Pap Res J 11(1):15–21
Boufi S, Gandini A (2015) Triticale crop residue: a cheap material for high performance nanofibrillated cellulose. RSC Adv 5(5):3141–3151. https://doi.org/10.1039/C4RA12918K
Boufi S, González I, Delgado-aguilar M, Tarrés Q, Pèlach MÀ, Mutjé P (2016) Nanofibrillated cellulose as an additive in papermaking process : A review. Carbohydr Polym 154:151–166
Carrasco F, Mutje P, Pelach MA (1998) Control of retention in paper-making by colloid titration and zeta potential techniques. Wood Sci Technol 32(2):145–155
Delgado-Aguilar M, González I, Pèlach MA, De La Fuente E, Negro C, Mutjé P (2015a) Improvement of deinked old newspaper/old magazine pulp suspensions by means of nanofibrillated cellulose addition. Cellulose 22(1):789–802. https://doi.org/10.1007/s10570-014-0473-2
Delgado-aguilar M, González I, Tarrés Q, Alcalà M, Pèlach MÀ (2015b) Approaching a low-cost production of cellulose nanofibers for papermaking applications. BioResourses 10(3):5345–5355
Delgado-Aguilar M, Tarrés Q, Puig J, Boufi S, Blanco A, Mutjé P (2015c) Enzymatic refining and cellulose nanofiber addition in papermaking processes from recycled and deinked slurries. BioResources 10(3):5730–5743
Delgado-Aguilar M, González I, Tarrés Q, Pèlach MA, Alcalà M, Mutjé P (2016) The key role of lignin in the production of low-cost lignocellulosic nanofibers for papermaking applications. Ind Crops Prod 86:295–300. https://doi.org/10.1016/j.indcrop.2016.04.010
Desharnais L, Chabot B, Daneault C, Montplaisir D, Croteau L (2002) Thermomechanical pulp washing effect of retention and drainage. Pulp Pap Can 103(4):44–48
Dunham AJ, Sherman LM, Alfano JC (2002) Effect of dissolved and colloidal substances on drainage properties of mechanical pulp suspensions. J Pulp Pap Sci 28(9):298–304
Espinosa E, Tarrés Q, Delgado-Aguilar M, González I, Mutjé P, Rodríguez A (2016) Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose. https://doi.org/10.1007/s10570-015-0807-8
Fernandes Diniz J, Gil MH, Castro JAAM (2004) Hornification—its origin and interpretation in wood pulps. Wood Sci Technol 37:489–494. https://doi.org/10.1007/s00226-003-0216-2
Garver TM, Xie T, Boegh KH (1997) Variation of white water composition in a TMP and DIP newsprint paper machine. Tappi J 80(8):163–173
Gill RIS (1996) Chemical control of deposits: scopes and limitations. Pap Technol 37(6):23–31
González I, Boufi S, Pèlach MA, Alcalà M, Vilaseca F, Mutjé P (2012) Nanofibrillated cellulose as paper additive in eucalyptus pulps. BioResources 7(4):5167–5180
Hietala M, Ämmälä A, Silvennoinen J, Liimatainen H (2015) Fluting medium strengthened by periodate–chlorite oxidized nanofibrillated celluloses. Cellul. https://doi.org/10.1007/s10570-015-0801-1
Hubbe MA (2014) Prospects for maintaining strength of paper and paperboard products while using less forest resources: A review. BioResources 9(1):1634–1763
Hubbe MA, Venditti RA, Rojas OJ (2007) What happens to cellulosic fibers during papermaking and recycling? A Review. BioResourse 2:739–788
Hubbe MA, Sundberg A, Mocchiutti P, Ni Y, Pelton R (2012) Dissolved and colloidal substances (dcs) and the charge demand of papermaking process waters and suspensions: a review. BioResources 7(4):6109–6193
Hurter RW (2002a) Nonwood fiber content papers-Part1: Corrugating medium physical properties. HurterConsult
Hurter RW (2002b) Nonwood fiber content papers-Part2: Unbleached papers physical properties. HurterConsult
ISO (1990) ISO 187:1990: Paper, board and pulps—Standard atmosphere for conditioning and testing and procedure for monitoring the atmosphere and conditioning of samples
ISO (1999) ISO 5267-1:1999: Pulps—Determination of drainability—Part 1: Schopper-riegler method
ISO. (2008a). ISO 1924-2:2008: Paper and board—Determination of tensile properties—Part 2: constant rate of elongation method (20 mm/min)
ISO (2008b) ISO 5269-2:2004 Pulps—Preparation of laboratory sheets for physical testing—Part 2: Rapid-Köthen method
