Abstract
We report here a simple and effective method applying a combination of chitosan (Cs) and Cu(II) ion to fabricate antibacterial cotton fabric with a remarkable durability against laundering. The antibacterial fabric was prepared by grafting Cs onto cotton fibers through a succinic acid linkage, following with loading Cu(II) ions by the coordination effect. The modified fabric achieved 100% bacterial reduction (BR) rates against both S. aureus and E. coli, and remarkable laundering durability was confirmed even after 100 washing cycles. Moreover, longer Cs chains grafted on fibers shown enhanced chelating capability with Cu(II) ions. When compared to copper nanoparticles, our strategy has advantages in terms of low dosage of Cu(II), reasonable cost, simple process, reduced environmental hazards, and improved antibacterial durability. This work is believed to be a practical strategy for developing environment-friendly and cost-effective long-acting antibacterial cotton textiles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Public health issues driven by emerging infectious diseases constitute the forefront of global safety concerns (Metcalf and Lessler 2017), the demand and requirements for medical protective textiles are also increasing. Wearing comfort and high-effective antibacterial activity are the two key characteristics of a medical protective textiles. Cotton fabric has been widely used in medical protective textiles (Gao et al. 2021) due to its availability and low cost. However, its susceptibility for bacterial growth severely limits the service life of cotton textiles and even increases infection risk of the users (Hajipour et al. 2012; Rojas-Andrade et al. 2017). Therefore, various strategies have been developed to endow cotton fabric with antibacterial function (Emam 2019; Roman et al. 2020).
The mostly commonly reported technique is embedding of metal nanoparticles onto cotton fabric (Ali et al. 2018; Awais et al. 2021; Emam 2019; Zhang et al. 2016), and silver nanoparticles (AgNPs) have attracted the most attention (Chernousova and Epple 2013; Mohamed et al. 2017). For example, Sadanand et al. prepared antibacterial cotton fabrics through in situ syntheses of AgNPs, and realized a broad spectrum of antibacterial activities and low toxicity (Sadanand et al. 2017). Another representative class of metal nanoparticles is copper-based nanoparticles, which has been accepted in the field of antimicrobial textiles due to its low cost and outstanding antibacterial activity (Gouda and Hebeish 2009; Roman et al. 2020). Actually, elementary substance and oxide forms of Cu have been widely used as antibacterial reagents (Markovic et al. 2018; Nabila and Kannabiran 2018; Radetić and Marković 2019; Vasantharaj et al. 2019; Xu et al. 2018a, b). Despite of the high antibacterial activity, application of these inorganic nanoparticles in the textile industries still are limited by complicated preparation process, large dosage and poor durability (El-Nahhal et al. 2018; Roman et al. 2020). Generally, metal ions releasing from the metal nanoparticles play a primordial role for the antibacterial effect (Applerot et al. 2012; Godoy-Gallardo et al. 2021; Meghana et al. 2015). Therefore, immobilization of active metal ions onto fabrics may be a reasonable way to achieve an antibacterial function using mini-doses and simple treatment process. Especially, Cu(II) ion can be a candidate because it has high antibacterial activity, acceptable biocompatibility and low toxicity (Godoy-Gallardo et al. 2021). However, this expectation faces a challenge in achieving biocidal activity and durability simultaneously, which is a basic feature of the ideal antibacterial textile (Andreeva and Shchukin 2008; Si et al. 2018). Most Cu salts are water-soluble, which are hardly to stabilized on cotton fabrics to realize a satisfying durability against laundering (El-Ajaily et al. 2007; Qin et al. 2010). Therefore, it is still the biggest challenge to find an effective way to immobilize Cu(II) ion onto cotton fibers to overcome the durability challenging.
Chitosan is one of the most sustainable and abundant polysaccharides that has been studied for its antimicrobial properties for many years (Badawy et al. 2016; Dutta et al. 2012; Li et al. 2020; Ma et al. 2017; Shukla et al. 2013; Verma et al. 2021). Numbers studies have demonstrated that chitosan can be used as a good binder for immobilizing antibacterial reagents (An et al. 2014; El.Shafei and Abou-Okeil 2011; Haldorai and Shim 2013; Murali et al. 2019). Moreover, chitosan is able to chelate with a broad spectrum of metal ions, in particular transition elements (Khan et al. 2013; Qin 1993). The chelating ability of chitosan with Cu(II) ion has been well-documented and extensively studied (Gritsch et al. 2018). Interestingly, the chelation ability of chitosan with Cu(II) ions has been applied to form coordination complexes to act as therapeutic metal ions (Mourino et al. 2012), which play a positive role in promoting tissue regeneration and inhibiting the growth of prokaryotes. However, to our knowledge, no previous study has addressed the application of the chitosan with Cu(II) ion complex for fabricating antibacterial cotton fabrics that combined both the biocompatibility and antimicrobial activity.
