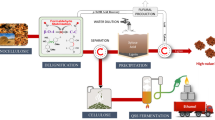
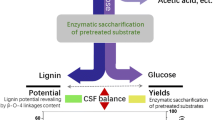
Abstract
Pretreatment to enhance the enzymatic digestibility of cellulose usually alters the structure of lignin, resulting in subsequent inferior depolymerization and utilization. Herein, a physicochemical pretreatment strategy, specifically, using a recyclable acid hydrotrope (p-toluenesulfonic acid, p-TsOH) followed by 10 s of ultrasonic treatment, was developed to facilitate high-value lignin extraction from lignocellulosic biomass and improve enzymatic hydrolysis for high-titer ethanol production. The wood material (poplar) was first treated with the recyclable p-TsOH aqueous solution under mild conditions (C80T80t15) to extract the lignin. The obtained lignin exhibited excellent properties, including a high hydroxyl (OH) content (4.19 and 4.07 mmol/g of aliphatic and phenolic OH, respectively), abundant β-O-4 aryl ether linkages (60%), a low Mw (3357 ± 121 g/mol), and a narrow polydispersity (2.28, Mw/Mn), according to the results from FTIR spectroscopy, TGA, 31P NMR spectroscopy, 2D-HSQC NMR spectroscopy, and GPC. The pretreated substrates were then subjected to 10 s of ultrasonication to improve the enzymatic saccharification and finally afford ethanol by quasi-simultaneous enzymatic saccharification and fermentation (Q-SSF). The highest ethanol concentration (40.08 ± 3 g/L) was obtained after 60 h of fermentation, and the residual glucose concentration was only 4.22 ± 1 g/L; this experimental ethanol yield was equivalent to the theoretical ethanol yield of 81.87 ± 4% based on the glucan content. In short, this pretreatment method simultaneously enhanced the accessibility of cellulose to enzymatic hydrolysis and provided high-value lignin.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, with the increasing depletion of fossil carbon resources and the deterioration of the environment due to pollution, researchers have paid increasing attention to exploring renewable energy strategies to replace fossil resources. Lignocellulosic biomass, the most abundant renewable organic material, can be converted into various products, such as chemicals, materials, and fuels (Cesário and Almeida 2015), and it has been considered a sustainable alternative to fossil carbon resources. In terms of chemical composition, lignocellulose mainly consists of three major polymers: cellulose, hemicellulose and lignin. Due to the extremely complex and variable structures of these three components, the barrier to the hydrolysis of cellulose is quite high. Therefore, an appropriate pretreatment is needed to remove physical and chemical barriers that account for native biomass recalcitrance.
Many pretreatment methods have been developed over the last few decades, and they are classified into physical, chemical, biological and physicochemical pretreatments. Physical pretreatments are based on mechanical comminution (milling), microwaves and extrusion (Sukkaew et al. 2017). Chemical pretreatments involve dilute acid or base, ionic liquids and organic solvents (Liwan et al. 2011). Biological pretreatments employ microorganisms (white-rot fungi) and their machinery to degrade lignin and alter lignocellulose structures (Isroi et al. 2011). Physicochemical pretreatments usually refer to ammonium fiber explosion (AFEX) (Krishnan et al. 2010), steam explosion (Baeyens et al. 2015) and hydrothermal treatments (Pu et al. 2013). These pretreatment strategies effectively reduce biomass recalcitrance, facilitate cellulose exposure, and decrease lignin content and cellulose crystallinity (Zhao et al. 2010), thus significantly improving the enzymatic hydrolysis of cellulose. However, typical physical pretreatments, such as mechanical comminution and microwaves, require substantial energy input, which limits their large-scale application. Chemical pretreatments, such as dilute acid/base treatments, are usually carried out in corrosion-resistant reactors (Sukkaew et al. 2017). Furthermore, the challenges of biological pretreatments, such as the long pretreatment times and the harsh conditions required for degradation, still have not been resolved (Narayanaswamy et al. 2013). Physicochemical pretreatments, such as steam explosion and hydrothermal treatment, require harsh conditions, such as high reaction temperature and pressure, long