Abstract
Natural antibacterial agents have attracted increasing attention due to safety, ecological, and environmental protection concerns. In this study, hinokitiol-grafted-chitosan (HTCS) was prepared via the Mannich reaction and used as an antibacterial agent for the treatment of cotton fabric. The results showed that, compared with chitosan (CS) and hinokitiol (HT) alone, HTCS exhibited a lower value of minimum inhibitory concentration (MIC). Compared with the fabrics treated by CS and HT, the antibacterial rate of the treated fabric against Escherichia coli using HTCS as antibacterial agent increased by approximately 90% and 27%, the bacterial reduction rate against Staphylococcus aureus increased by about 58% and 39%. The HTCS-treated cotton fabric possessed good antibacterial properties even after 25 washing cycles. Moreover, the antibacterial cotton fabric retained original hydrophilicity, handle, and strength. Consequently, HTCS has great potential as a natural antibacterial agent for textiles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the continuous improvement of people’s living standards, the demand for textiles is no longer limited to the original features of fabrics, such as warmth and comfort, but also tends to pursue their health care, protection, and other functions. In our daily life, the human body always secrete sweat. Lactic acid, urea, and other secretions in sweat will breed the growth of bacteria within fabric, which can, in turn, cause harm to human health. Cotton fabric is soft, breathable, and hygroscopic. It is more likely to breed bacteria and mold which cause discoloration, mildew, embrittlement, degradation, and other problems. These may induce a variety of human body ailments (Chen et al. 2016). Consequently, antibacterial finishing is an important approach to improve the comfortability of cotton fabric.
Although a variety of antibacterial agents have been developed, there are still problems to be resolved. Further researches should be implemented to make the properties of antibacterial agents better. At present, there have been three main categories of antibacterial agents for textiles on the market, namely, synthetic organic antibacterial agents (Muñoz-Bonilla and Fernández-García 2012; Hasan et al. 2013; Bai et al. 2015), inorganic antibacterial agents (Li et al. 2012; Raza et al. 2021), and natural antibacterial agents (Gao and Cranston 2008). However, only a few can be widely used for the antibacterial finishing of textiles. Quaternary ammonium salts containing 12 to 18 carbon atoms are often used as bactericides and disinfectants for fibers. The advantages of these salts are their strong bactericidal power and abundant sources, but their binding force to fibers is poor and, therefore, they require to be combined with reactive resins (Shahid-ul-Islam and Kumar 2020; Lin et al. 2018). This method of combining is used to improve their durability, but they also have defects such as poor chemical stability (Buffa et al. 2020), high toxicity (Mumcuoglu et al. 2008), and short antibacterial aging (Han et al. 2020). Silver is an inorganic antibacterial agent which is often used in fabric finishing (Chen et al. 2012; Shameli et al. 2010). Silver ions are combined with a carrier by ion exchange or adsorption. This produces good antibacterial performance, but also has many problems, such as the large amount of silver required, high cost, uneven dispersion, and easy discoloration (Deng et al. 2015). With increasing awareness of environmental protection, natural antibacterial agents have attracted increasing attention from related scientific research departments. Natural antibacterial agents cause little harm to human beings, animals, or the environment during their production and usage, such as aloe (Xu and Deng 2011), chitosan (CS) (No et al. 2002), tea polyphenols (Wen et al. 2020), hinokitiol (HT) (Phan et al. 2020), and so on. They are safe and environmentally friendly. Nevertheless, they also have some problems, such as a short life, poor heat resistance, a narrow application range, limited production conditions, and so on (Chen et al. 2016).
To the best of our knowledge, the ideal antibacterial finishing agent should not only kill harmful microorganisms and prevent the spread of disease, but also meet the following requirements: low toxicity to the human body and the environment, little negative impact on wearability of fabrics, and antibacterial durability (Gao et al. 2019). Accordingly, the new antibacterial agent with high-efficient, low-toxicity, durable, and environmental properties.
