Abstract
The synthesis of multi-function flame retardants is widely increasing to fulfill industrial and economic goals. In this work, a novel flame retardant, melamine salt of tannic phosphate (MTP) was prepared and characterized. MTP was mixed with polyvinyl alcohol (PVA) solution and used as a coating for cotton fabrics. In addition, tannic acid (TA) and melamine phosphate (MP) were mixed with PVA solution and applied as a coating for cotton fabrics. Vertical and horizontal flammability tests showed that the flame did not propagate in samples treated with PVA/MTP. In contrast, samples treated with PVA/TA/MP burnt completely. Limiting oxygen index (LOI) data indicated that samples treated with PVA/30%MTP reached LOI value 68.4%, while the control sample had LOI value 17.1%. Smoke density results presented that PVA/MTP succeeded in reducing the maximum specific optical density (Ds max) of cotton fabrics. FTIR gas analyzer results manifested that the addition of PVA/MTP to cotton fabrics decreased the emission of CO, CO2, C3H8, C2H6, C6H14, and formaldehyde in the gas phase. Fractional effective dose (FED) and lethal toxic potency (LC50) showed that samples coated with PVA/MTP are less toxic than blank. In addition, these fabrics exhibited a remarkable antibacterial property against gram-positive and gram-negative bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabrics are excessively used in applications like clothing and house furniture due to their fabulous properties which include comfort, biodegradability, hydrophilicity, and good breathability (Chen et al. 2021; Zhu et al. 2020a). However, cotton fabrics are highly flammable and this problem restricts their industrial applications. For instance, the LOI of cotton fabrics is in the range of 16–18%, which makes fabrics able to combust at ambient conditions (where O2 in the air is 20.95%) (Chen et al. 2020). To overcome this problem, many studies gave great interest to improve flame retardancy of cotton fabrics using different kinds of flame retardant materials. As the cotton fabric is a naturally occurring material, an after-finish method is used to improve its flame retardancy (Pan et al. 2015). Halogenated flame retardants such as pentabromodiphenyl ether, decabromodiphenyl ether, and (Polychlorinated Biphenyl) showed good flame retardancy characteristics. However, the environmental regulations in many countries restricted their use because these materials manifested harmful effects to humans and animals (Van der Veen and De Boer 2012). Metal hydroxides such as Al(OH)3 and Mg(OH)2 were introduced as alternatives for halogenated flame retardants because they can take in a large amount of heat at high temperatures. But these materials should be added at high concentrations to obtain good flame retardancy action. The high loading levels of these materials deteriorate the mechanical properties (Hu et al. 2020; Zhu et al. 2020b; Laoutid et al. 2009). Intumescent flame retardants (IFRs) were used by many researchers to improve the flame retardancy of cotton fabrics because they are environmentally friendly (halogen-free compounds) and produce low amounts of smoke and toxic gases (Wang et al. 2021). Traditional IFRs are consisting of acid source (like phosphoric acid), charring agent (like pentaerythritol), and blowing agent (such as melamine). The ingredients of the IFR system are decomposed during the combustion process of polymer composite to form an insulating, cellular, and intumescent char layer on the polymer surface which protects the polymer from the effect of heat and flame. Moreover, it reduces the evolving of volatile organic compounds to the combustion zone (Wang et al. 2021; Chan et al. 2018; Fang et al. 2015). Liu et al. (2018a, b) coated cotton fabrics with polyethylenimine (PEI)/melamine and phytic acid (PA) using layer by layer assembly. Then, coated fabrics were dipped in a dilute solution of poly(dimethylsiloxane) (PDMS) for improving fire- retardancy and water repellent behaviour. The results indicated that cotton-4BL-PDMS samples achieved self-extinguishing in the vertical flammability test. Cone calorimeter (CC) data showed that PEI/melamine-PA/PDMS coating decreased the peak of heat release rate (pHRR) of cotton-4BL-PDMS sample by more than 50% in comparison with the control sample. Also, the samples presented a primary contact angle more than 130° and nearly did not show significant change over time which was explained as good water repellent behaviour. Li et al. (2020a) used layer by layer deposition method to coat cotton fabric with chitosan (CS) and ammonium phytate (AP). The authors reported that at 8 wt% weight gain from CS/AP, samples had LOI value 27% and achieved self-extinguishing in the vertical burning test. In addition, CC data indicated that CS/AP coating decreased the pHRR and THR of cotton by 55.8% and 64.1%, respectively. Li et al. (2020b) coated cotton fabrics with lignosulfonate (LS) and CS using layer by layer method. The interesting results in this work were that samples with 25.2% weight gain were able to achieve LOI value 26%. MCC data showed that cotton/LS/CS-25.2% samples had pHRR value 75 W/g while the control sample displayed pHRR value 266 W/g. Nabipour et al. (2020a) prepared flame retardant cotton fabric by deposition of guanazole complexes with silver and zinc metal ions on fabrics through self-assembly technique. Fabrics treated with guanazole-Zn and guanazole-Ag showed LOI values 29.5% and 27.5%, respectively. Also, they displayed self–extinguishing in the vertical flame test after removing the ignition source. Nabipour et al. (2020b) coated cotton fabrics with coatings containing ammonium hexametaphosphate (NH4-HMP), laponite (LAP), and hexadecyltrimethoxysilane which were synthesized through the sol–gel method. The effect of LAP concentrations on flame retardancy of the coated samples was evaluated. MCC and LOI values presented that as the loading level of LAP increased, pHRR and THR values decreased while LOI values increased. When LAP concentration was 2.5 wt%, LOI reached 29% while pHRR and THR decreased by 66.1% and 36.6%, respectively. Liu et al. (2018a, b) coated cotton fabrics with CS, sodium phytate and 3-Aminopropyl triethoxysilane (APTES) using layer-by-layer (LBL) assembly. Cone calorimeter data indicated that pHRR of the control sample was decreased from 223 to 40 kW/m2 in cotton-15BL sample. Also, coated samples showed lower production from smoke; and CO and CO2 gases in comparison with the control sample. Recently, much research work was directed to combine the IFRs ingredients in one molecule to reduce the polarity of traditional IFRs and preparing single-molecule intumescent flame retardant (SMIFR) (Makhlouf et al. 2020; Yang et al. 2019; Jian et al. 2019). In our previous work, we prepared different melamine salts such as melamine salt of montmorillonite phosphate (MMP), melamine salt of pentaerythritol phosphate montmorillonite (MPPM), and melamine salt of chitosan phosphate (MCHP) as SMIFRs and they showed good flame retardancy action on polypropylene and polyethylene (Makhlouf et al. 2017a, b; Hassan et al. 2016).