residence times, and substantial energy consumption, making the efficient application of these strategies at the industrial scale difficult (Tsubakiac et al. 2012). For example, the total energy consumption for steam explosion (1977 MJ/ton wood) was much higher than that of the organosolv (1254 MJ/ton wood) and SPORL (1503 MJ/ton wood) methods for an equivalent glucose yield (Zhu and Pan 2010). Moreover, damage to the lignin structure caused by pretreatment to improving enzyme saccharification to obtain a more digestible substrate is usually ignored, but this reduces the utilization value of the lignin. Lignin, an aromatic polymer with a three-dimensional network structure, accounts for approximately 30% of the organic carbon in the biosphere (Vadivel et al. 2017). Linkages between monolignols are the primary factors controlling the reactivity of lignin. As β-O-4 (β-aryl ether) is the most frequent linkage in lignin, its content generally dictates the chemical digestion of the lignin. Another important factor that affects the reactivity is the content of functional groups, including methoxyl, benzyl alcohol, phenolic and aliphatic hydroxyl, acyclic benzyl ether, carboxyl and carbonyl groups (Qiu et al. 2019). Currently, lignin with high reactivity has been shown to have broad application potential in the preparation of biojet fuel, phenolic resins, green composite hydrogels, concrete additives, wastewater treatment, etc. (Wang et al. 2015; Yong et al. 2017). However, most pretreatment methods usually cause structural changes in the lignin by cleaving ether bonds (e.g., β-O-4 bonds with low bond dissociation energies of 54–72 kcal/mol) and forming stable C–C bonds (high bond dissociation energies of 86–91 kcal/mol) (Rinaldi et al. 2016). These structural changes make depolymerization and utilization of the resulting lignin more difficult (Huang et al. 2018; Li et al. 2015). Therefore, exploring pretreatment technologies and processes for efficient lignin valorization is of great importance. In previous studies, a recyclable acid hydrotrope (p-toluenesulfonic acid, p-TsOH) has been highlighted for its unparalleled performance in delignification at low temperatures (below the boiling point of water) (Chen et al. 2017; Ji et al. 2016). A high lignin and hemicellulose removal rate (poplar wood, approximately 90%) by p-TsOH pretreatment significantly improved the enzymatic hydrolysis of cellulose (Ji et al. 2017). The p-TsOH was recycled using a commercially established crystallization technique by cooling the concentrated spent acid solution to ambient temperature; thus, environmental sustainability could be achieved (Chen et al. 2017; Ji et al. 2016). However, the structure of the separated lignin was altered even under these relatively mild pretreatment conditions, as revealed by the decreased content of β-O-4 bonds. Developing an efficient pretreatment strategy is a prerequisite for improving enzymatic hydrolysis and high-value lignin extraction.
Ultrasonic pretreatments without acid or base additives or pollutant formation during the application are considered a green strategy for lignocellulose treatment (Liyakathali et al. 2016; Revin et al. 2016). Numerous studies have demonstrated that ultrasonic pretreatment significantly promotes structural damage to cellulose by increasing surface area and enzymatic accessibility, thereby significantly increasing enzymatic saccharification efficiency and bioethanol yield (Wongsorn et al. 2010). Nevertheless, ultrasonic pretreatment is usually limited by the challenges associated with high energy consumption. The energy consumption of the pretreatment can be dramatically reduced via an auxiliary treatment in conjunction with chemical pretreatment.
In this study, a recyclable acid hydrotrope, p-TsOH, was used for pretreatment under mild conditions to extract high-value lignin. Subsequently, a 10 s ultrasonic pretreatment was used to improve the enzymatic hydrolysis efficiency of the cellulose. The lignin structure was characterized by FTIR spectroscopy, thermogravimetric analysis (TGA), 2D-HSQC NMR spectroscopy, 31P NMR spectroscopy, and GPC. The effects of pretreatment conditions on the enzymatic hydrolysis of glucan and lignin structure were evaluated. The obtained glucan-rich solids were finally converted into high-titer ethanol by a Q-SSF process.