Among the many natural antimicrobial agents, CS has attracted wide attention due to its excellent properties, such as being antibacterial, low cost, good film forming, biocompatible, biodegradable (Liu et al. 2004; Raza et al. 2020a, b), and contains rich amino and hydroxy function groups within its structure. However, CS generally only displays antibacterial activity in an acidic medium (which can only inhibit individual bacteria) and its antibacterial performance drops sharply after film formation (Yin et al. 2020). Therefore, improving the antibacterial activity and broad spectrum of CS is an urgent problem to be overcome.
The structure of CS contains rich amino and hydroxy function groups, which is convenient for chemical modification to improve its antibacterial properties (Mittal et al. 2021; Raza et al. 2020a, b). At present, the main purpose of CS modification is to improve its broad-spectrum antibacterial activity. Most modification schemes designed by researchers have focused on introducing different chemical functional groups into the molecular structure of CS. Some researchers have tried to introduce natural molecules (catechin, curcumin, geraniol, lysine, and so on) into the structure of CS. But very few have studied the application of antibacterial agents in textiles. Zhou et al. prepared protocatechuic acid-grafted-quaternized chitosan with excellent antibacterial properties against E. coli, S. aureus and MRSA (Zhou et al. 2021). Yadav et al. fabricated a biobased antibacterial polymer film by grafting of vanillin-furfuryl amine benzoxazine on CS using Schiff-base chemistry (Yadav et al. 2021).
HT, a natural compound isolated from Japanese cypress and western red cedar, has the tropolone structure. It possesses good broad-spectrum antibacterial properties, strong antibacterial, antifungal, and insect prevention effects (Zhang et al. 2018) as well as no significant cytotoxicity against human endothelial and epithelial cells (Chang et al. 2019). Thus, it has been widely used in cosmetics, medicine, agriculture, textiles, and other fields. However, HT owns bad water solubility and was not suitable to be used as antibacterial agent in textile finishing. It can be considered the combination of CS and HT to achieve high-efficient antibacterial properties. The study of grafting HT onto CS has not been reported.
In this work, HT was grafted onto CS using the Mannich reaction. The properties of hinokitiol-grafted-chitosan (HTCS) were initially investigated and then HTCS was applied as an antibacterial agent for the treatment of cotton. The antibacterial and wearing properties of the treated fabrics were studied to evaluate the application potential of HTCS as an antibacterial agent.
Experimental
Materials
Cotton fabrics (thread density 14.578 tex, warp 133 threads/inch, weft 72 threads/inch) were kindly supplied by Shanghai Hualun Printing and Dyeing Co., Ltd.
CS (Viscosity 100 ~ 200 mPa·s, DA ≥ 95%), HT, paraformaldehyde, dibasic sodium phosphate, and potassium dihydrogen phosphate were obtained from Shanghai Macklin Biochemical Co., Ltd. Acetic acid, ethanol, N-hexane, ethyl acetate, sodium chloride, and N,N-Dimethylformamide (DMF) were supplied by Hangzhou Gaojing Fine Chemical Industry Co., Ltd. Diethyl ether and acetone were supplied by Hangzhou Shuanglin Chemical Reagent Co., Ltd. Citric acid was supplied by Chengdu Dongjin Chemical Reagent Co., Ltd. Dimethyl sulfoxide (DMSO) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. All the chemicals were of analytical grade.
S. aureus (ATCC 6538) and E. coli (ATCC 25,922) were purchased from Shanghai Luwei Technology Co., Ltd. Agar bacteriological (BR) was supplied by Hangzhou Baisi Biotechnology Co., Ltd.
Preparation of HTCS antibacterial agent
In an oil-bath reactor equipped with a magnetic stirrer and a reflux condenser, 1 mmol of CS and 50 mL 2% v/v of acetic acid were mixed in a round-bottomed flask and stirred at 80 °C until the CS dissolved. Paraformaldehyde was then added and the mixture stirred for 30 min before HT of the same mol as paraformaldehyde was added into the flask at the specific temperature required for reaction. The end point of the reaction was monitored through thin layer chromatography (TLC). N-hexane and ethyl acetate were selected as the developing agent, and their volume ratio was 1:1. The spots of the samples after the reaction were compared with those before the reaction. When the spots of the raw materials disappeared or remained stable, the reaction was over. After cooling, the unreacted HT and paraformaldehyde were extracted with diethyl ether. No raw material spots were found in the extracted samples by TLC, indicating that the extraction was completed. Since the reaction takes place in a aqueous medium, the product remains in water after the extraction of HT with ether. Finally, hinokitiol-grafted-chitosan (HTCS) liquor, the solid content of which was 0.738 wt%, was obtained by rotary evaporation to remove residual ether. The preparation route (Step a) is presented in Fig. 1.