Tannic acid (TA) is one of the hydrolyzable tannins, commercially available and has an estimated chemical structure C76H52O46. It is composed of an inner layer containing glucose ring and outer layers consisting of gallic acid units. TA is used in different industries such as inks, plastic resins, adhesives, surface coatings and dyes. Besides; it is used in the water treatment process (Das et al. 2020; Ramakrishnan and Krishnan, 1994). Pantoja-Castro and González-Rodríguez (2011) used the thermogravimetric analysis technique to differentiate between TA and condensed tannins. The authors reported that tannins were decomposed through three decomposition steps meanwhile TA showed five decomposition steps. This was referred to that TA has a less complex structure and its bonds may be easily broken to form smaller structures. Also, the char residue at 600 °C was higher in the case of condensed tannins due to tannins have more carbon atoms in their chemical structure than TA. Pyrolysis of tannins at 600 °C yields mainly catechol as a peculiar fragment (Nam et al. 2017; Galletti and Reeves 1992). On the other hand, TA produces 1,2-benzene diol and 1,2,3-benzene triol from the degradation of the outer layer that containing gallic acid units. The inner layer was characterized by stability to higher temperatures and at temperature > 700 °C, it crosslinked to form intumescent carbonaceous char (Nam et al. 2017; Xia et al. 2015). Tributsch and Fiechter (2008) stated that the presence of tannins in the bark of trees makes them naturally fire-resistant due to tannins have antioxidant effects and are able to neutralize radicals through their electron donation properties. Therefore, tannins seem to be new bio-based materials that can be used in the development of fire retardant additives (Das et al. 2020; Singh and Kumar, 2020; Nam et al. 2017). Nam et al. (2017) treated cotton fabric with TA and TA/sodium hydroxide solution as IFR. The authors stated that, on one hand, treating cotton samples with 20% TA alone was able to alter the combustion behaviour of cotton. Microscale calorimeter results showed that 20%TA decreased the heat release capacity (HRC) of cotton by 35.8% and increased the char residue from 6.3% to 16.9%. However, TA alone was not sufficient to make improvement in the LOI value of cotton fabric. On the other hand, when treating cotton fabrics with 20%TA/1% sodium hydroxide LOI value increased to 30.2%, and the HRC of the control sample was reduced by 81.8% and the char residue was increased to 21.5%. But the durability, mechanical and vertical flammability properties were not studied. According to our literature survey, combining TA as a charring agent with other IFR ingredients to prepare SMIFR to impart different functions (such as flame retardancy and antibacterial effects) to cotton fabric was not studied.
Fire toxicity is considered the main reason for death and harm from unexpected fires. In the UK, during the period from 1955 to 2015, there has been a gradual transfer for the reason of death from burn to toxic gases, and the injuries due to fire toxicity increased greatly (Stec 2017; Stull 2008). Exposure to toxic gases (such as CO, CO2, NOx, SO2, acrolein, HCHO, NH3, HCl and HBr) which are produced from fatal fires drives to a set of physiological and behavioural effects. For instance, physical incapacitation and loss of sense of direction can take place when the person is subjected to these gases, and these effects are likely to threaten life because they can hinder safe escape. In the last decades, chemical analysis (without refuge to experiments on animals) was used to predict the main effects of fire toxicity like incapacitation by quantifying the toxic gases that evolved during different fire conditions in small scale tests (Stec 2017). Fractional Effective Dose (FED) is used in creating toxic potency data using chemical analysis of fire effluents, by taking into account the toxic effects of CO, CO2, HCl, HBr, NOx, SO2, anoxia and other toxic gases. FED is defined as the ratio of the concentration and time product for a toxic gas released in a given test to the concentration and time product of that toxicant that has been statistically defined from distinct experimental data to cause lethality in 50% of test animals during a fixed exposure and post-exposure time (Stec 2017; Chow et al. 2020). FED was calculated following Eq. (1) (ISO 13344: 1996; ISO 13344: 2015; Chow et al. 2020).
But this equation was not sufficient to consider the effect of CO2 concentration on fire toxicity. Therefore, FED calculations were updated (see Eq. 2) by including CO2 concentration; and the slope (m) and intercept (b) of plotting the curve of [CO] against [CO2] to describe the growing up of CO toxicity as the [CO2] increases. In addition, O2 concentration was included in this update (ISO 13,344:2015).
Besides Eqs. (1, 2), the Purser model in Eq. (3) was applied also to calculate FED where the concentration of each toxic gas was divided by its lethal concentration and multiply the result by\({\mathrm{V}}_{{\mathrm{CO}}_{2}}\). Where, \({\mathrm{V}}_{{\mathrm{CO}}_{2}}\) is a multiplication factor for CO2-driven hyperventilation, equal to {1 + e[(0.14. CO2) − 1]/2} and A is an acidosis factor, equal to {[CO2] × 0.05}.
When the FED value is equal to 1, the mixture of the toxic gases is cable of causing death to 50% of the animals that inhaled this mixture (Stec 2017; Chow et al. 2020). The fire toxicity of materials can also be presented by lethal concentration 50 (LC50). It is defined as the concentration of fire effluent that able to cause death in 50% of test animals at a defined exposure time. LC50 can be calculated based on FED value, mass loss during combustion (ΔM) and the volume of the combustion chamber (V) following Eq. (4) (Chow et al. 2020).
where, M (in g) is the specific mass loss and V (in m3) is the total air volume at standard temperature and pressure.
The aims behind this work were to combine TA with melamine and phosphoric acid to form a new multifunction single-molecule intumescent flame retardant, MTP, for cotton fabrics. In addition, comparing the efficiency of the new SMIFR with other traditional flame-retardant systems that were based on MP and TA. The thermal stability and flammability properties of the different samples were studied by TGA, UL94, LOI, single flame source and smoke box chamber. The toxic gases that evolved during the combustion process were determined by FTIR gas analyser and the fire toxicity of the samples was evaluated based on FED and LC50 data. Tensile strength and antibacterial properties of the treated cotton samples were evaluated.
Experimental
Materials
Natural cotton fabrics (100%; 120 g/m2) were supplied by Texmar Company, Egypt. Tannic acid (C76H52O46) with M.W 1701.22 g/mol was obtained from S D Fine-Chem Limited, India. Poly (vinyl alcohol) with M.W 115,000 g/mol and degree of polymerization 1700–1800 was purchased from Oxford Lab Chem, India. Melamine (99%) was purchased from Alfa Aesar Company, Germany. Phosphoric acid (85%) and methanol (99%) were supplied by Sigma-Aldrich Company, Germany. The chemicals were used without further purification. Deionized water was collected from select fusion water purification unit supplied by Purite limited Company, UK.
Synthesis of MTP
MTP was synthesized by adding 50 g (0.5 mol) phosphoric acid to 20 g (0.01 mol) TA to form tannic phosphate (TP) as an intermediate. The reactants were stirred on a hot plate with a stirrer at 100 °C and the reaction continued for 1 h till a black solution was obtained (pH = 0.01). In a round bottom flask with a condenser, 30 g (0.24 mol) of melamine powder was dispersed in 500 mL of methanol. TP solution was added dropwise to melamine (dispersed in methanol) within 30 min, the hot plate temperature remained at 120 °C and the reaction continued for 6 h. The product (MTP) was filtered, washed with methanol, dried at room temperature for 72 h and grinded to a fine powder. The final pH value of MTP in methanol after the washing process was 6.75 pH unit. Figure 1a shows the synthetic route of MTP.
Preparation of MP
MP was synthesized according to the procedure in Abdelkhalik et al. (2019). In a round bottom flask linked to a condenser, 30 g (0.24 mol) of melamine was scattered in 250 mL methanol and agitated for 30 min at room temperature using a hot plate with a stirrer. Then, 25 g (0.25 mol) phosphoric acid was added to melamine, the hot plate temperature was regulated at 120 °C and the reaction was kept for 3 h. Finally, MP was obtained, filtered, washed with 500 mL methanol to remove the excess of phosphoric acid and dried for 72 h at room temperature.