Materials and methods
Materials
Poplar wood chips were kindly provided by Shandong Sun Paper Industry Joint Stock Co., Ltd. (Jining, China); p-TsOH was purchased from Macklin Biochemical Co., Ltd. (Shanghai, China); H2SO4 (95% to 98%, wt%) was obtained from Yantai Far Eastern Fine Chemical Co., Ltd. (Shandong, China); 1,4-dioxane and other organic solvents were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. DMSO-d6 was supplied by Cambridge Isotope Laboratories, Inc. (MA, United States). 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane was purchased from Sigma-Aldrich, Inc. (MA, USA). Cellulase with an enzyme activity of 147 FPU/mL (Cellic® CTec2) was kindly provided by Novozymes (Beijing, China). All chemical reagents used were of analytical grade.
Recyclable acid hydrotrope and subsequent 10 s ultrasonic pretreatment of biomass
Fifty milliliters of p-TsOH solution at various concentrations (60%, 70%, and 80%) and 4 g of 40–60 mesh poplar wood powder were added into a 150 mL flask. The pretreatment reactions were performed in an oil bath at different temperatures (70 °C and 80 °C) and times (15 min, 30 min, and 60 min). Each pretreatment was repeated 3 times. At the end of each reaction, the liquor and solid were separated using medium-speed filter paper (Hangzhou, China). The solid was washed using deionized water to a neutral pH and freeze-dried for subsequent enzymatic hydrolysis. For comparison, the neutral solid was subjected to 10 s ultrasonication and then freeze-drying. The residual monosaccharides in the liquor were measured by a high-performance liquid chromatography (HPLC, Ultimate 3000, Thermo Scientific) system equipped with a separating column (Aminex HPX-87H, Bio-Rad, CA, United States) and a refractive index detector (RID-20A, Shimadzu, Japan).
The enzymatic saccharification of glucan
The pretreated substrate (1 g) and sodium citrate buffer (pH = 4.8, 50 mL) were combined in a 250-mL conical flask. The enzymatic hydrolysis was carried out in a shaking bed incubator (TS-100B, Sichuan Experimental Instruments CO., Ltd., Hangzhou, China) with a rotation speed of 180 rpm, a temperature of 50 °C, and a cellulase loading of 15 FPU/g glucan. The enzymatic saccharification of each cellulose sample was conducted in triplicate. At predetermined intervals, 400-μL samples were withdrawn to analyze the glucose concentration using a biosensor analyzer (SBA-40E, Biological Institute of Shandong Academy Sciences, Jinan, China).
Lignin characterization
The extracted lignin (EL) obtained using p-TsOH as a hydrotrope and the residual lignin in the pretreated substrate (also called the cellulolytic enzyme lignin, CEL) were characterized by FTIR spectroscopy, TGA, 31P NMR spectroscopy, 2D-HSQC NMR spectroscopy, and GPC. To separate the lignin from the pretreated substrate, the glucan-rich solid was first milled by a planetary ball mill at a rotation speed of 400 rpm for 4 h. The obtained powder was hydrolyzed by cellulase to remove the cellulose and hemicellulose (180 rpm, 50 °C, and 24 h). Then, the liquor and solid were separated by centrifugation at 5000 rpm for 5 min. The residual solid was washed with distilled water three times and then freeze-dried. The lignin sample was extracted with dioxane/water (94:6, v/v) in a shaking bed incubator in the dark (180 rpm, 35 °C, 24 h).
For FTIR analysis, lignin was scanned using a spectrophotometer (ALPHA, BRUKER, Germany) over the wavenumber range from 500 to 4000 cm−1 at a scanning resolution of 4 cm−1. For TGA, the thermal stability of the lignin was measured using a simultaneous thermal analyzer (STA449-F3, NETZSCH Scientific Instruments Trading CO., Ltd., Germany) over a temperature range from 35 to 800 °C and a heating rate of 10 °C/min (Shen et al. 2016).
NMR spectra were recorded on a Bruker 600 MHz spectrometer (AVANCE III, BRUKER, Germany) at 25 °C. 31P NMR spectra were acquired according to a previous publication (Ji et al. 2017). For 2D-HSQC NMR experiments, 40 mg of lignin was dissolved in 0.60 mL DMSO-d6 and then transferred into an NMR tube for subsequent analysis.