Antibacterial treatment of cotton fabric
Cotton fabric of dimension 15 × 15 cm2 was impregnated into a solution containing 18.8 wt% HTCS aqueous solution and 1.2 wt% citric acid at a bath ratio of 1: 30 at 90 °C for 10 min. The treated cotton fabric was padded by a padder at the weight gain percentage about 85%. The antibacterial cotton fabric (F-HTCS) was obtained after drying initially at 80 °C for 3 min and then baking at 140 °C for 2 min. The process (Step b) is shown in Fig. 1.
Correspondingly, the antibacterial cotton fabrics using CS, HT, and HTCS as agents were marked as F-CS, F-HT, and F-HTCS, respectively.
Determination of degree of substitution
The element content of HTCS and CS was measured via an elemental analyzer (Vario MICRO cube, Elementar, Germany). The degree of substitution (DS) was calculated using Eq. 1 and Eq. 2 (Bi et al. 2021):
where DA denotes the acetyl degree of CS; MN and MC are the relative molecular masses of carbon and nitrogen atoms, respectively; RC/N means the ratio of carbon to nitrogen of HTCS; R’C/N represents the ratio of carbon to nitrogen of CS.
FTIR spectroscopy
The functional groups of the antibacterial agents were examined within the range 4000–400 cm−1 using a Fourier Transform Infrared Instrument (Nicolet 5700, USA).
Scanning electron microscopy (SEM)
The shape changes of S. aureus and E. coli were observed by SEM (Vltra55, USA).
Solubility test
The dissolution of CS, HT, and HTCS in distilled water and six other organic solvents (acetone, anhydrous ethanol, acetic acid, diethyl ether, DMF, and DMSO) at room temperature were explored. In addition, in order to investigate the effects of pH on the solubility of CS and HTCS, the samples were dissolved in 0.1 mol/L HCl solution, respectively. The pH values were adjusted with 0.1 mol/L NaOH solution. The pH range was from 1 to 10. The transmittance of the solution at 600 nm was recorded using a UV–Vis spectrophotometer (Lambda 35, Elmer Perkin, USA) (Bi et al. 2021).
Antibacterial testing
Minimum inhibitory concentration (MIC) was used as an effective qualitative method of antibacterial ability assessment. The MIC values of the antibacterial agents were determined against S. aureus and E. coli by a continuous dilution method according to the literature (Su et al. 2020). The minimum concentration at which the microplate reader could not observe the bacterial growth was taken as the MIC of the antibacterial agent.
The antibacterial activity of the treated cotton fabric was evaluated by the continuous dilution method according to AATCC test method 100–2012. The antibacterial rate of the treated cotton fabric was calculated using Eq. 3 (Lin et al. 2018):
where A represents the number of viable bacteria per unit volume of culture for the untreated fabrics and B represents the number of viable bacteria per unit volume of culture for the treated fabrics.
Antibacterial durability test
The treated cotton fabrics were washed using the laundering method according to AATCC test method 61–2013. The antibacterial durability was evaluated through the antibacterial rate of the treated cotton fabrics after 5, 10, 15, 20, and 25 washing cycles.
Tensile strength test
The breaking strength and elongation of the fabric in both the warp and weft directions were measured according to GB/T 3923.1-1997.
Hydrophilicity test
The capillary effect of the fabric was tested to access the hydrophilicity of the fabric according to GB/T 21,655.1-2008.
Hand Parameters
According to AATCC test method 202-2012, its hand parameters were tested by a fabric sensory instrument (PhabrOmeter, Nucybertek, USA).