Preparation of flame retardant cotton fabrics
Cotton fabric samples were washed using deionized water and were dried at 60 °C for 1 h before use. Cotton samples with appropriate size were weighted and the results were recorded. A beaker containing deionized water and PVA was heated to 150 °C for 30 min using a hot plate with a mechanical stirrer at 650 rpm until PVA was dissolved in deionized water. Then, MTP was added into the PVA solution with continuous stirring at 70 °C. PVA/MTP finishing solutions with different MTP concentrations (10%MTP, 20%MTP and 30%MTP) were prepared. The cotton fabric samples were soaked in these solutions at 70 °C for 10 min and 90% pick-up was obtained within three pads. After that, the coated samples were dried at 100 °C for 5 min and cured at 160 °C for 3 min. The other samples which were coated with PVA/MP, PVA/TA and PVA/MP/TA were prepared according to the previous method.. Figure 1(B) shows the structure of cotton fabric/PVA/MTP prepared by the dip-pad-dry method. The weight gain (WG) of MTP-coated cotton fabrics were calculated based on Eq. (5).
where, Wa and Wb are the weight of control sample and MTP coated cotton fabrics, respectively. Table 1 presents the coating composition of cotton fabric samples.
Characterization and measurements
1H NMR spectra were reported on Varian Mercury (400 MHz) spectrometer and dimethyl sulphoxide (DMSO-d6) was selected as a solvent. FTIR spectra were recorded by Nicolet 380 spectrophotometer in the optical range of 4000–400 cm−1. Thermogravimetric analysis (TGA) was executed by Shimadzu TGA50 under nitrogen atmosphere with a flow rate 30 mL/min. TGA measurements were started from ambient temperature to 750 °C, the heating rate was10 °C/min and the samples weights were approximately 6–7 mg. Vertical and horizontal flammability tests were performed using the UL94 flame chamber (purchased from FTT—company, UK). The vertical test was carried out according to the standard test method ASTM D 6413 (2015) where samples with dimensions 250 mm × 90 mm were subjected to vertical flame with 38 mm height for 12 s. The horizontal burning rate of the samples was evaluated according to the standard test method ISO 3795 (1989) where samples were exposed horizontally to flame with 38 mm height for 15 s. The ignitability of specimens was determined by single flame source apparatus (manufactured by FTT-Company, UK). In this test, a small flame with 20 mm height directly impinged with a vertically oriented test specimen for 30 s according to the standard test method ISO 11925–2 (2020). LOI values were determined by oxygen index (supplied by Reohmetric Scientific Ltd, UK) according to the standard test method ISO 4589–2:(2017). The samples dimensions were 120 mm × 50 mm. Specific optical density was determined by the smoke density chamber (manufactured by FTT—Company, UK) according to the standard test method ISO 5659–2:(2017). The specimens’ dimensions were 75 mm × 75 mm. Each specimen was wrapped in aluminium foil and subjected horizontally to external heat flux 25 kW/m2 under non-flaming conditions. The smoke density chamber was linked to FTIR gas analyser (supplied by FTT Company, UK) to measure continuously, during the smoke density test, the concentrations of the following gases: CO, CO2, H2O, NO, NO2, HCN, NH3, HCHO, C3H8, C2H6 and C6H14. The concentration of O2 gas was measured by Testo gas analyser. The recorded concentrations for gases are the average of three tested samples results. SEM images were collected by Quanta 250 FEG (Field Emission Gun) produced by FEI Company, the Netherlands. The assessment of bacteriostatic activity against Gram-positive (Staphylococcus aureus, S. aureus) and Gram-negative (Escherichia coli, E-Coli) bacteria was assessed using agar diffusion test according to AATCC test method 147–2004 (Parallel Steak Method) and expressed as zone inhibition (mm). Fresh inoculants for antibacterial assessment were prepared on nutrient at 37 °C for 24 h. Any prominent zone of inhibition around the samples was recorded as an inhibitory effect against the above mentioned bacterial species. Tensile strength was measured in the strip method by using universal testing machine (Tinlus Olsen, model H5KT) according to the standard test method ISO 13934–1:(2013). The durability study was performed using tape water, without adding any detergent, at 30 °C in a small washing machine. Ten washing cycles were applied where each washing cycle continued for 5 min. Then, samples were dried in an oven at 60 °C for 1 h.
Results and discussion
Characterization of MP, MTP, control and coated samples
1H NMR of TA and MTP
1H NMR of melamine and phosphoric acid were presented in our previous work Abdelkhalik et al. (2019) and Makhlouf et al. (2020) where 1H NMR of melamine showed a characteristic peak at 6.1 ppm due to NH2 protons and phosphoric acid spectrum displayed a peak at 7.7 ppm for P(OH3). 1H NMR of TA and MTP are presented in Fig. S1(A, B). TA spectrum shows a peak at 5.3 ppm for OH protons and peaks around 6.9 ppm due to the aromatic protons. 1H NMR of MTP presents that TA peak at 5.3 ppm was disappeared. Also, the intensity of the peaks at 6–8 ppm was increased. This may be referred to interference between aromatic ring protons of TA, NH2 protons from melamine, and the protons of (OH) in phosphate groups.
FTIR analysis of melamine, TA, MP and MTP
FTIR spectra of melamine, TA, MP and MTP are presented in Fig. 2a. The main absorption peaks and the corresponding functional groups are presented in Table 2. The data in Table 2 for melamine and TA indicates good consistency with literature data. FTIR absorption peaks of MP agree well with the data recorded in Abdelkhalik et al. (2019) and Chen and Wang (2007). When comparing FTIR spectra of TA and MTP in Fig. 2a, it can be seen formation of new peaks in MTP spectrum at 3420 cm−1and 3470 cm−1 (for NH2); 3140 cm−1and 1406 cm−1 (for +NH3); 3370 cm−1 (for hydrogen-bonded to OH); 2680 cm−1 (for OH of O = P-O–H); 1670 cm−1, 1560 cm−1 and 817 cm−1 (for triazine ring); 1250 cm−1, 1170 cm−1 and 1110 cm−1 (for P=O, P–O and P–O–C); 499 cm−1 and 619 cm−1 (for O–P–O stretching). In addition, the absorption peaks of TA at 1620 cm−1 and 1425 cm−1 (for C–C aromatic); 780 cm−1 (for C–H aromatic); 2360 cm−1 (for O–C =O); 1710 cm−1 (for C=O) are also found. These data confirm the interaction between phosphoric acid, TA and melamine to form MTP.
FTIR analysis of control and coated samples
FTIR spectra of C0 and C7 samples are presented in Fig. 2b. It is clearly seen in FTIR spectrum of C0 absorption peaks at 3443 cm−1 (for H bonded OH groups), 2800–3000 cm−1 (for C–H stretching), 2920 cm−1 (for CH2 of long alkyl chain), 1630 cm−1 (for adsorbed water), 1389 cm−1 (for C–H bending), 1120 cm−1 (for C–O–C bridge), 1044 cm−1 (for C–O stretch) and 894 cm−1 (for out-of-phase ring stretch: C1–O–C4; β glucosidic bond) (Chung et al. 2004). FTIR spectrum of C7 shows nearly the same absorption peaks as C0. However, there are some differences where the intensity of the peak 1630 cm−1 increased which attributed to the presence of triazine ring in MTP. The peaks at 1160 cm−1, 1120 cm−1 and 1044 cm−1 are merged in one broad peak in the range 1000 cm−1–1174 cm−1. Moreover, a new peak appeared at 466 cm−1 which refers to O–P–O groups (Dayanand et al. 1996). It is stated by Wang et al. (2013) and Xu et al. (2018) that the construction of hydrogen bonds can drive to enhancing the intensity of the peak of hydroxyl radicals in the FTIR spectrum. The relative height of the peak in the range 3000–3676 cm−1 was calculated. The weight gain from PVA/MTP is expected to affect the result of peak height (Wang et al. 2013; Xu et al. 2018). Figure 3b shows that the relative peak height of the control sample increased with the addition of PVA/MTP which confirms the formation of hydrogen bonds.