The weight-average molecular weight (Mw), number-average molecular weight (Mn), and polydispersity (Mw/Mn) of the lignin samples were measured on a gel permeation chromatography (GPC) system (Waters CO., USA) after acetylation. Each measurement was made in triplicate. Specifically, 50 mg of dry lignin was dissolved in 4 mL of pyridine/acetic anhydride (1:1, v/v) and stirred without light radiation at room temperature for 24 h. After evaporating the excess pyridine and acetic anhydride, 10 volumes acidified water (pH = 2, HCl) was slowly dropped into the obtained concentrated liquid to precipitate the acetylated lignin. The freeze-dried lignin was dissolved in tetrahydrofuran (THF) (2 mg/mL) and filtered through a 0.22-μm filter. Solution-state samples were analyzed by chromatography (Styragel® HR 4 THF, 7.80 × 300 mm, Ireland) with an injection volume of 20 μL and a column temperature of 35 °C. THF was used as the mobile phase, and the flow rate was 0.60 mL/min. Polystyrene was used for calibration.
High-titer ethanol production by a Q-SSF process
Q-SSF with a 15% (w/w) total solid loading was carried out at 32 °C with the addition of 15 FPU/g glucan CTec 3 and 2‰ (g/v) active dry yeast (Angel Yeast CO., Ltd. Hubei, China). Each Q-SSF experiment was conducted in triplicate. Before fermentation, the solid substrate was first enzymatically hydrolyzed for approximately 6 h at 50 °C and 180 rpm.
Samples were periodically withdrawn (0, 12, 24, 36, 48, and 60 h) and centrifuged at 8000 rpm for 3 min. The sugar and ethanol contents in the supernatant were analyzed using an HPLC system equipped with a refractive index detector (RID-20A) and a separating column (Aminex HPX-87H, Bio-Rad, CA, United States) (Zhang et al. 2015).
Results and discussion
Pretreatment and enzymatic saccharification
The effects of different pretreatment conditions on the contents of the three components are shown in Fig. 1 and Table S1. Compared with the raw material, the total contents in the samples after pretreatment were significantly reduced due to the removal of hemicellulose and lignin. Nearly 80.70% of the hemicellulose and 53.71% of the lignin were dissolved under the optimal condition (C80T80t15) with negligible degradation of cellulose. Moreover, the dissolution of hemicellulose and lignin was significantly improved with increasing severity of the pretreatment; only 4.69 ± 1% of the hemicellulose and 11.34% of the lignin were retained at C80T80t15. The elimination of natural barriers between enzymes and cellulose would efficiently enhance the enzymatic digestibility of the substrates. However, a harsh pretreatment would inevitably cause chemical changes in the structure of the lignin, decreasing its potential value. Therefore, to facilitate the extraction of functional lignin from lignocellulose, mild pretreatment conditions using p-TsOH and a subsequent 10 s ultrasonic treatment for the pretreated substrate were employed to further improve the enzymatic saccharification of the cellulose. Under the same pretreatment conditions as shown in Fig. 1, ultrasonic treatment did not cause any loss of cellulose.
After pretreatment with p-TsOH as the hydrotrope, the obtained substrates were subsequently treated with and without 10 s ultrasonic treatment, and the enzymatic saccharification yields were compared. The morphology of the pretreated substrates before and after 10 s of ultrasonic treatment and the results of the enzymatic saccharifications are displayed in Fig. 2. Under the same pretreatment conditions, the 10 s of ultrasonic treatment significantly enhanced the enzymatic hydrolysis efficiency of the cellulose. For the C70T70t60 pretreatment conditions (Fig. 2b), the enzymatic saccharification yield increased from 52.15 ± 2% (without ultrasonic treatment) to 72.51 ± 2% (with ultrasonic treatment) after 72 h. A high enzymatic hydrolysis efficiency of 93.36 ± 2% after 72 h was observed under pretreatment condition of C80T80t15 and subsequent ultrasonic treatment, and this yield was equivalent to that obtained with a harsher pretreatment, namely C80T80t30 (enzymatic saccharification yield of 93.61 ± 3%). Some studies have demonstrated that the pore volume and surface area of the residual glucan-rich solids are obviously increased by ultrasound treatment, and the digestibility of the cellulose is enhanced (Zhang et al. 2011). With a similar lignin extraction efficiency and enzymatic hydrolysis yield, the milder pretreatment did not notably impact the native chemical structure of the lignin. In the following study, the structural properties of the EL and the residual lignin in the pretreated solids (CEL) under the pretreatment conditions of C80T80t15 with 10 s of ultrasonic treatment and C80T80t30 without ultrasonic treatment were characterized by FTIR spectroscopy, TGA, 31P NMR spectroscopy, 2D-HSQC NMR spectroscopy, and GPC.