Determination of formaldehyde release of the fabric
The formaldehyde content of the treated cotton fabric was evaluated according to GB/T 2912.1-2009. Then, the safety grade of the treated cotton fabric was evaluated according to GB 18,401-2010.
Results and discussion
Modification of CS by HT
Effect of molar ratio of HT to CS on the antibacterial properties of HTCS
A series of HTCS antibacterial agents were prepared by changing the molar ratios of HT and CS at 90 ℃. In order to observe the influence of HT and CS proportions on the polymer grafting rate, an elemental analysis test was conducted, as shown in Table 1. The RC/N of the nine products were all higher than that of CS, indicating the successful grafting of HT onto CS. From the table, it can be seen that the HT and CS proportions had an obvious effect on the DS of derivatives. As the proportions gradually increased, the RC/N and DS of the product also gradually increased and reached the maximum value when the proportion was 2:1.
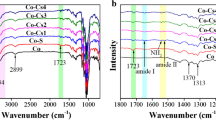
In order to explore the effect of HT and CS proportions on the polymer structure, an FTIR test was conducted. Figure 2a shows the FTIR spectra of HT, CS, and nine HTCS antibacterial agents. The overall FTIR spectrum of the HTCS antibacterial agent was similar to that of CS. For CS, the broad peak from 3200 to 3500 cm−1 was assigned to be the absorbance of –OH and –NH stretching vibrations, and the weak peak from 2871 to 2917 cm−1 showed the stretching vibration of –CH. The peak at 1155 cm−1 was assigned to be the characteristics of its polysaccharide structure. The peak from 1028 to 1073 cm−1 was assigned to be the absorbance peak of C6 and C3 hydroxyl groups (Su et al. 2020), respectively. For HT, the peaks at 1600 cm−1, 1500 cm−1, and 1269 cm−1 characterized C=O stretching, C=C stretching, and C–O stretching (Phan et al. 2020). When the HT and CS proportion was 2:1, the characteristic peaks of HT could be clearly observed on the polymer, indicating that this graft condition was best. This result was consistent with the results of elemental analysis.
In order to explore the influence of proportion on the antibacterial activity of antibacterial agents, MIC values were determined, as shown in Fig. 2b. It demonstrated that CS had a poor antibacterial effect on E. coli, but after grafting HT, the antibacterial activity improved noticeably. When the HT and CS proportion was 2:1, the HTCS antibacterial agent showed better antibacterial activity (0.043 and 0.054 mg/mL against S. aureus and E. coli, respectively) than HT alone (0.079 and 0.068 mg/mL against S. aureus and E. coli, respectively). Therefore, this proves that the grafting of HT onto CS greatly improved antibacterial activity.
Effect of reaction temperature on the antibacterial properties of HTCS
When the ratio of HT to CS was 2:1, a series of HTCS antibacterial agents were prepared by changing the reaction temperature. In order to observe the influence of temperature on the polymer grafting rate, an elemental analysis test was conducted, as shown in Table 2. The table shows that the reaction temperature had an obvious effect on the DS of the derivatives. As the reaction temperature increased, the DS of the product gradually increased at first, and reached the maximum value when the temperature was 90 °C.
In order to explore the effect of temperature change on the polymer structure, an FTIR test was conducted. Figure 3a shows the FTIR spectra of HT, CS, and four HTCS antibacterial agents. The overall FTIR spectrum of the HTCS antibacterial agents was similar to that of CS. It suggested that the reaction temperature had an obvious effect on the polymer structure. When the reaction temperature was 90 °C, the characteristic peaks of HT could be clearly observed on the polymer, indicating that this graft condition was best. This result was consistent with the results of elemental analysis.
In order to explore the influence of reaction temperature on the antibacterial activity of antibacterial agents, MIC values were determined, as shown in Fig. 3b. It was clearly seen that when the reaction temperature was 90 °C, the HTCS antibacterial agent had the best antibacterial activity (0.043 and 0.054 mg/mL against S. aureus and E. coli, respectively).