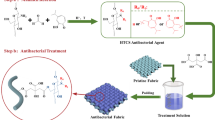
Aiming at a highly durable antibacterial cotton fabric with a mini-dose loading of Cu(II) ions, we investigate the combination effect of Cu(II) ion and chitosan grafted on cotton fibers. A very simple treatment process is employed to preparing the chitosan-Cu(II) based antibacterial cotton fabric. Cotton fabric is first pretreated with succinic acid (SA), and then, the chitosan is linked onto cotton fibers by using SA as the bridging agent. Finally, Cu(II) ions are immobilized on the modified fiber surfaces through the coordination effect of the amine groups of chitosan. According to the previous literatures, the coordination models for Cu(II) and NH2 group could be a bridge model (Schlick 1989), a pendant model (Ogawa et al. 1993), or the coexisting model (Rhazi et al. 2002). Therefore, the grafted Cs chains should be able to provide long enough free segments to allow the amino groups movable for the ordered arrangement. We suggest that Cs chain length might be an important factor to affect the movable Cs segments. To test this hypothesis, the chain length effect of the grafted chitosan on chelating capability with Cu(II) ion is further studied. The structure and morphology of the grafted chitosan as well chitosan-Cu(II) complexes layer were characterized utilizing Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and Field emission scanning electron microscopy (FE-SEM). The release behavior of Cu(II) ions was also evaluated by Atomic absorption spectroscopy (AAS). Experimental data show that longer chitosan chains are beneficial for immobilization of Cu(II) ions, and the modification gives outstanding antibacterial durability to cotton fabric without sacrificing of the desired cotton properties. We believe that this work will stimulate further research interests in developing antibacterial cotton textiles having a low toxicity but more efficient, flexible and economical.
Experimental
Materials
Cotton fabric (100 g/m2) was obtained from Suzhou Ke Chuan Textile Co., Ltd (China). Before modification, the cotton fabric was cleaned with an aqueous solution of sodium dodecyl sulfate (1 wt%, 1 h) and ethanol (99.5 wt%, 30 min) at 60 °C to remove impurities, rinsed with distilled water (100 mL × 3 times), and dried at 80 °C for 1 h. Chitosan samples (extracted and purified from crab shell) were purchased from Zhejiang Golden-shell Pharmaceutical Co. Ltd (China); their viscosity average molar mass (Mv) and degree of deacetylation (DD) were determined by the viscometry and potentiometric titration, as shown in Supporting Information. The Mv values determined for chitosan samples amounted to 5 kDa, 40 kDa, 160 kDa and 320 kDa, and the corresponding DD were 81%, 82%, 81% and 91%. Sodium dodecyl sulfate (98%), succinic acid (99.50%), glacial acetic acid (99.50%), sodium chloride (99.50%), ethanol (99.50%), and cupric sulfate anhydrous (99%) were purchased from Shanghai Aladdin Co. Ltd (China), and used as received without further purification. Other reagents are described in the Supporting Information (SI).
Preparation of Co–S fabrics
The antibacterial cotton fabrics were prepared by a pad-dry-cure process shown in Scheme 1. Firstly, the pre-cleaned cotton fabrics (5 cm × 5 cm, 10 pieces) were immersed in an aqueous solution of succinic acid (100.00 mL, 0.03 mol/L) at ambient temperature for 10 min, pad-rolled to 190 ± 10 wt% in wet weight, heated at 160 °C for 20 min, washed using distilled water (100 mL × 3 times) and dried in an oven at 80 °C for 1 h to obtain the Co–S fabric.
Preparation of Co–Cs fabrics
Chitosan (Cs) powder (1.00 g) was dissolved in an aqueous solution of acetic acid (99.00 mL, 1 wt%) at ambient temperature with stirring. The Co–S fabrics (5 cm × 5 cm, 2 pieces) were soaked in the Cs solution for 5 min, pad-rolled to 190 ± 10 wt% in wet weight, heated at 120 °C for 20 min, washed using distilled water (100 mL × 3 times), and dried at 80°C for 1 h to obtain the Co–Cs samples, they are named as Co–Cs1, Co–Cs2, Co–Cs3 and Co–Cs4 according to the Cs Mv values, i.e., 5 kDa, 40 kDa, 160 kDa and 320 kDa, respectively.