SEM images of control and coated samples
Figure 3a–d shows the surface morphology of the control sample and the treated samples (C5, C7 and C8). The surface of the control sample is smooth and clear as there is no coating on it (see Fig. 3a). SEM images of treated samples show the presence of a flame-retardant coating on the surface and the samples are thicker than the control sample. Figure 3b, c shows that the coating particles were able to penetrate into the interior of cotton fibre of C5 and C7 samples. Figure 3d presents that intensive particles of MTP are found on the surface of C8 sample where the sample is coated with MTP only.
Flammability properties
Vertical and horizontal flammability tests
The data in Table 3 shows the results of horizontal and vertical burning tests. In the horizontal burning rate test, the control sample burnt completely and its rate of burning was 214 mm/min. In the vertical flammability test, the control sample burnt completely where after flame time (taf) and afterglow time (tag) were 9 s and 36 s, respectively; and there was no residual char after the test. The addition of PVA to cotton fabric, C1 sample, did not show a significant change in horizontal and vertical flammability properties of the control sample (see Table 3). C2 sample data indicated that PVA/TA system reduced the rate of burning of the control sample by 26.6% and increased its taf to 19 s. C3 sample showed that (PVA/MP) decreased the rate of burning of control sample by 35.5%, while taf raised to 30 s, tag increased to 40 s and the char length was 250 mm. According to the data in Table 3, C4 failed to achieve better or even the same flammability results of C3. This indicates that the addition of TA to PVA/MP decreased the efficiency of PVA/MP as a flame retardant coating for cotton fabrics. The next data for LOI and smoke density for C3 and C4 showed the same behavior also. This may be referred to that MP and TA were decomposing separately at the same time and the presence of TA may be decreased the possibility of interaction between MP and PVA to form a coherent char layer on the sample surface. On the contrary, C5, C6, C7 and C8 samples had a burning rate 0 mm/min. Moreover, Tafand Tagfor C5, C6, C7 and C8 samples were 0 s. This confirms that PVA/MTP and MTP alone are highly efficient as flame retardants compared with PVA/TA, PVA/MP and PVA/MP/TA. Figure 4 shows the different samples after the vertical flammability test. It is clearly seen in Fig. 4a–c that C0, C1 and C2 burnt completely without leaving char residue. The flame propagated to the end of samples C3 and C4 and the char length values in C3 and C4 were 250 mm (Fig. 4d, e). In contrast, Fig. 4f–i shows that the flame did not propagate in samples C5, C6, C7 and C8 and the char lengths of these samples were 120 mm, 90 mm, 75 mm and 65 mm, respectively. It is expected that the single-molecule intumescent flame retardant MTP decomposed to give CO, CO2, NH3 and water vapor in the gas phase. In the condensed phase, phosphate groups decomposed to produce phosphoric acid and polyphosphoric acid which interacted with PVA, TA and cotton fabric to form a coherent and compact char layer which prevented cotton from the effect of flame. In conclusion, PVA/MTP and MTP alone presented high efficiency as a flame retardant for cotton fabrics in comparison with PVA/TA, PVA/MP and PVA/TA/MP. The order of different flame retardants based on their efficiency was as follow:
PVA/MTP and MTP > PVA/MP > PVA/MP/TA > PVA/TA > PVA.
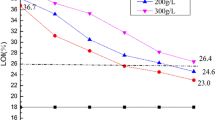
LOI data
LOI is defined as the minimum concentration of oxygen, in oxygen and nitrogen mixture, which can support flame propagation in a vertical test sample under particular test conditions (ISO 4589–2 (2017)). The higher LOI values of samples relative to blank reflect the increase in flame retardancy of specimens. LOI data are presented in Table 3 and it shows that, on one hand, the control sample had LOI value 17.1%. The addition of PVA (C1), PVA/TA (C2) and PVA/MP (C3) increased the LOI value of the control sample to 19.4%, 22.3% and 28.5%, respectively. C4 sample showed LOI value 23.2%. This indicates that the coating in C4 sample (PVA/TA/MP) failed to improve LOI value compared to C3 (where PVA/MP was used as a coating). Therefore, adding TA to PVA/MP was not the best choice to improve the flame retardancy of cotton fabrics. On the other hand, C5, C6 and C7 samples showed LOI values 39.4%, 50.6% and 68.4%, respectively. This means that the addition of PVA/MTP to cotton fabric enhanced greatly its flame retardancy. And as the loading level of MTP increased in the coating formulation, the LOI value was increased. It is clearly seen in Table 3 that PVA/MTP system (C5, C6, C7) was highly efficient compared with PVA/MP system (C3). In addition, the coating of C7 was more efficient as a flame retardant than the coating of C8 sample (MTP alone) which had LOI value 48.1%. This means that PVA participated with MTP in improving the flame retardancy of the control sample through fixing MTP inside cotton fabric and enhancing the formation of a thermally stable char layer on the samples surface.
Single flame source test
The single flame source test was performed to subject the control and coated samples to direct flame for a time of more than 12 s. This test is a part of fire tests which are used to evaluate the fire performance of building materials. According to ISO 11925–2:(2020), the flame can be applied to the sample for 30 s or 15 s. Also, the flame can be applied at the midpoint of the bottom edge of the sample or at the sample surface after 40 mm from the bottom edge. In this work, the flame was applied at the midpoint of the bottom edge for 30 s and the total test duration was 60 s. The sample is considered pass the test when the flame front doesn’t exceed the reference mark at 150 mm within 60 s (30 s as flame application time + 30 s after removing the ignition source away from the sample). It is clearly seen in Table 4 that the samples C0, C1, C2, C3 and C4 failed to pass the test where the flame front reached the reference mark at 150 mm within 60 s. In contrast, the samples C5, C6, C7 and C8 did not ignite and passed the test. This means that the addition of PVA/MTP and MTP (alone) to cotton fabrics enhanced greatly its flame retardancy. Moreover, the single-molecule intumescent flame retardant, MTP, in PVA/MTP system was more efficient than PVA/TA, PVA/MP and PVA/MP/TA mixtures. According to BS EN 13501-1:(2018), the samples C5, C6, C7 and C8 fulfil the requirements of classes B, C, D, E concerning single flame source test only. But other large scale fire tests such as the single burning item test will be necessary to consider the samples achieve completely class B, C and D requirements (BS EN 13501-1:(2018)).