Solubility of HTCS
In textile finishing, it is important for the antibacterial agents to be resolved in water. Table 3 lists the solubility of CS, HT, and HTCS. It was found that CS was soluble in DMSO, slightly soluble in water, DMF and acetic acid, and insoluble in acetone, ethanol, and ether. HT was also slightly soluble in water, but soluble in other solvents. Compared to CS, the solubility of HTCS in water, DMF, and anhydrous ethanol was improved due to the grafting of HT. The water solubility of HTCS was beneficial for the treatment of fabric.
Figure 4 shows the dissolution of HTCS in the pH range of 1–10. The results exhibited that HTCS had good solubility in the pH range of 3–8, but their solubility decreased in alkaline environments and highly acidic environments. It was understood that the general pH of human sweat is 4.2–7.5, indicating that HTCS had a certain level of dissolution in this environment, thereby playing an antibacterial role.
Antibacterial mechanism of HTCS
To investigate the mechanism of the interactions between HTCS and bacterial cells, we chose S. aureus and E. coli as model bacterium. The untreated S. aureus cells and E. coli cells (Fig. 5a–d) can be seen to exhibit a spherical shape and a rodlike shape, respectively, with a smooth cell surface. Additionally, it was seen from Fig. 5e–g that some of the cell walls were indeed destroyed or disintegrated.
Antibacterial activity of cotton fabric using HTCS as antibacterial agent
The antibacterial activity of F-CS, F-HT, and F-HTCS were confirmed via the spread plate method, as shown in Fig. 6a. The calculated antibacterial rates of all samples against two test strains, S. aureus and E. coli, are shown in Fig. 6b. The antibacterial rate of F-HTCS against S. aureus was 99.9% and against E. coli was 99.8%. The antibacterial rate of CS and HT against S. aureus were 63.2% and 71.7%, respectively. And the antibacterial rate of CS and HT against E. coli were 52.5% and 78.5%, respectively. It can, therefore, be confirmed that F-HTCS displayed excellent antibacterial activity.
Other related literature had reported that the antibacterial activity of CS against S. aureus was better than E. coli because the reaction on quaternary ammonium groups limited antibacterial activity against E. coli (No et al. 2002). Meanwhile, HT had good antibacterial activity against E. coli due to the presence of phenolic hydroxyl (Morita et al. 2007). Therefore, it can be inferred that the antibacterial activity of HTCS had a synergistic effect with CS and HT, and that the antimicrobial activity was greatly improved due to the grafting of HT onto CS.
Durability of cotton fabric using HTCS as antibacterial agent
The antibacterial durability of antibacterial cotton fabric (F-HTCS) was assessed by determining the change of the antibacterial rate after the washing durability test. From Fig. 7, it was found that the antibacterial rate of the F-HTCS against S. aureus and E. coli was slightly reduced with an increase in the number of washing cycles. With the increase in the number of washing cycles, the change of antibacterial rate became smaller and the antibacterial rate of the F-HTCS against two test strains remained above 80%, indicating that the treated cotton fabrics could retain their antibacterial activity. Due to the addition of citric acid in the finishing solution, citric acid acted as a crosslinking agent to fix HTCS onto the fabric (Jabar et al. 2021). As a result, the durability of the antibacterial cotton fabric was excellent.
Wearability of cotton fabric using HTCS as antibacterial agent
Taking into consideration that antibacterial cotton fabrics should be comfortable on the skin, the nature of cotton fabrics, such as its water absorbability and softness, should be reserved in the finishing process. Therefore, these properties were tested in this study.
The wicking effect was measured to investigate the hydrophilicity of the antibacterial cotton fabric, as shown in Fig. 8a. The wicking height of the antibacterial cotton fabrics showed a slightly faster rate in comparison with the untreated cotton fabrics.
The hand parameters were tested to compare the stiffness, softness, and smoothness of the untreated cotton fabric and antibacterial cotton fabric, as shown in Fig. 8b. The decrease amplitudes of stiffness and smoothness and the increase of softness were very low. It could be considered as only slight changes of the handle of the untreated and treated fabric. The antibacterial treatment using HTCS as agents had little impact on the handle of the fabric.