Preparation of Co–Cs–Cu fabrics
The aforementioned Co–Cs fabrics were further soaked into an aqueous solution of CuSO4 (20.00 mL, 12.5 mmol/L) for 10 min at ambient temperature, pad-rolled to 190 ± 10 wt%, heated at 120 °C for 20 min, washed using distilled water (100 mL × 3 times), and dried at 80 °C for 1 h to obtain the Co–Cs–Cu fabrics, i.e., Co–Cs1–Cu, Co–Cs2–Cu, Co–Cs3–Cu and Co–Cs4–Cu samples (Scheme 1), respectively. Meanwhile, the original cotton fabric was also treated with the CuSO4 solution using similar process as a control sample, named as Co–Cu.
Characterizations
Surface morphology of the modified cotton fabrics was observed using FE-SEM, and the energy dispersive X-ray spectrometer (EDS) was used to measure element distribution and content. Other instruments, such as FTIR-ATR, XRD and XPS were used to characterize the structural changes of modified cotton fabrics, atomic absorption spectrophotometer (AAS) is employed to measure the releasing of Cu ion. Additionally, the antibacterial, laundering durability, thermo-oxidative aging and wearing properties were also evaluated, specific method are described in the Supporting Information.
Results and discussion
The present work aims at developing an effectively antibacterial cotton textile via the incorporation of natural biomaterial and metal ions onto the fabric surface. The preparation route we tried was shown in Scheme 1. Cs was linked on the surface of the cotton fabric through SA linkages, the grafted Cs chains offer NH2 groups to act as the ligands for coordinate with Cu(II) ions, which exert an antibacterial effect. To demonstrate the feasibility and effectiveness of the functional finishing strategy, the surface coating structure, antibacterial effect, and fabric properties were investigated.
Coating structure and surface morphology
ATR-FTIR spectra of the modified and untreated cotton fabrics are compared in Fig. 1. After the treatment using SA, the resulting Co–S fabric shows a new peak corresponding to the C=O stretching vibration at 1723 cm−1, confirming that a considerable number of SA molecules have been linked onto the cotton fabric. Further treatment using Cs allows the Co-Cs fabric showed new peaks at 1540 cm−1, 1650 cm−1 and 1523 cm−1, which are attributed to the NH2 group, the amide I (C=O) and amide II (C–N) bonds, respectively (Khalilzadeh et al. 2020; Liu et al. 2016). Besides, the overlapped peaks at 3334 cm−1 corresponding to the stretching vibrations of O–H and N–H groups (Li et al. 2019; Pires et al. 2021; Xue et al. 2019b) changed to broader when the Co-S fabric was converted to Co–Cs fabric. Finally, the loading of Cu(II) ions onto the Co-Cs fabric led to the two peaks at 1545 cm−1 and 3340 cm−1 (all for N–H stretching vibration of the NH2 group) shifted (Fig. S1), which due to the coordination effect of the Cu(II) ions with the NH2 groups of Cs (Qu et al. 2011). We also investigated the effect of polymer chain length of the grafted Cs by preparing a variety of Co–Cs fabrics modified using different Cs samples with the Mv values ranged from 5 to 320 kDa. As shown in Fig. 1b, the peak depth at 1540 cm−1 increases in the order, suggesting that the Cs Mv has a positive effect on the quantity of Cs grafted on the cotton fibers.
Figure 2 presents representative SEM images of Co-Cs4 and Co–Cs4–Cu fabrics to compare to pristine cotton fabric (Co). The low-magnification SEM images show insignificant differences between the modified fabrics and the untreated fabric. Especially, it is clear that the interspaces between the yarns are not occupied by the Cs polymer. This is beneficial to the comfort fabric properties, such as water vapor transmission, water absorption and softness. In the high-magnification SEM images, however, the smooth fiber surfaces of the untreated fibers (Fig. 2b) significantly changed to rough after the modifications to Co–Cs4 (Fig. 2d). In addition, the Co–Cs1, Co–Cs2 and Co–Cs3 fabrics all exhibit the similar surface morphological changes of their fibers (Fig. S3a2–c2). Subsequently, the element mappings images reveal that carbon (C) and oxygen (O) elements are homogenously distributed on the fiber surfaces of all the fabrics, whereas, nitrogen (N) element only exits in the Co-Cs fabric (Figs. S2b3 and S3a3, b3, and c3). Moreover, as the Mv of Cs increases, the coverage density of N element in the surface Co–Cs fibers increases. In summary, SEM and mapping images suggest that Cs molecules are immobilized on the fiber surfaces but not in the interspaces between yarns.