Smoke density
The data in Fig. 5 shows the maximum specific optical density (Dsmax) for all samples at 25 kW/m2 and non-flaming conditions. Dsmax of the control sample was 80.5 and it decreased in C1 and C2 samples by 22.8% and 23.3%. PVA/MP and PVA/MP/TA minimized Dsmax of cotton fabrics by 62.7% and 51.4% as it is shown in C3 and C4 results. This indicates that PVA/MP mixture was more efficient as a smoke suppressant than PVA/MP/TA mixture. The samples C5, C6, C7 and C8 showed the maximum reductions in Dsmax and the amounts of these reductions were 79.7%, 76.7%, 72.2 and 82.9%, respectively. This is referred to the formation of a protective char layer on the cotton fabric surface which saved the underlying polymer from the effect of heat. The digital photographs in Fig. 6 for the samples after the smoke density test confirmed this suggestion. It is clearly seen in Fig. 6 that C0, C1 and C2 samples approximately bunt completely. The char residue of C3 sample was coherent and covered the whole sample meanwhile the char residue of C4 was discontinuous and the sample burnt completely at certain parts. This confirms that PVA/MP mixture was more efficient as a flame retardant than PVA/MP/TA. The char residue of samples C5, C6, C7 and C8 in Fig. 6 was continuous, coherent, and compact and were able to protect the underlying cotton samples from the effect of heat and oxygen. This led to higher reductions in Dsmax values of C5, C6, C7 and C8 relative to C0. According to smoke density data, the order of the efficiency of different additives as a smoke suppressant is MTP > PVA/MTP > PVA/MP > PVA/MP/TA > PVA/TA > PVA.
FTIR gas analyser data and flame retardant mechanism
FTIR gas analyser connected to the smoke density chamber was used to identify the combustion products in the gas phase. The data in Table 5 shows the average values for the concentrations of gases that are detected by FTIR gas analyser. According to the data in Table 5, the control sample decomposed and gave out mainly CO, CO2, water vapour, HCHO, C2H6 and C6H14. In addition, NO, HCN, CH4, C2H4 and C3H8 were detected at minor concentrations. The presence of NO and HCN in combustion products of C0 sample is referred to the oxidation of nitrogen in the air which was found inside the smoke density chamber. The addition of PVA to cotton fabric, sample C1, increased the concentrations of water vapour, CO, C3H8, C6H14 and C2H6. The gases HCHO and CO2 were also produced, but at lower concentrations compared to the control sample. The increase in hydrocarbon gases (C3H8, C6H14 and C2H6) concentrations is referred to the decomposition of PVA. It was reported that PVA decomposition takes place through dehydration, depolymerization and polyene formation. Then, pyrolysis of polyene formed and producing certain volatile organic compounds (Zaikov and Lomakin, 1998; Gaikwad et al. 2015; Dong et al. 2016). FTIR gas analyser data for C2, C3 and C4 samples showed that the addition of PVA/TA, PVA/MP and PVA/MP/TA to cotton fabric succeeded in decreasing its CO and CO2 production during combustion. The production of hydrocarbon gases (C3H8, C6H14and C2H6) was also reduced. The maximum reductions were observed in C3 sample where PVA/MP retarded the effect of heat through the formation of a coherent char layer on the cotton sample surface. But the presence of MP in the samples C3 and C4 led to an increase in the concentrations of NH3 and HCN relative to the control sample. This is referred to the decomposition of melamine in MP structure. The data in Table 5 manifested that C5, C6, C7 and C8 samples which contain PVA/MTP and MTP alone as coatings produced the highest concentrations of NH3 and water vapour relative to the other samples. In addition, the production of CO, HCHO and hydrocarbon gases were decreased relative to C0, C1, C2, C3, and C4 samples. This indicates that degradation of MTP produced water vapour, CO2 and NH3 (in the gas phase) which diluted the concentrations of volatile organic compounds and oxygen in the combustion zone. Moreover, the great reduction in the concentrations of HCHO and hydrocarbon gases indicates that PVA and cotton fabrics in C5, C6, C7 and C8 samples maintained most of their main structure during combustion. This is referred to the formation of coherent, continuous and compact char layer on cotton fabric; see Fig. 7a. This char acted as a physical barrier and protected the cotton fabric from the effect of heat and flame; and decreased the emission of toxic gases.
SEM image of char residue
Figure 7b–d shows the surface morphology of the char residue of C7 sample after the smoke density test. It is clearly seen that the fibres are grouped, and the surface was wrapped and protected by a char layer with a cellular structure. These properties are referred to that PVA/MTP coating produced during C7 combustion polyphosphoric acid which promoted the dehydration and carbonization of cotton fibre. Moreover, the degradation of PVA/MTP sent out NH3, CO2 and water vapour. These gases led to char intumesce. Besides, these gases dilute volatile organic compounds and oxygen in the combustion zone. The intumescent char layer acted as a barrier and protected the cotton from the effect of heat and flame, and reduced the escaping of volatile organic compounds from the substrates to the combustion zone, see Fig. 7a.
FTIR analyses of char residue
The char residue after the smoke density test for C7 sample was analysed by FTIR. Figure 7(e) shows a broad peak at 2800–3600 cm−1 due to interfering between the peak at 3450 cm−1 which is referred to hydrogen-bonded OH group and the peak at 2920 cm−1which was attributed to the CH2 group. This confirms the presence of carbon–hydrogen chains in the char structure. The peak at 1630 cm−1 is for aromatic structure, the peak at 1400 cm−1 is for C–N and C=N groups, the peak at 1160 cm−1 is for P–O, the peak at 1050 cm−1 is for P–O–C, the peak at 984 cm−1is for polyphosphate and/or pyrophosphate, the peak at 881 cm−1is for alkene, the peak at 492 cm−1 is for O–P–O group (Abdelkhalik et al. 2020, 2019; Wahyono et al. 2019; Makhlouf et al. 2017a, b; Viswanath et al. 2015; Chen and Wang 2007).
Smoke toxicity
Fire toxicity for all samples was evaluated based on FED and LC50 data. FED values were calculated using Eqs. (1,2,3) where the following gases were taken into account: CO, CO2, NO, NO2, O2, HCN, and HCHO. Figure 8a shows that FED values of control and treated samples are lower than 1 regardless the equation used in the calculation. Following Eq. (1), the samples C4, C5, C6 and C7 have FED values greater than the control sample, but the maximum difference between blank and these samples is very low (0.04) as it is obvious in Fig. 8a. According to Eq. (2), FED values for treated samples are lower than the control sample which means lower toxicity. Figure 8a presents that calculating FED using Eq. (3) leads to lower FED values for all treated samples relative to cotton fabric. The only exception was C7 which has FED value (0.2) equal to FED value of blank. The data of Eqs. (2, 3) indicates that the different formulations for coating were able to decrease the fire toxicity of cotton fabrics. LC50 values were calculated according to Eq. (4) where FED values were obtained following Eq. (3). Figure 8b shows that all samples have LC50 value greater than the control sample. This indicates that coated samples are less toxic than the control sample. The maximum values for LC50 were observed in C5, C6, C7 and C8 which means that PVA/MTP and MTP coatings were able to reduce fire toxicity of cotton fabrics.
Antibacterial properties
The data in Table 6 show antimicrobial activity inhibition zone in (mm) for C0, C5, C6, C7 and C8. The results prove that there is no zone of inhibition (ZOI) on uncoated cotton (C0), indicating that it has no antibacterial property. However, the antibacterial activity was enhanced when the concentration of MTP increased in the coating formulation. This may be referred to the amine groups in MTP, which are in the form of +NH3 and have positive charges, interacted with the negative charges on the surface of bacteria cells. This interaction led to expanded changes in the cell surface and cell permeability and causes leakage of thiol of protein. Figure 9A, a, B, b displays the effect of G + ve and G−ve bacteria on the sample treated by MTP in the absence as well as the presence of PVA. It is clearly seen that the bacteria die on the surface of the coated fabric and the growth of bacteria around the coated fabric was stopped. According to the data in Table 6, inhibition of S. aureus is more dominant than E. coli. This is attributed to the mode of action of MTP on bacteria includes binding of the cationic MTP with the anionic cell surface. The results of antibacterial properties indicated that MTP can be used in home and antimicrobial textile.