Figure 8c shows the mechanical properties of cotton fabrics finished with HTCS as compared with the untreated cotton fabrics. It was found that the maximum breaking elongation was improved from around 7.99 to 9.22% in the weft direction (an increase of 1.23%) while it was reduced slightly from around 17.0 to 16.2% in the warp direction (a decrease of 0.78%). The effective concentration of HTCS in the finishing solution was 1.3 g/L. Its content was very low and pH value of the finishing liquid was about 6.5, which caused little damage to the fabric. Consequently, the hand parameters and mechanical properties of the treated fabric using HTCS as antibacterial agent retained the level of the untreated fabric.
Formaldehyde release of cotton fabric using HTCS as antibacterial agent
In order to verify the safety of the antibacterial cotton fabric, the formaldehyde release of the treated cotton fabric using HTCS as an antibacterial agent was carried out at different pH values (Fig. 9). The formaldehyde release of antibacterial cotton fabric kept at 33 mg/kg at different pH values. According to the safety grades of classification standard, the antibacterial cotton fabric complied with Grade B textile requirements (< 75 mg/kg), which products can be in direct contact with the skin. It demonstrated that the treated cotton fabric using HTCS as an antibacterial agent was relatively safe and had greatly potential application prospect.
Conclusions
HT was successfully grafted onto CS using the Mannich reaction to form HTCS. The optimal grafting condition was at the molar ratio 2:1 of HT to CS at 90 °C. HTCS had good solubility in the pH range 3–8 and much higher antibacterial activity against both S. aureus and E. coli in comparison with CS and HT. Using HTCS as an antibacterial agent for the treatment of cotton fabric, the antibacterial rate of cotton fabric against S. aureus and E. coli achieved more than 99% and the remarkable increase in comparison with CS and HT, respectively. In addition, F-HTCS maintained good antibacterial durability after 25 washing cycles and preserved the inherent mechanical and physical properties of the untreated cotton fabric. The formaldehyde release test proved the safety of F-HTCS. HTCS is, therefore, expected to be a good natural antibacterial agent with the potential for many applications.
References
Bai HT, Yuan HX, Nie CY, Wang B, Lv FT, Liu LB, Wang S (2015) A supramolecular antibiotic switch for antibacterial regulation. Angew Chem Int Ed 54:13208–13213. https://doi.org/10.1002/anie.201504566
Bi R, Yue L, Niazi S, Khan IM, Sun D, Wang WZP, Jiang QX, Xia WS (2021) Facile synthesis and antibacterial activity of geraniol conjugated chitosan oligosaccharide derivatives. Carbohydr Polym 251:117099. https://doi.org/10.1016/j.carbpol.2020.117099
Buffa R, Hermannova M, Sojka M, Svozil V, Sulc P, Halamkova P, Pospísilova M, Krejcí H, Velebný V (2020) Hyaluronic acid chloramide—synthesis, chemical structure, stability and analysis of antimicrobials. Carbohydr Polym 250:116928. https://doi.org/10.1016/j.carbpol.2020.116928
Chang KC, Lin DJ, Wu YR, Chang CW, Chen CH, Ko CL, Chen WC (2019) Characterization of genipin-crosslinked gelatin/hyaluronic acid-based hydrogel membranes and loaded with hinokitiol: in vitro evaluation of antibacterial activity and biocompatibility. Mater Sci Eng 105:110074. https://doi.org/10.1016/j.msec.2019.110074
Chen SG, Guo YJ, Chen SJ, Yu HM, Ge ZC, Zhang X, Zhang PX, Tang JN (2012) Facile preparation and synergistic antibacterial effect of three-component Cu/Tio2/CS nanoparticles. J Mater Chem 22:9092–9099. https://doi.org/10.1039/C2JM00063F