The grafted Cs chains provide a lot of NH2 groups acting as ligand to form coordination bonds with Cu(II) ions. We further compared the fiber surface morphology of Co–Cs fabrics before and after coordinating Cu(II) ions, the low-magnification SEM images indicate that the fiber surfaces changed insignificantly (Fig. 2c and e). However, the high-magnification SEM images (Fig. 2d and f) reveal that the surfaces of the Co–Cs4–Cu fibers are rougher than those of Co–Cs4 fabrics, suggesting that adsorption of the Cu(II) ions caused grafted the Cs coating changed. The similar fiber morphological changes can also be found in Co–Cs1–Cu, Co–Cs2–Cu and Co–Cs3–Cu fabrics (Fig. S4a2–c2). Moreover, the mapping images (Figs. 2j and S4a3–c3) suggest that Cu ions are distributed on the Co–Cs–Cu fabrics uniformly, and the EDS spectrum of Co–Cs4–Cu fabric means the existence of C, O, N, and Cu elements on the surfaces of fibers (Fig. 2k). As shown in Table 1, the content of Cu element before and after washing at Co–Cu sample are 0.54% and 0.13%, respectively. However, the content of Cu element increases with the Mv value of Cs increase, and reached the maximum of 1.19% at Co-Cs4-Cu sample, it is still reminding 1.15% even after 100 washing times. In summary, Cu(II) ions are immobilized onto the cotton fabrics by forming coordination bonds with the NH2 groups of Cs, the higher the Mv value of Cs, the stronger the chelating ability of Cu ions. Whereas the surface morphology of the fibers changed insignificantly.
XPS is a widely used technique to investigate chemical structure of the material surfaces. Here, we collected the XPS spectra of the treated fabrics to analyses their chemical compositions of the surfaces to a depth of tens of nanometers. As shown in Fig. 3a, all of the fabrics (Co, Co–Cs4 and Co–Cs4–Cu samples) show C 1 s and O 1 s signals in their full XPS survey spectra, whereas, the Co-Cs4 fabric exhibits a new N1s signal, and the Co–Cs4–Cu fabric new Cu 2p and N 1 s signals. Peak deconvolution of the elemental signals was further performed to reveal the chemical environments corresponding to the elements. The high-resolution spectrum of the C 1 s peak of Co fabric can be deconvoluted into three peaks at 284.6 eV (C–C), 286.3 eV (C–OH), and 287.6 eV (C–O–C) (Fig. 3b) (Jiang et al. 2019; Xue et al. 2019a). In contrast, the Co–Cs4 (Fig. 3c) and Co–Cs4–Cu (Fig. 3d) fabrics, showed two new deconvolution peaks at 285.3 eV and 287.8 eV, which are ascribed to C-N and C=O/C–O–C bonds, respectively. As shown in Fig. 3e, the deconvolution of N 1 s peak showed two peaks corresponding to N–H and N–C bonds. It is worth noting that the deconvolution peak corresponding to N–H bond positive shifted by 0.4 eV by loading Cu(II) ions, suggesting again that the coordination effect causes an electron transfer at the N atoms (Artyushkova et al. 2013; Metson 1999). As shown in Fig. 3f, the high-resolution spectrum of the Cu 2p peak has two signals at 953.09 eV and 933.05 eV, which are corresponding to Cu 2p 1/2 and Cu 2p 3/2, respectively (Komeily-Nia et al. 2013; Xu et al. 2019). It provides credible evidence for the presence of the Cu(II) valence state. These XPS spectra demonstrated the existence of Cs and Cu(II) ions on the fiber surfaces, and the coordination bonds between the Cu(II) ions and the NH2 groups of the grafted Cs.
It is well known that most properties of cotton fabric are related to their crystalline structure of the cotton fibers. Hence, X-ray diffraction performed on the original and modified fabrics to confirm their crystalline structure. As shown in Fig. 4, the typical peaks for the cellulose I crystalline form, for instance, 2θ = 14.7°, 16.5°, 22.6°, and 34.5° (Nam et al. 2016), were observed in the XRD curves of all fabrics. Clearly, the crystalline structure of cotton fibers has not been significantly damaged after the heating process for the grafting chitosan and coordination complexation Cu(II) ions, which is in good agreement with our preview studies (Duan et al. 2020; Xu et al. 2019; Zhou et al. 2019).
Antibacterial effect and durability
In the present work, Cs chains were covalently linked onto cotton fibers via amidation reactions (Scheme 1). However, the antimicrobial activities of the resulting Co–Cs fabrics were so poor, that the BR values against S. aureus and E. coli were lower than 46.00% and 38.25%, respectively (Figs. 5a and S5). Therefore, we tried to immobilize Cu(II) ions by forming coordination bonds with the NH2 groups of the grafted Cs chains. As a result, a high-efficiency and durable antibacterial effect was achieved on the modified fabrics. Figures 5b, c and S6a show that the BR values of Co–Cu and Co–Cs–Cu fabrics against S. aureus and E. coli can reach to 100%, suggesting that the Cu(II) ions immobilized on the cotton fiber surfaces not only inhibit bacterial reproduction but also can kill the bacteria. However, in the repeating washing tests, the fabrics show significant difference in the term of durability against laundering. The Co–Cu fabric, which was prepared by a simple loading treatment of Cu(II) ions on the original cotton fabric, showed a rapid decrease in BR values. In contrast, the Co–Cs–Cu fabrics exhibit an improved durability against washing. Particularly, the BR rate of Co–Cs4–Cu fabrics against E. coli and S. aureus were kept higher than 98.48% even after 100 washing cycles.