Lignin characterization
FTIR spectroscopy
Figure 3 shows the structural properties of CEL and EL under different pretreatment conditions as determined by FTIR spectroscopy. The signals were assigned according to a previous publication (Ji et al. 2017). The absorption band at approximately 3450 cm−1 was attributed to the hydroxyl O–H stretching vibration of aromatic and aliphatic species. The absorption peaks at 2937 and 2841 cm−1 were assigned to the C–H asymmetric and symmetric vibrations of methyl (–CH3) and methylene (–CH2–) groups, respectively. The following characteristic absorption peaks of lignin were also found: the absorption peak at 1720 cm−1 is the stretching vibration of carboxyl C=O groups, and the absorption bands at 1606, 1512, and 1422 cm−1 were attributed to the aromatic skeletal vibrations of lignin. Other characteristic signals of lignin were also detected at 1458 cm−1 (the C–H deformation combined with the aromatic ring vibrations), 1320 cm−1 (breathing vibration of syringyl and condensed guaiacyl moieties), 1270 cm−1 (guaiacyl units), 1222 cm−1 (C–C plus C=O stretching), 1033 cm−1 (the aromatic C–H in-plane deformation vibrations), and 834 cm−1 (the C–H out-of-plane stretching). The appearance of the signals of syringyl (S) units and guaiacyl (G) units further confirmed that poplar lignin is a GS-type lignin. Generally, the IR characteristic peaks of EL and CEL under pretreatment conditions of C80T80t15 with 10 s of ultrasonic treatment and C80T80t30 without ultrasonic treatment were similar. In Fig. 3, the similar spectral profiles indicated that EL and CEL under different pretreatment conditions maintained the core structure of native lignin.
TGA
The thermal stabilities of CEL and EL subjected to different pretreatment conditions were measured by TGA. As shown in Fig. 4, the TGA curves directly reflected the relationships between their thermal stabilities and temperature. Generally, the thermal stability of lignin is closely related to its chemical structure and the contents of certain functional groups. In the early stage of lignin thermal decomposition, the weight loss was not obvious. The chemical bonds that were broken were mainly the weak ether bonds in the β-O-4 linkages. In the region of 200–400 °C, endothermic decomposition along with an obvious weight loss was observed. As the temperature increased from 200 to 300 °C, the cleavage of aryl ether linkages among the lignin molecules produced various phenolic substances. In the region of 300–350 °C, carbonylation or carboxylation due to the oxidation of aliphatic hydroxyl groups occurred. When the temperature was increased above 350 °C, aromatic rings and C–C bonds began to be cleaved, which led to the formation of H2O and small volatile substances. Finally, when the temperature was 500 °C or above, the oxygen in the lignin was depleted, and the remaining carbon formed biochar, which was revealed by the slow decomposition rate and the flattened TGA curve. Obviously, the “core” of the chemical structures of CEL and EL did not change significantly. CEL showed a relatively high thermal decomposition rate due to its high content of β-O-4 linkages. As the pretreatment severity increased (pretreatment time increased from 15 to 30 min), the thermal decomposition rates of EL decreased. Essentially, a large portion of the ether bonds were broken, and stable C–C bonds were formed, decreasing the pyrolysis rate of the lignin. Therefore, milder pretreatment conditions are required to obtain more valuable lignin.