Mechanical properties
The data in Table 6 shows the tensile strength of control and treated samples. It can be seen that the tensile strength value of the control sample decreases with the addition of PVA/MTP and MTP alone. In addition, as the loading level of MTP increases in PVA/MTP system, the tensile strength value decreases relative to blank. The reductions in tensile strength are 6.5%, 9.1%, 18.1% and 3.6% in C5, C6, C7 and C8, respectively, which means that PVA/MTP and MTP coatings have a slightly negative effect on the tensile strength.
TGA of control and treated samples
TGA was used to study the thermal degradation of MTP, PVA, C0, C1, C7 and C8 samples. The data for thermal analysis are presented in Table 7 and Fig. 10a, b. MTP shows, in Fig. 10a, two decomposition steps. The first degradation step locates between 25 °C and 205 °C and includes 5.6% weight loss which is signed to losing moisture. The second decomposition step is between 208 °C and 493 °C and it involves 54.2% weight loss. During this step, T50% (at 414 °C) and Tmax (at 390 °C) are attained. This step includes dehydration of OH group and abstracting NH2 group to form non-flammable gases besides evolving CO and CO2. In addition, phosphate ester groups decomposed to form polyphosphoric acid which interacted with other elements (C, O, N) to form a carbonous char layer. The gradual growing up in temperature after 493 °C leads to a gradual increase in weight loss and the residual char at 750 °C is 24.6%. PVA sample reaches T10%, T50% and Tmax at 224 °C, 269 °C and 266 °C, respectively, and it leaves 2.5% as char residue at 750 °C. The thermal degradation of PVA takes place through two steps, where the first stage includes dehydration, depolymerization of PVA, and polyene formation. The second decomposition step involves pyrolysis of polyene and it is accompanied by producing certain volatile organic compounds (Zaikov and Lomakin, 1998; Gaikwad et al. 2015; Dong et al. 2016). C0 sample attains T10%, T50% and Tmax at 300 °C, 347 °C and 348 °C, respectively. The main decomposition stage of the cotton sample locates between 280 °C and 400 °C and it is accompanied by 68.5% weight loss. Thermal degradation of C0 sample takes place either through depolymerization from sugar-based units to form volatile compounds (chiefly including levoglucosan, furan, and furan derivatives) or by glycosyl unit dehydration to form aromatic char layer which is a thermally stable compound (Carosio et al. 2015; Wan et al. 2020). Coating cotton fabric with PVA alone decreased its T10% and T50% by 24 °C and 1 °C, respectively, and increased Tmax by 3 °C. The reduction in T10% of C1 relative to C0 is referred to dehydration and depolymerization of the PVA layer on the cotton fabric surface. TGA data of C7 show that it attains T10% at 251 °C. This great reduction in T10% relative to C0 is attributed to the earlier degradation of PVA/MTP coating which accompanied by evolving of water vapour, ammonia and other volatile compounds. T50% of C7 is increased by 24 °C relative to C0 and by 25 °C compared with C1. Tmax of C7 is attained at a lower temperature (304 °C) compared with C1 and C7. This may be referred to decomposition of MTP to form polyphosphoric acid which can accelerate decomposition and interaction with O and C atoms to form an insulating char layer containing polyaromatic structure. C8 sample shows a higher value for T10% (273 °C) relative to C7. This is attributed to that coating of C7 sample contains PVA which is decomposing at earlier temperatures (T10% of PVA is 224 °C). T50% and Tmax of C8 are attained at 365 °C and 336 °C. The maximum mass loss rate was 1.19 mg/min in C0 and it decreased to 0.5 mg/min and 0.51 mg/min in C7 and C8. Moreover, the char residue at 750 °C increased from 0.3% in C0 to 4.4%, 25.5% and 26.6% in C1, C7 and C8, respectively. This indicated that MTP assisted in the formation of an insulating char layer which protected cotton fabric from the effect of heat, oxygen and slowed down the degradation process of C7 and C8 samples. Moreover, the emission of gases such as NH3, CO2 and water vapour expelled degradation heat and decreased the temperature of the treated cotton sample surface (Levchik et al. 1996; Xu et al. 2019; Wan et al. 2020).
Durability study
The flammability properties of coated samples were studied after washing samples using tap water and drying them at 60 °C for 1 h. The results of horizontal and vertical flammability tests after washing are presented in Table 8 (in supplementary data). In the horizontal flammability test, the flame propagated in samples C5 and C8 and the rate of burning was 65 mm/min and 206.1 mm/min. Meanwhile, flame failed to spread in C6 and C7 and the burning rate was 0 mm/min. In the vertical flammability test, C5 sample results manifested that the flame attained the far end of the sample (char length 250 mm) and Taf was 7 s. The flame propagation was decreasing as the concentration of MTP in PVA/MTP coating increased. C6 and C7 showed char lengths values 110 mm and 90 mm, and Taf results were 0 s. This means that C6 and C7 coatings still have high flame retardancy action after the washing process. Fig. S2 shows C5, C6 and C7 samples after the vertical flammability test, and it confirms that PVA/20%MTP (C6) and PVA/30%MTP (C7) coatings still have good flame retardancy action after the washing process. The data in Table 8 indicated that within flame application time, the flame reached the far end of C8 without leaving char residue. The results of horizontal and vertical flammability tests indicated that C8 samples lost nearly all the flame-retardant material during the washing process. In contrast, PVA in C6 and C7 samples played an important role in fixing the flame retardant (MTP) on the cotton fabric surface during the washing process. LOI data in Table 8 confirmed the horizontal and vertical flammability tests results. It can be seen that the LOI of C8 after washing process is 18.5%, meanwhile, LOI values of C5, C6 and C7 are 24.2%, 33.5% and 49.8%, respectively.
Single flame source test data in Table 9 (in supplementary data) are also promoted the previous flammability tests. C8 and C5 failed in the single flame source test, where the flame front reached the reference mark at 150 mm within 60 s. In contrast, C6 and C7 pass the test and showed a higher degree of flame retardancy compared to C8 and C5 samples. It can be concluded that PVA plays a vital role in fixing MTP on the cotton fabric surface and as the concentration of MTP in PVA/MTP mixture increases, the flame retardancy of the cotton fabric increases after the washing process. Applying MTP alone, as a coating for cotton fabric, is not sufficient to impart flame retardancy to the fabric after the washing process.