This enhanced durability is in good agreement with the Cu ion releasing results shown in Fig. 5d. When the modified fabrics were soaked in water for 24 h, the Co–Cu fabric leads the concentration of Cu(II) ion to be almost constant at approximately 0.58 ppm, whereas the Co–Cs–Cu fabrics with except of Co–Cs1–Cu fabric released Cu(II) ions to a concentration more than 4.5 ppm. Interestingly, the laundering durability of the Co–Cs–Cu fabrics semes influenced by the Mv of the Cs used. After soaking for 24 h, the leaching Cu(II) concentration of the Co–Cs2–Cu, Co–Cs3–Cu and Co–Cs4–Cu fabrics are 5.95, 6.54 and 5.44 ppm, respectively. Especially, the Co–Cs4–Cu fabric shows the greatest affinity for Cu(II) ions, as it loaded the maximum quantity of Cu(II) ions, and keep more than 96.70% of Cu(II) ions even after 100 washing cycles (Table 1). We speculated that the enhanced affinity is due to the Cs grafted on the fiber surfaces. The effect of polymer chain length of Cs grafted on the cotton fibers on antibacterial durability is explained by a coordination mechanism shown in Scheme 2. In general, one Cu(II) ion maybe need four or six NH2 ligands to form a spatially stable complex. It is indisputable that longer Cs polymer leads to longer segments movable, offering more NH2 groups for the assembly with Cu(II) ions. Therefore, the longer Cs chains would be able to form more coordination bonds with Cu(II) ions. From another perspective that one Cu(II) ion moving from deep in the grafted Cs coating to outside, the Cu(II) ion will experience numerous cycles of the formation/deformation of the coordination complex with NH2 groups, thus longer Cs chains provide more NH2 groups to chelate with the Cu(II) ion, causing lower releasing rate of Cu(II) form the grafted Cs layer. This suggestion is in good agreement with the experimental results that longer Cs chains show better antibacterial durability against laundering.
On the other hand, textiles often be exposed to the sun light or dried at a high temperature after washing, which make copper nanoparticles easily oxidized to copper oxides. It is necessary to use an inert atmosphere of nitrogen or argon, and organic coatings to prevent the oxidation (Kanninen et al. 2008) when Cu NPs were used as the antibacterial reagent. This is the main reason for the limited applications of Cu NPs in the textile industry. In this regard, thermal oxygen aging tests of the antibacterial fabrics that modified by Cu(II) ions are very important. In the present work, Co–Cs4–Cu fabric was selected to the tests because its excellent laundering durability. As a result, after the thermal oxygen aging test at 90 °C for 72 h, Co–Cs4–Cu fabric still has strong antibacterial capability (Figs. 6a and S7), as the BR values against E. coli and S. aureus are 100% and 99.25%, respectively. Moreover, as shown in Fig. 6b, the XRD spectrum of Co–Cs4–Cu fabric after the thermal oxygen aging test shows insignificant changes when compared to Fig. 4, and the characteristic peaks assignable to copper oxides are not found. These results imply that the Cs polymer chains offer a protective effect to stabilize the coordinated Cu(II) ions to be hardly oxidized. The wide range XPS spectrum and Cu 2p spectrum of Co-Cs4-Cu fabric shown in Figs. 6c and d also suggest that the coordinated Cu(II) ions did not change after the thermal oxygen aging at 90 °C for 72 h.
Another merit for the present work is the low dosage of Cu(II) ions. As show in Table S2, the antibacterial cotton fabrics based on Cu-based NPs generally require high copper precursor dosages that ranged from 0.25 to 2.5 g/gcotton. In contrast, the present work achieved a durable antibacterial effect by only using 0.18 g/gcotton. In summary, the grafted Cs with high Mv value (320 kDa) offer a suitable number of NH2 groups for coordinating Cu(II) ions, imparting durably antibacterial effect to the cotton fabric. The low dosage of Cu(II) ions in the present work has advantages in the terms of low mass production cost and alleviated environmental concerns (Tamayo et al. 2016), which is in line with the development requirements of eco-friendly and green environmental protection.