Chen SG, Yuan LJ, Li QQ, Li JN, Zhu XL, Jiang YG, Sha O, Yang XH, Xin JH, Wang JX (2016) Durable antibacterial and nonfouling cotton textiles with enhanced comfort via zwitterionic sulfopropylbetaine coating. Small 12:3516–3521. https://doi.org/10.1002/smll.201600587
Deng XL, Nikiforov AY, Coenye T, Cools P, Aziz G, Morent R, De Geyter N, Leys C (2015) Antimicrobial nano-silver non-woven polyethylene terephthalate fabric via an atmospheric pressure plasma deposition process. Sci Rep 5:10138. https://doi.org/10.1038/srep10138
Gao Y, Cranston R (2008) Recent advances in antimicrobial treatments of textiles. Text Res J 78:60–72. https://doi.org/10.1177/0040517507082332
Gao DG, Li YJ, Lyu B, Lyu LH, Chen SW, Ma JZ (2019) Construction of durable antibacterial and anti-mildew cotton fabric based on P(DMDAAC-AGE)/Ag/ZnO composites. Carbohydr Polym 204:161–169. https://doi.org/10.1016/j.carbpol.2018.09.087
Han H, Liu C, Zhu J, Li FX, Wang XL, Yu JY, Qin XH, Wu DQ (2020) Contact/release coordinated antibacterial cotton fabrics coated with N-halamine and cationic antibacterial agent for durable bacteria-killing application. Int J Mol Sci 21:9489. https://doi.org/10.3390/ijms21186531
Hasan J, Crawford RJ, Ivanova EP (2013) Antibacterial surfaces: the quest for a new generation of Biomaterials. Trends Biotechnol 31:31–40. https://doi.org/10.1016/j.tibtech.2013.01.017
Jabar S, Mardiana L, Rahmah Z, Ajrina M, Imanda MR, Rahmi R, Khairi RM, Marlina M (2021) Preparation and characterization of chitosan-starch Janeng membranes cross-linking with citric acid. IOP Conf Ser Mater Sci Eng R Rep 1087:012066. https://doi.org/10.1088/1757-899X/1087/1/012066
Li Y, Zhang W, Niu JF, Chen YS (2012) Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 6:5164–5173. https://doi.org/10.1021/nn300934k
Lin J, Chen XY, Chen CY, Hu JT, Zhou CL, Cai XF, Wang W, Zheng C, Zhang PP, Cheng J, Guo ZH, Liu H (2018) Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Appl Mater Inter 10:6124–6136. https://doi.org/10.1021/acsami.7b16235
Liu H, Du YM, Yang JH, Zhu HY (2004) Structural characterization and antimicrobial activity of chitosan/betaine derivative complex. Carbohydr Polym 55:291–297. https://doi.org/10.1016/j.carbpol.2003.10.001
Mittal A, Singh A, Benjakul S, Prodpran T, Nilsuwan K, Huda N, Caba KDL (2021) Composite films based on chitosan and epigallocatechin gallate grafted chitosan: characterization, antioxidant and antimicrobial activities. Food Hydrocoll 111:106384. https://doi.org/10.1016/j.foodhyd.2020.106384
Morita Y, Sakagami Y, Okabe T, Ohe T, Inamori Y, Ishida N (2007) The mechanism of the bactericidal activity of hinokitiol. Biocontrol Sci 12:101–110. https://doi.org/10.4265/bio.12.101
Mumcuoglu KY, Gabbay J, Borkow G (2008) Copper oxide-impregnated fabrics for the control of house dust mites. Int J Pest Manage 54:235–240. https://doi.org/10.1080/09670870802010856
Muñoz-Bonilla A, Fernández-García M (2012) Polymeric materials with antimicrobial activity. Prog Polym Sci 37:281–339. https://doi.org/10.1016/j.progpolymsci.2011.08.005
No HK, Park NY, Lee SH, Meyers SP (2002) Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 74:65–72. https://doi.org/10.1111/j.1365-2621.2002.tb10314.x
Phan DN, Rebia RA, Saito Y, Kharaghani D, Khatri M, Tanaka T, Lee H, Kim IS (2020) Zinc oxide nanoparticles attached to polyacrylonitrile nanofibers with hinokitiol as gluing agent for synergistic antibacterial activities and effective dye removal. J Ind Eng Chem 85:258–268. https://doi.org/10.1016/j.jiec.2020.02.008