Conclusions
A novel single-molecule intumescent flame retardant, MTP, was synthesized and characterized by FTIR and 1H NMR. MTP was mixed with PVA solution and used as a coating for the cotton fabric to improve its flame retardancy and antibacterial properties. Horizontal flammability test data showed that coated samples C5, C6, C7 and C8 had a burning rate equal to 0 mm/min while the burning rate of the control sample was 214 mm/min. Vertical burning test results indicated that applying PVA/MTP and MTP alone as coatings for cotton fabric prohibited flame propagation in cotton fabric samples. LOI of the control sample was 17.1% and it increased to 39.4%, 50.6%, 68.4% and 48.1% in C5, C6, C7 and C8 samples, respectively. Single flame source test results showed that samples C5, C6, C7 and C8 were able to pass the test and the flame did not propagate through the samples. The maximum specific optical density of the control sample was decreased by 79.7%, 76.7%, 72.2% and 82.9% in C5, C6, C7 and C8 samples, respectively. FTIR gas analyser results indicated that the coatings PVA/MTP and MTP (alone) decreased the emission of CO, CO2 and HCHO gases from cotton fabric, while NH3 concentrations increased due to decomposition of melamine part in MTP. Digital photos and SEM images for char residue after smoke density test showed that MTP was able to form coherent and insulating char layer on the surface of cotton fabric. This char protected the fabric from the effect of heat and oxygen, and prevented evolving of volatile organic compounds to the combustion zone. FED and LC50 calculations indicated that coated samples are less toxic than the control sample. In addition, samples C5, C6, C7 and C8 showed antibacterial characteristic against G + ve (S.aureus) and G−ve (E. coli) bacteria. TGA data presented that PVA/MTP mixture enhanced the thermal stability of cotton fabric at high temperature (T50%) and increased the char residue at 750 °C from 0.3% in the control sample to 25.5% in C7 sample. Durability study manifested that C6 and C7 samples can maintain good flame retardancy action after washing them with tap water. In contrast, C8 sample lost its flame retardancy due to losing MTP particles during the washing process. This indicated that PVA played an important role in preserving MTP on cotton samples during the washing process.
References
Abdelkhalik A, Makhlouf G, Hassan MA (2019) Manufacturing, thermal stability, and flammability properties of polypropylene containing new single molecule intumescent flame retardant. Polym Adv Technol 30:1403–1414. https://doi.org/10.1002/pat.4573
Abdelkhalik A, Makhlouf G, Abdel-Hakim A (2020) Fire behavior of natural rubber filled with intumescent flame retardant containing graphite. J Vinyl Addit Technol 26(2):155–164
ASTM D6413 (2015) Standard test method for flame resistance of textiles (vertical test)
Carosio F, Negrell-Guirao C, Blasio AD, Alongi J, David G, Camino G (2015) Tunable thermal and flame response of phosphonated oligoallylamines layer by layer assemblies on cotton. Carbohydr Polym 115:752–759. https://doi.org/10.1016/j.carbpol.2014.06.066
Chan SY, Si L, Lee KI, Ng PF, Chen L, Yu B, Hu Y, Yuen RKK, Xin JH, Fei B (2018) A novel boron–nitrogen intumescent flame retardant coating on cotton with improved washing durability. Cellulose 25:843–857. https://doi.org/10.1007/s10570-017-1577-2
Chen Y, Wang Q (2007) Reaction of melamine phosphate with pentaerythritol and its products for flame retardation of polypropylene. Polym Adv Technol 18(8):587–600
Chen Y, Wang D, Liu S, Lu Y, Zhang G, Zhang F (2020) A novel P-N-based flame retardant with multi-reactive groups for treatment of cotton fabrics. Cellulose 27:9075–9089. https://doi.org/10.1007/s10570-020-03387-0
Chen Y, Wan C, Liu S, Wang P, Zhang G (2021) A novel flame retardant based on polyhydric alcohols and P-N synergy for treatment of cotton fabrics. Cellulose. https://doi.org/10.1007/s10570-020-03615-7
Chow CL, Han SS, Han GY, Hou GL, Chow WK (2020) Assessing smoke toxicity of burning combustibles by four expressions for fractional effective dose. Fire Mater 44:804–813. https://doi.org/10.1002/fam.2875
Chung C, Lee M, Choe EK (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohyd Polym 58:417–420
Das AK, Islam MN, Faruk MO, Ashaduzzaman M, Dungani R, Rosamah E, Hartati S, Rumidatul A (2020) Hardwood tannin: sources, utilizations, and prospects. In: Aires A (ed) tannins structural properties, biological properties and current knowledge. IntechOpen Limited, London, pp 1–18
Dayanand C, Bhikshamaiah G, Tyagaraju VJ, Salagram M, Murthy ASRK (1996) Structural investigations of phosphate glasses: a detailed infrared study of the x(PbO)-(1–x)P2O5 vitreous system. J Mater Sci 31:1945–1967
Dong S, Wu F, Chen L, Wang Y, Chen S (2016) Preparation and characterization of Poly(vinyl alcohol)/graphene nanocomposite with enhanced thermal stability using PEtVIm-Br as stabilizer and compatibilizer. Polym Degrad Stab 131:42–52
BS EN 13501–1:2018. Fire classification of construction products and building elements. Classification using data from reaction to fire tests.
Fang F, Zhang X, Meng Y, Gu Z, Bao C, Ding X, Li S, Chen X, Tian X (2015) Intumescent flame-retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf Coat Technol 262:9–14. https://doi.org/10.1016/j.surfcoat.2014.11.011
Gaikwad KK, Lee JY, Lee YS (2015) Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J Food Sci Technol 53(3):1608–1619
Galletti GC, Reeves JB (1992) Pyrolysis/gas chromatography/ion-trap detection of polyphenols (vegetable tannins): preliminary results. Org Mass Spectrom 27:226–230
Hassan M, Nour M, Abdelmonem Y, Makhlouf G, Abdelkhalik A (2016) Synergistic effect of chitosan-based flame retardant and modified clay on the flammability properties of LLDPE. Polym Degrad Stab 133:8–15. https://doi.org/10.1016/j.polymdegradstab.2016.07.011
Hu S, Tan ZW, Chen F, Li JG, Shen Q, Huang ZX, Zhang LM (2020) Flame-retardant properties and synergistic effect of ammonium polyphosphate/aluminum hydroxide/mica/silicone rubber composites. Fire Mater 44:673–682. https://doi.org/10.1002/fam.2831
ISO 11925–2:2020. Reaction to fire tests—Ignitability of products subjected to direct impingement of flame—Part 2: Single-flame source test.
ISO 13344: 1996. Determination of the lethal toxic potency of fire effluents.
ISO 13934–1:2013. Textiles—Tensile properties of fabrics—Part 1: Determination of maximum force and elongation at maximum force using the strip method.
ISO 3795: 1989. Road vehicles, and tractors and machinery for agriculture and forestry—Determination of burning behaviour of interior materials.
ISO 4589–2:2017. Plastics—Determination of burning behaviour by oxygen index—Part 2: Ambient-temperature test.
ISO 5659–2:2017. Plastics—Smoke generation—Part 2: Determination of optical density by a single-chamber test.
ISO 13344: 2015. Estimation of the lethal toxic potency of fire effluents.