Wearing performance analysis
Cotton fabric is the most commonly used material in the textile industry. Thus, it is necessary to evaluate the change of wearing properties before and after modification. By comparing to the breaking strength of Co fabric (31.33 MPa), the tensile strength (Fig. 7a) of Co–Cs4 (27.62 MPa) and Co–Cs4–Cu fabric (25.56 MPa) shown a slight decreased due to the small amount of acetic acid (1%) used in the modification process. On the other hand, decent softness and air permeability are the typical comfort properties of cotton fabric (Xu et al. 2018c). In this work, no significant changes occurred in the crease recovery angle (110.9°), water absorption (81.8%), and water vapor transmission (21.6 g/h/m2) of the Co–Cs4-Cu fabrics (Fig. 7b, c, and d). These results indicated that the modification process did not damage the wearing comfort properties of pristine cotton fabric.
Conclusion
In summary, durably antibacterial cotton fabrics with high antibacterial efficiency were obtained basing on coordination effect between Cu(II) ions and the Cs chains grafted on cotton fiber surfaces. The antibacterial fabrics can be prepared by a very simple treatment process. Cs was grafted onto the cotton fabric using SA as the bridging agent, following with immersion treatment in a Cu(II) solution to immobilize Cu(II) ions by the coordination effect. Longer Cs chains grafted on fiber surfaces showed positive effect on antibacterial durability against laundering. The fabrics modified by the combination of Cu(II) ion and grafted Cs can achieve 100% BR rates against S. aureus and E. coli. Excellent laundering durability was proofed by the BR value against E. coli and S. aureus after 100 washing cycles, which are kept at 98.48% and 99.20%, respectively. The Cu(II) ions immobilized by this way are stable at 90 °C for oxidation conditions. The wearing properties of the modified fabrics, including water vapor transmission, water absorption, and softness, were almost undamaged during the modification process. The combination of Cu(II) ion and Cs grafted on cotton fibers shows remarkable advantages in terms of low cost, simple process, reduced environmental hazards, and improved antibacterial durability, thus is believed to be instructive in developing antibacterial cotton textiles.
References
Ali N, Awais KT, Ul-Islam M, Khan A, Shah SJ, Zada A (2018) Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int J Biol Macromol 111:832–838
An J, Ji Z, Wang D, Luo Q, Li X (2014) Preparation and characterization of uniform-sized chitosan/silver microspheres with antibacterial activities. Mater Sci Eng C 36:33–41
Andreeva DV, Shchukin DG (2008) Smart self-repairing protective coatings. Mater Today 11:24–30
Applerot G, Lellouche J, Lipovsky A, Nitzan Y, Lubart R, Gedanken A, Banin E (2012) Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small 8:3326–3337
Artyushkova K, Kiefer B, Halevi B, Knop-Gericke A, Schlogl R, Atanassov P (2013) Density functional theory calculations of XPS binding energy shift for nitrogen-containing graphene-like structures. Chem Commun 49:2539–2541
Awais AN, Khan A, Asiri AM, Kamal T (2021) Potential application of in-situ synthesized cobalt nanoparticles on chitosan-coated cotton cloth substrate as catalyst for the reduction of pollutants. Environ Technol Innov 23:101675
Badawy ME, Rabea EI, Taktak NE, El-Nouby MA (2016) The antibacterial activity of chitosan products blended with monoterpenes and their biofilms against plant pathogenic bacteria. Scientifica 2016:1796256
Chernousova S, Epple M (2013) Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl 52:1636–1653
Duan P, Xu Q, Zhang X, Chen J, Zheng W, Li L, Yang J, Fu F, Diao H, Liu X (2020) Naturally occurring betaine grafted on cotton fabric for achieving antibacterial and anti-protein adsorption functions. Cellulose 27:6603–6615
Dutta J, Tripathi S, Dutta PK (2012) Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: a systematic study needs for food applications. Food Sci Technol Int 18:3–34
El-Ajaily M, Abdlseed F, Ben-Gweirif S (2007) Preparation, characterization and antibacterial activity of some metal ion complexes. E-J Chem 4:461–466
El-Nahhal IM, Elmanama AA, Amara N, Qodih FS, Selmane M, Chehimi MM (2018) The efficacy of surfactants in stabilizing coating of nano-structured CuO particles onto the surface of cotton fibers and their antimicrobial activity. Mater Chem Phys 215:221–228