31P NMR analysis
Quantitative 31P NMR spectroscopy can be used to accurately monitor structural changes and the content of hydroxyl groups in the CEL and EL obtained under different pretreatment conditions. The signals were assigned according to previous publications (Ji et al. 2017; Li et al. 2017). Fig. S1 displays the 31P NMR spectra, and the contents of different types of OH are summarized in Table 1. The cleavage of β-O-4 bonds as a function of the reaction intensity can be evaluated based on the changes in the hydroxyl content. As shown in Table 1, the aliphatic hydroxyl content in CEL was generally higher than that of EL under the same pretreatment conditions. However, the phenolic hydroxyl content exhibited the opposite trend, which was attributed to the CEL structure being less damaged. In regard to EL, as the pretreatment time increased from 15 to 30 min, the content of phenolic hydroxyl groups increased from 4.07 to 4.83 mmol/g. The increase in phenolic OH groups was mainly attributed to the cleavage of β-O-4 aryl ether linkages to benzene rings, resulting in side-chain hydroxyl groups. In addition, the aliphatic hydroxyl content decreased from 4.19 to 3.68 mmol/g, as shown in Table 1. The high content of aliphatic hydroxyl functionalities (4.19 mmol/g) in EL makes them highly applicable in the synthesis of polyurethanes, polyesters, epoxide resins, and phenolic resins as macromonomers (Upton and Kasko 2015). In addition, the content of COOH groups also decreased with increasing pretreatment severity. In summary, a milder pretreatment (C80T80t15) can reduce the degree of damage to the lignin structure, enhancing its utilization value. This statement was further verified by the following 2D-HSQC NMR analysis.
2D-HSQC NMR analysis
The structural features of CEL and EL obtained from different pretreatment conditions could be further clarified by 2D-HSQC NMR analysis, which provided important structural information related to the lignan, such as the main chemical structures, the content of β-O-4 linkages, and the S/G ratio. The main cross-peaks were assigned according to previous reports (Ji et al. 2017). In the side-chain region (Fig. 5), β-O-4′ substructures were identified based on the cross-peaks of δC/δH 71.90/4.85 (Cα–Hα, A unit), δC/δH 83.90/4.33 and δC/δH 85.90/4.11 ppm (Cβ–Hβ, A unit), and δC/δH 59.50/3.69 ppm (Cγ–Hγ, A unit). In addition, β–β′ resinol substructures were identified based on the cross-peaks of δC/δH 84.90/4.64 (Cα–Hα, B unit) and δC/δH 71.00/3.88–4.17 ppm (Cγ–Hγ, B unit). Phenylcoumaran (β-5′) substructures were also observed and resulted in cross-peaks at δC/δH 85.50/4.60 ppm (Cα–Hα, C unit) and δC/δH 62.30/3.70 ppm (Cγ–Hγ, C unit). Table 2 summarizes the quantitative results related to the major inter-unit linkages in the lignin samples. Apparently, increasing the pretreatment severity from C80T80t15 to C80T80t30 significantly improved lignin depolymerization, as revealed by the decease in the β-O-4 linkage content. The EL subjected to C80T80t15 had a higher content of β-O-4 linkages (60.0%) than did that subjected to C80T80t30 (31.9%), which was in agreement with 31P NMR results. As shown in the spectra of CEL and EL subjected to C80T80t30, most signals, such as those of Aα, Aβ, and Bα, were absent. Therefore, a mild pretreatment method (C80T80t15) was suggested to extract the lignin. The enzymatic saccharification of cellulose can be improved by a subsequent 10-s ultrasonic treatment.
In the aromatic region (Fig. 5), cross-peaks between the syringyl (S) and guaiacyl (G) units were clearly observed at δC/δH 104.00/6.70 (C2, 6–H2, 6, S unit), 111.10/6.98 (C2–H2, G unit), 114.70/6.71 (C5–H5, G unit) and 118.90/6.80 (C6–H6, G unit). The p-coumarate acid was identified by the cross-peaks at δC/δH 129.80/7.50 (C2, 6–H2, 6, PCA unit). In addition, p-benzoate was identified based on the cross-peaks at δC/δH 131.60/7.70 ppm (C2, 6–H2, 6, PB unit). Table 2 lists the ratio of S/G units in lignin samples. The S/G ratio gradually increased from 2.8 (C80T80t15) to 3.4 (C80T80t30), which was ascribed to the rapid extraction of the S units under harsh pretreatment conditions. Based on the cross-peaks in both the side-chain region and the aromatic region, the lignin structure was more drastically damaged at C80T80t30. To obtain lignin samples suitable for high-value utilization, mild pretreatment condition (C80T80t15) should be used, which is in accordance with the results of 31P NMR analysis.