Jian R, Ai Y, Xia L, Zhao L, Zhao H (2019) Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J Hazard Mater 371:529–539
Laoutid F, Bonnaud L, Alexandre M, Lopez-Cuesta JM, Dubois P (2009) New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng R Rep 63:100–125. https://doi.org/10.1016/j.mser.2008.09.002
Levchik SV, Levchik GF, Balabanovich AI, Camino G, Costa L (1996) Mechanistic study of combustion performance and thermal decomposition behaviour of nylon 6 with added halogen-free fire retardants. Polym Degrad Stab 54:217–222. https://doi.org/10.1016/s0141-3910(96)00046-8
Li P, Wang B, Liu Y-Y, Xu Y-J, Jiang Z-M, Dong C-H, Zhang L, Liu Y, Zhu P (2020a) Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohydr Polym 237:116173. https://doi.org/10.1016/j.carbpol.2021.118263
Li P, Liu C, Xu Y-J, Jiang Z-M, Liu Y, Zhu P (2020b) Novel and eco-friendly flame-retardant cotton fabrics with lignosulfonate and chitosan through LbL: Flame retardancy, smoke suppression and flame-retardant mechanism. Polym Degrad Stab 181:109302. https://doi.org/10.1016/j.polymdegradstab.2020.109302
Liu L, Huang Z, Pan Y, Wang X, Song L, Hu Y (2018a) Finishing of cotton fabrics by multi-layered coatings to improve their flame retardancy and water repellency. Cellulose 25:4791–4803. https://doi.org/10.1007/s10570-018-1866-4
Liu Y, Wang Q, Jiang Z-M, Zhang C-J, Li Z-F, Chen H-Q, Zhu P (2018b) Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J Anal Appl Pyrolysis 135:289–298. https://doi.org/10.1016/j.jaap.2018.08.024
Makhlouf G, Hassan M, Nour M, Abdelmonem Y, Abdelkhalik A (2017a) Evaluation of fire performance of linear low-density polyethylene containing novel intumescent flame retardant. J Therm Anal Calorim 130(2):1031–1041. https://doi.org/10.1007/s10973-017-6418-x
Makhlouf G, Hassan M, Nour M, Abdelmonem Y, Abdelkhalik A (2017b) A novel intumescent flame retardant: synthesis and its application for linear low-density polyethylene. Arab J Sci Eng 42(10):4339–4349. https://doi.org/10.1007/s13369-017-2443-0
Makhlouf G, Abdelkhalik A, Hassan MA (2020) Combustion toxicity of polypropylene containing melamine salt of pentaerythritol phosphate with high efficiency and stable flame retardancy performance. Process Saf Environ Prot 138:300–311. https://doi.org/10.1016/j.psep.2020.04.012
Nabipour H, Wang X, Rahman M, Song L, Hu Y (2020a) An environmentally friendly approach to fabricating flame retardant, antibacterial and antifungal cotton fabrics via self-assembly of guanazole-metal complex. J Clean Prod 273:122832. https://doi.org/10.1016/j.jclepro.2020.122832
Nabipour H, Wang X, Song L, Hu Y (2020b) Hydrophobic and flame-retardant finishing of cotton fabrics for water–oil separation. Cellulose 27:4145–4159. https://doi.org/10.1007/s10570-020-03057-1
Nam S, Condon BD, Xia Z, Nagarajan R, Hinchliffe DJ, Madison CA (2017) Intumescent flame-retardant cotton produced by tannic acid and sodium hydroxide. J Anal Appl Pyrolysis 126:239–246. https://doi.org/10.1016/j.jaap.2017.06.003
Pan H, Wang W, Pan Y, Zeng W, Zhan J, Song L, Hu Y, Liew KM (2015) Construction of layer-by-layer assembled chitosan/titanate nanotubes based nanocoating on cotton fabrics: flame retardant performance and combustion behaviour. Cellulose 22:911–923. https://doi.org/10.1007/s10570-014-0536-4
Pantoja-Castro MA, González-Rodríguez H (2011) Study by infrared spectroscopy and thermogravimetric analysis of tannins and tannic acid. Rev Latinoamer Quím 39(3):107–112
Ramakrishnan K, Krishnan MRV (1994) Tannin—classification, analysis and applications. Anc Sci Life XII I(3–4):232–238
Singh AP, Kumar S (2020) Applications of tannins in industry. In: Aires A (ed) Tannins structural properties, biological properties and current knowledge. IntechOpen Limited, London, pp 1–19
Stec AA (2017) Fire toxicity—the elephant in the room? Fire Saf J 91:79–90. https://doi.org/10.1016/j.firesaf.2017.05.003
Stull JO (2008) U.S. Fire Administration Firefighter Autopsy Protocol, Federal Emergency Management Agency.
Tributsch H, Fiechter S (2008) The material strategy of fire-resistant tree barks. In: De Wilde WP, Brebbia CA (eds) High performance structures and materials IV. WIT Press Southampton, Boston, pp 43–52
Van der Veen I, de Boer J (2012) Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Viswanath V, Leo VV, Prabha SS, Prabhakumari C, Pott, VP, Jisha MS (2015) Thermal properties of tannin extracted from Anacardium occidentale L. using TGA and FT-IR spectroscopy. Nat Prod Res 30(2):223–227. https://doi.org/10.1080/14786419.2015.1040992
Wahyono T, Astuti DA, Wiryawan IKG, Sugoro I, Jayanegara A (2019) Fourier transform mid-infrared (FTIR) spectroscopy to identify tannin compounds in the panicle of sorghum mutant lines. IOP Conf. Ser Mater Sci Eng 546042045. https://doi.org/10.1088/1757-899X/546/4/042045
Wan C, Liu M, Tian P, Zhang G, Zhang F (2020) Renewable vitamin B5 reactive N-P flame retardant endows cotton with excellent fire resistance and durability. Cellulose 27:1745–1761. https://doi.org/10.1007/s10570-019-02886-z
Wang D, Ma J, Liu J, Tian A, Fu S (2021) Intumescent flame-retardant and ultraviolet-blocking coating screen-printed on cotton fabric. Cellulose. https://doi.org/10.1007/s10570-020-03669-7
Xia Z, Singh A, Kiratitanavit W, Mosurkal R, Kumar J, Nagarajan R (2015) Unraveling the mechanism of thermal and thermo-oxidative degradation of tannic acid. Thermochim Acta 605:77–85
Xu F, Zhong L, Xu Y, Zhang C, Zhang FX, Zhang GX (2019) Highly efficient flame-retardant and soft cotton fabric prepared by a novel reactive flame retardant. Cellulose 26:4225–4240. https://doi.org/10.1007/s10570-019-02374-4
Yang B, Chen Y, Zhang M, Yuan G (2019) Synergistic and compatibilizing effect of octavinyl polyhedral oligomeric silsesquioxane nanoparticles in polypropylene/intumescent flame retardant composite system. Compos A Appl Sci Manuf 123:46–58. https://doi.org/10.1016/j.compositesa,2019.04.032
Zaikov GE, Lomakin SM (1998) Flame retardants: poly(vinyl alcohol) and silicon compounds. In: Pritchard G (eds) Plastic additives. Polymer Science and Technology Series. Springer, Dordrecht, pp 55–56.
Zhu WJ, Yang MY, Huang H, Dai Z, Cheng BW, Hao SS (2020a) A phytic acid-based chelating coordination embedding structure of phosphorus-boron-nitride synergistic flame retardant to enhance durability and flame retardancy of cotton. Cellulose 27:4817–4829. https://doi.org/10.1007/s10570-020-03063-3
Zhu XK, Pang HC, Zheng N, Tian P, Ning GL (2020b) High effects of smoke suppression and char formation of Ni-Mo/Mg(OH)2 for polypropylene. Polym Adv Technol 31:1688–1698. https://doi.org/10.1002/pat.4896
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Makhlouf, G., Abdelkhalik, A. & Ameen, H. Synthesis of a novel highly efficient flame-retardant coating for cotton fabrics with low combustion toxicity and antibacterial properties. Cellulose 28, 8785–8806 (2021). https://doi.org/10.1007/s10570-021-04076-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04076-2