El.Shafei A, Abou-Okeil A (2011) ZnO/carboxymethyl chitosan bionano-composite to impart antibacterial and UV protection for cotton fabric. Carbohydr Polym 83:920–925
Emam HE (2019) Generic strategies for functionalization of cellulosic textiles with metal salts. Cellulose 26:1431–1447
Gao D, Li X, Li Y, Lyu B, Ren J, Ma J (2021) Long-acting antibacterial activity on the cotton fabric. Cellulose 28:1221–1240
Godoy-Gallardo M, Eckhard U, Delgado LM, de Roo Puente YJD, Hoyos-Nogues M, Gil FJ, Perez RA (2021) Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioact Mater 6:4470–4490
Gouda M, Hebeish A (2009) Preparation and evaluation of CuO/Chitosan nanocomposite for antibacterial finishing cotton fabric. J Ind Text 39:203–214
Gritsch L, Lovell C, Goldmann WH, Boccaccini AR (2018) Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr Polym 179:370–378
Hajipour MJ, Fromm KM, Ashkarran AA, Aberasturi DJd, Larramendi IRd, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30:499–511
Haldorai Y, Shim J-J (2013) Multifunctional chitosan-copper oxide hybrid material: photocatalytic and antibacterial activities. Int J Photoenergy 2013:1–8
Jiang C, Liu W, Yang M, Liu C, He S, Xie Y, Wang Z (2019) Robust multifunctional superhydrophobic fabric with UV induced reversible wettability, photocatalytic self-cleaning property, and oil-water separation via thiol-ene click chemistry. Appl Surf Sci 463:34–44
Kanninen P, Johans C, Merta J, Kontturi K (2008) Influence of ligand structure on the stability and oxidation of copper nanoparticles. J Colloid Interface Sci 318:88–95
Khalilzadeh MA, Hosseini S, Rad AS, Venditti RA (2020) Synthesis of grafted nanofibrillated cellulose-based hydrogel and study of its thermodynamic, kinetic, and electronic properties. J Agric Food Chem 68:8710–8719
Khan A, Mehmood S, Shafiq M, Yasin T, Akhter Z, Ahmad S (2013) Structural and antimicrobial properties of irradiated chitosan and its complexes with zinc. Radiat Phys Chem 91:138–142
Komeily-Nia Z, Montazer M, Latifi M (2013) Synthesis of nano copper/nylon composite using ascorbic acid and CTAB. Colloids Surf A 439:167–175
Li P, Wang B, Liu Y, Xu Y, Jiang Z, Dong C, Zhang L, Liu Y, Zhu P (2020) Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohydr Polym 237:116173
Li P, Wang B, Xu Y, Jiang Z, Dong C, Liu Y, Zhu P (2019) Ecofriendly flame-retardant cotton fabrics: preparation, flame retardancy, thermal degradation properties, and mechanism. ACS Sustain Chem Eng 7:19246–19256
Liu L, Liao Q, Xie J, Qian Z, Zhu W, Chen X, Su X, Meng R, Yao J (2016) Synthetic control of three-dimensional porous cellulose-based bioadsorbents: correlation between structural feature and metal ion removal capability. Cellulose 23:1–17
Ma Z, Garrido-Maestu A, Jeong KC (2017) Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: a review. Carbohydr Polym 176:257–265
Markovic D, Deeks C, Nunney T, Radovanovic Z, Radoicic M, Saponjic Z, Radetic M (2018) Antibacterial activity of Cu-based nanoparticles synthesized on the cotton fabrics modified with polycarboxylic acids. Carbohydr Polym 200:173–182
Meghana S, Kabra P, Chakraborty S, Padmavathy N (2015) Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv 5:12293–12299
Metcalf CJE, Lessler J (2017) Opportunities and challenges in modeling emerging infectious diseases. Science 357:149–152
Metson JB (1999) Charge compensation and binding energy referencing in XPS analysis. Surf Interface Anal 27:1069–1072
Mohamed AL, Hassabo AG, Shaarawy S, Hebeish A (2017) Benign development of cotton with antibacterial activity and metal sorpability through introduction amino triazole moieties and AgNPs in cotton structure pre-treated with periodate. Carbohydr Polym 178:251–259
Mourino V, Cattalini JP, Boccaccini AR (2012) Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J R Soc Interface 9:401–419
Murali S, Kumar S, Koh J, Seena S, Singh P, Ramalho A, Sobral AJFN (2019) Bio-based chitosan/gelatin/Ag@ZnO bionanocomposites: synthesis and mechanical and antibacterial properties. Cellulose 26:5347–5361
Nabila MI, Kannabiran K (2018) Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal Agric Biotechnol 15:56–62
Nam S, French AD, Condon BD, Concha M (2016) Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr Polym 135:1–9