Molecular weight determination
To investigate the change in the molecular weight of lignin under different pretreatment conditions, the weight-average molecular weight (Mw), number-average molecular weight (Mn), and polydispersity index (PDI, Mw/Mn) of the lignin molecules were determined by GPC, and the results are shown in Table 3. The Mw of EL decreased from 3313 to 3188 g/mol when the pretreatment severity increased from C80T80t15 to C80T80t30, which indicated enhanced depolymerization and cleavage of β-O-4 linkages during the extraction. The residual lignin in the pretreated substrates (CEL) had a higher Mw (3977–4089 g/mol) than that of EL. The obtained lignin samples were suitable for hydrogenolysis due to their lower molecular weight and slight recondensation (Shu et al. 2015). Moreover, the low polydispersity index (Mw/Mn < 3) of the obtained lignin fractions indicated that pretreatment with p-TsOH aqueous solution resulted in homogeneous lignin fragmentation, which is improves its industrial applicability. Notably, the extracted lignin fractions obtained under mild pretreatment condition (C80T80t15) exhibited excellent properties, such as low Mw (3357 ± 121 g/mol), Mn (1473 ± 76 g/mol), and PDI (2.328) values. In summary, a mild pretreatment condition (C80T80t15) is suggested for extracting functional lignin for value-added utilization based on FTIR spectroscopy, TGA, 31P NMR spectroscopy, and 2D-HSQC NMR spectroscopy.
High-titer ethanol production by a Q-SSF process
After pretreatment using p-TsOH, the obtained substrates were subsequently subjected to 10 s of ultrasonic treatment to improve their enzymatic saccharification. The final glucan-rich solids were converted into ethanol by a Q-SSF process. Figure 6 displays the changes in the glucose concentration and the ethanol and alcohol yields against time in the Q-SSF process. The glucose consumption and the ethanol yield rapidly increased within the first 24 h, and the maximum ethanol production was reached at approximately 36 h. No significant inhibitory effect was observed due to the formation of inhibitory compounds, such as acetic acid, furfural (hemicellulose hydrolysate), and aromatic compounds (lignin hydrolysate), during the Q-SSF process. The highest ethanol concentration (40.08 ± 3 g/L) was reached after 60 h of fermentation, and the residual glucose concentration was only 4.22 ± 1 g/L; this yield was equivalent to the theoretical ethanol yield of 81.87 ± 4% based on the glucan content in the treated biomass. Considering our results, this strategy, pretreatment by recyclable p-TsOH aqueous solution followed by 10 s of ultrasonic treatment, shows enormous potential for providing functional lignin and high-titer ethanol.
Conclusion
In this study, a pretreatment strategy based on recyclable p-TsOH aqueous solution followed by 10 s of ultrasonic treatment for lignocellulosic biomass was developed. Pretreatment with recyclable p-TsOH aqueous solution under mild conditions (C80T80t15) facilitated the extraction of high-value lignin. The obtained lignin exhibited excellent properties, such as a high hydroxyl (OH) content (4.19 mmol/g of aliphatic hydroxyl and 4.07 mmol/g of phenolic OH groups), a high content of β-O-4 aryl ether linkages (60%), a low Mw (3357 ± 121 g/mol), and a low polydispersity index (Mw/Mn, 2.28), according to FTIR spectroscopy, TGA, 31P NMR spectroscopy, 2D-HSQC NMR spectroscopy, and GPC. The pretreated substrates were then subjected to ultrasonic treatment for 10 s to improve their enzymatic saccharification and finally converted into ethanol by a Q-SSF process. The highest ethanol concentration (40.08 ± 3 g/L) was reached after 60 h of fermentation, and the residual glucose concentration was only 4.22 ± 1 g/L; this yield is equivalent to the theoretical ethanol yield of 81.87 ± 4% based on the glucan content in treated biomass. In short, this study provides a constructive reference for the enzymatic saccharification of cellulose for high-titer ethanol production and the extraction of high-value lignin using a recyclable acid hydrotrope and ultrasonic pretreatment.