Raza ZA, Khalil S, Ayub A, Banat IM (2020a) Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr Res 492:108004. https://doi.org/10.1016/j.carres.2020.108004
Raza ZA, Anwar F, Abid S (2020b) Sustainable antibacterial printing of cellulosic fabrics using an indigenous chitosan-based thickener with distinct natural dyes. Int J Cloth Sci Tech 33:914–928. https://doi.org/10.1108/IJCST-01-2020-0005
Raza ZA, Taqi M, Tariq MR (2021) Antibacterial agents applied as antivirals in textile-based PPE: a narrative review. J Text I 2:1–13. https://doi.org/10.1080/00405000.2021.1889166
Shahid-ul-Islam BBS, Kumar A (2020) Green chemistry based in-situ synthesis of silver nanoparticles for multifunctional finishing of chitosan polysaccharide modified cellulosic textile substrate. Int J Biol Macromol 152:1135–1145. https://doi.org/10.1016/j.ijbiomac.2019.10.202
Shameli K, Ahmad MB, Yunus WMZW, Rustaiyan A, Ibrahim NA, Zargar M, Abdollahi Y (2010) Green synthesis of silver/montmorillonite/chitosan bionanocomposites using the UV irradiation method and evaluation of antibacterial activity. Int J Nanomed 5:875–887. https://doi.org/10.2147/IJN.S13632
Su ZW, Han QM, Zhang F, Meng XH, Liu BJ (2020) Preparation, characterization and antibacterial properties of 6-deoxy-6-arginine modified chitosan. Carbohydr Polym 230:115635. https://doi.org/10.1016/j.carbpol.2019.115635
Wen Y, Zhao RF, Yin XQ, Shi YD, Fan HJ, Zhou Y, Tan L (2020) Antibacterial and antioxidant composite fiber prepared from polyurethane and polyacrylonitrile containing tea polyphenols. Fiber Polym 21:103–110. https://doi.org/10.1007/s12221-020-9497-4
Xu YH, Deng YJ (2011) Study on preparation and properties of cotton fabric modified by anthraquinone extract from aloe. Adv Mater Res 287–290:2705–2708. https://doi.org/10.4028/www.scientific.net/AMR.287-290.2705
Yadav N, Monisha M, Niranjan R, Dubey A, Patil S, Priyadarshini R, Lochab B (2021) Antibacterial performance of fully biobased chitosan-grafted-polybenzoxazine films: Elaboration and properties of released material. Carbohydr Polym 254:117296. https://doi.org/10.1016/j.carbpol.2020.117296
Yin ML, Wang YF, Zhang Y, Ren XH, Qiu YY, Huang TS (2020) Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr Polym 232:115823. https://doi.org/10.1016/j.carbpol.2019.115823
Zhang J, Wei W, Yang LL, Pan YK, Wang XH, Wang TL, Tang SC, Yao Y, Hong H, Wei J (2018) Stimulation of cell responses and bone ingrowth into macro-microporous implants of nano-bioglass/polyetheretherketone composite and enhanced antibacterial activity by release of hinokitiol. Colloids Surf B 164:347–357. https://doi.org/10.1016/j.colsurfb.2018.01.058
Zhou C, Ao HY, Han X, Jiang WW, Yang ZF, Ma L, Deng XY, Wan YZ (2021) Engineering a novel antibacterial agent with multifunction: protocatechuic acid-grafted-quaternized chitosan. Carbohydr Polym 258:117683. https://doi.org/10.1016/j.carbpol.2021.117683
Acknowledgments
This work was supported by Zhejiang Province Public Welfare Technology Application Research Project (No. LGG19E030001) and the Fundamental Research Funds of Zhejiang Sci-Tech University (No. 2021Q003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Luo, Y., Zhao, X. et al. A natural antibacterial agent based on modified chitosan by hinokitiol for antibacterial application on cotton fabric. Cellulose 29, 2731–2742 (2022). https://doi.org/10.1007/s10570-022-04456-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04456-2