Ogawa K, Oka K, Yui T (1993) X-ray study of chitosan-transition metal complexes. Chem Mater 5:726–728
Pires AB, Vitali L, Tavares A, Germano CA, Amorim SM, Moreira RFPM, Peralta RA, Neves A (2021) Chitosan functionalized with heptadentate dinucleating ligand applied to removal of nickel, copper and zinc. Carbohydr Polym 256:117589
Qin Y (1993) The chelating properties of chitosan fibers. J Appl Polym Sci 49:727–731
Qin Z, Chen Y, Zhang P, Zhang G, Liu Y (2010) Structure and properties of Cu(II) complex bamboo pulp fabrics. J Appl Polym Sci 117:1843–1850
Qu J, Hu Q, Shen K, Zhang K, Li Y, Li H, Zhang Q, Wang J, Quan W (2011) The preparation and characterization of chitosan rods modified with Fe3+ by a chelation mechanism. Carbohydr Res 346:822–827
Radetić M, Marković D (2019) Nano-finishing of cellulose textile materials with copper and copper oxide nanoparticles. Cellulose 26:8971–8991
Rhazi M, Desbrières J, Tolaimate A, Rinaudo M, Vottero P, Alagui A (2002) Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 43:1267–1276
Rojas-Andrade MD, Chata G, Rouholiman D, Liu J, Saltikov C, Chen S (2017) Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 9:994–1006
Roman LE, Gomez ED, Solis JL, Gomez MM (2020) Antibacterial cotton fabric functionalized with copper oxide nanoparticles. Molecules 25:5802
Sadanand V, Tian H, Rajulu AV, Satyanarayana B (2017) Antibacterial cotton fabric with in situ generated silver nanoparticles by one-step hydrothermal method. Int J Polym Anal Charact 22:275–279
Schlick S (1989) Binding sites of Cu2+ in chitin and chitosan: an electron spin resonance study. Macromolecules 19:192–195
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol 59:46–58
Si Y, Zhang Z, Wu W, Fu Q, Huang K, Nitin N, Ding B, Sun G (2018) Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci Adv 4:eaar5931
Tamayo L, Azocar M, Kogan M, Riveros A, Paez M (2016) Copper-polymer nanocomposites: an excellent and cost-effective biocide for use on antibacterial surfaces. Mater Sci Eng C 69:1391–1409
Vasantharaj S, Sathiyavimal S, Saravanan M, Senthilkumar P, Gnanasekaran K, Shanmugavel M, Manikandan E, Pugazhendhi A (2019) Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B 191:143–149
Verma M, Gahlot N, Singh SSJ, Rose NM (2021) UV protection and antibacterial treatment of cellulosic fibre (cotton) using chitosan and onion skin dye. Carbohydr Polym 257:117612
Xu Q, Duan P, Zhang Y, Fu F, Liu X (2018a) Double protect copper nanoparticles loaded on l-cysteine modified cotton fabric with durable antibacterial properties. Fibers Polym 19:2324–2334
Xu Q, Ke X, Ge N, Shen L, Zhang Y, Fu F, Liu X (2018b) Preparation of copper nanoparticles coated cotton fabrics with durable antibacterial properties. Fibers Polym 19:1004–1013
Xu Q, Ke XT, Shen L, Ge N, Zhang Y, Fu F, Liu X (2018c) Surface modification by carboxymethy chitosan via pad-dry-cure method for binding Ag NPs onto cotton fabric. Int J Biol Macromol 111:796–803
Xu Q, Zheng W, Duan P, Chen J, Zhang Y, Fu F, Diao H, Liu X (2019) One-pot fabrication of durable antibacterial cotton fabric coated with silver nanoparticles via carboxymethyl chitosan as a binder and stabilizer. Carbohydr Polym 204:42–49
Xue C, Fan Q, Guo X, An Q, Jia S (2019a) Fabrication of superhydrophobic cotton fabrics by grafting of POSS-based polymers on fibers. Appl Surf Sci 465:241–248
Xue F, He H, Zhu H, Huang H, Wang S (2019b) Structural design of a cellulose-based solid amine adsorbent for the complete removal and colorimetric detection of Cr(VI). Langmuir 35:12636–12646
Zhang Y, Xu Q, Fu F, Liu X (2016) Durable antimicrobial cotton textiles modified with inorganic nanoparticles. Cellulose 23:2791–2808
Zhou J, Hu X, Zhu Y, Lyu H, Zhang L, Fu F, Liu X (2019) A hybrid binder of carboxymethyl chitosan and l-methionine enables a slight amount of Ag NPs to be durably effective on antibacterial cotton fabrics. Cellulose 26:9323–9333
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 51873195 and 51573167).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, Y., Shen, G., Zheng, W. et al. Remarkable durability of the antibacterial function achieved via a coordination effect of Cu(II) ion and chitosan grafted on cotton fibers. Cellulose 29, 1003–1015 (2022). https://doi.org/10.1007/s10570-021-04281-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04281-z