ISO. (2011). ISO 534:2011: Paper and board—Determination of thickness, density and specific volume
ISO. (2012). ISO 1974:2012: Paper—Determination tearing resistance—Elmendorf method
ISO. (2013). ISO 5636-5:2013: Paper and board—Determination of air permeance (medium range)—Part 5: Gurley method
ISO. (2014). ISO 2758:2014: Paper—Determination of bursting strength
Korhonen MH, Laine J (2014) Flocculation and retention of fillers with nanoceluloses. Nord Pulp Pap Res J 29(1):119–128
Lourenço AF, Gamelas JAF, Nunes T, Amaral J, Mutjé P, Ferreira PJ (2017) Influence of TEMPO-oxidised cellulose nanofibrils on the properties of filler-containing papers. Cellulose 24(1):349–362. https://doi.org/10.1007/s10570-016-1121-9
Miao Q, Huang L, Chen L (2013) Advances in the control of dissolved and colloidal substances present in papermaking processes: a brief review. BioResources 8(1):1431–1455
Neimo L (1999) Papermaking Chemistry. In: Gullichsen J, Paulapuro H (eds). Jyväskylä: Fapet Oy
Pöyry (2011) Average papermaking fibre furnish the world 1990–2025. Pulp and RP Consumption 1995–2025, World Fibre Outlook, Pöyry LLC
Rouger J, Mutje P (1984) Correlation between the cellulose fibres beating and the fixation of a soluble cationic polymer. Br Polym J 16(2):83–86
Salmi J (2009) Surface interactions in polyelectrolyte-cellulose systems and their implications for flocculation mechanisms. Helsinki University of Technology, TKK
Sehaqui H, Zhou Q, Berglund LA (2013) Nanofibrillated cellulose for enhancement of strength in high-density paper structures. Nord Pulp Pap Res J 28(2):182–189. https://doi.org/10.3183/NPPRJ-2013-28-
Shetty CS, Greer CS, Laubach GD (1994) A likely mechanism for pitch deposition control. Tappi J 77(10):91–96
Swerin A, Ödberg L (1996) Flocculation of cellulosic fibre suspensions by a microparticulate retention aid system consisting of cationic polyacrylamide and anionic montmotillonite. Nord Pulp Pap Res J 11(1):22–27
Taipale T, Österberg M, Nykänen A, Ruokolainen J, Laine J (2010) Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength. Cellulose 17(5):1005–1020. https://doi.org/10.1007/s10570-010-9431-9
TAPPI (2014) T569 om-14: internal bond strength (Scott type)
Tarrés Q, Saguer E, Pèlach MA, Alcalà M, Delgado-Aguilar M, Mutjé P (2016) The feasibility of incorporating cellulose micro/nanofibers in papermaking processes: the relevance of enzymatic hydrolysis. Cellulose. https://doi.org/10.1007/s10570-016-0889-y
Tarrés Q, Ehman NV, Evangelina M, Area MC, Delgado-aguilar M, Mutjé P (2017) Lignocellulosic nanofibers from triticale straw: the influence of hemicelluloses and lignin in their production and properties. Carbohyd Polym 163:20–27. https://doi.org/10.1016/j.carbpol.2017.01.017
Wang Y, Ni J, Chen C, Peng J, Liu H (2014) Anionic trash control in high-yield pulp (HYP) containing furnish by using a poly-DADMAC based commercial formulation. J Ind Eng Chem 20(6):4452–4456. https://doi.org/10.1016/j.jiec.2014.02.016
Whipple WL, Maltesh C (2002) Adsorption of cationic flocculants to paper slurries. J Colloid Interface Sci 256(1):33–40. https://doi.org/10.1006/jcis.2001.7867
Zhang X, Beatson RP, Cai YJ, Saddler JN (1999) Accumulation of specific dissolved and colloidal substances during white water recycling affects paper properties. J Pulp Pap Sci 25(6):206–210
Acknowledgments
The authors wish to acknowledge the financial support of the Economy and Competitiveness Ministry of the Spanish Government by the project CTQ2013–48090–C2–2–R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarrés, Q., Area, M.C., Vallejos, M.E. et al. Key role of anionic trash catching system on the efficiency of lignocellulose nanofibers in industrial recycled slurries. Cellulose 25, 357–366 (2018). https://doi.org/10.1007/s10570-017-1589-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1589-y