References
Baeyens J, Qian K, Appels L, Dewil R, Lv Y, Tan T (2015) Challenges and opportunities in improving the production of bio-ethanol. Prog Energy Combust Sci 47:60–88
Cesário MTF, Almeida MCMDD (2015) Lignocellulosic hydrolysates for the production of polyhydroxyalkanoates. Microbiol Monogr 26:79–104
Chen L, Dou J, Ma Q, Li N, Wu R, Bian H, Yelle DJ, Vuorinen T, Fu S, Pan X (2017) Rapid and near-complete dissolution of wood lignin at ≤80°C by a recyclable acid hydrotrope. Sci Adv 3(9):e1701735
Huang Y, Duan Y, Qiu S, Wang M, Ju C, Cao H, Fang Y, Tan T (2018) Lignin-first biorefinery: a reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain Energy Fuels 2(3):637–647
Isroi MR, Syamsiah S, Niklasson C, Nur Cahyanto M, Lundquist K, Taherzadeh MJ (2011) Biological pretreatment of lignocelluloses with white-rot fungi and its applications: a review. BioResources 6(4):5224–5259
Ji H, Chen L, Zhu J, Gleisner R, Zhang X (2016) Reaction kinetics based optimization of furfural production from corncob using a fully recyclable solid acid. Ind Eng Chem Res 55(43):11253–11259
Ji H, Song Y, Zhang X, Tan T (2017) Using a combined hydrolysis factor to balance enzymatic saccharification and the structural characteristics of lignin during pretreatment of hybrid poplar with a fully recyclable solid acid. Bioresour Technol 238:575–581
Krishnan C, Sousa LDC, Jin M, Chang L, Dale BE, Balan V (2010) Alkali-based AFEX pretreatment for the conversion of sugarcane bagasse and cane leaf residues to ethanol. Biotechnol Bioeng 107(3):441–450
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115(21):11559–11624
Li M, Pu Y, Tschaplinski TJ, Ragauskas AJ (2017) 31P NMR characterization of tricin and its structurally similar flavonoids. Chemistryselect 2(12):3557–3561
Liwan Y, Gekcheng N, Chua S, Hashim MA (2011) Comparison of ionic liquid, acid and alkali pretreatments for sugarcane bagasse enzymatic saccharification. J Chem Technol Biotechnol 86(10):1342–1348
Liyakathali NAM, Muley PD, Aita G, Boldor D (2016) Effect of frequency and reaction time in focused ultrasonic pretreatment of energy cane bagasse for bioethanol production. Bioresour Technol 200:262–271
Narayanaswamy N, Dheeran P, Verma S, Kumar S (2013) Biological pretreatment of lignocellulosic biomass for enzymatic saccharification. Green Energy and Technology pp 3–34
Pu Y, Hu F, Huang F, Davison BH, Ragauskas AJ (2013) Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol Biofuels 6(1):15–27
Qiu S, Guo X, Huang Y, Fang Y, Tan T (2019) Task-specific catalyst development for lignin-first biorefinery toward hemicellulose retention or feedstock extension. Chemsuschem 12:944–954
Revin V, Atykyan N, Zakharkin D (2016) Enzymatic hydrolysis and fermentation of ultradispersed wood particles after ultrasonic pretreatment. Electron J Biotechnol 20:14–19
Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PCA, Weckhuysen BM (2016) Wege zur verwertung von lignin: fortschritte in der biotechnik, der bioraffination und der katalyse. Angew Chem 128(29):8296–8354
Shen X, Wang B, Panli H, Wen J, Sun R (2016) Understanding the structural changes and depolymerization of eucalyptus lignin under mild conditions in aqueous AlCl3. RSC Adv 6(51):45315–45325
Shu R, Long J, Xu Y, Ma L, Zhang Q, Wang T, Wang C, Yuan Z, Wu Q (2015) Investigation on the structural effect of lignin during the hydrogenolysis process. Bioresour Technol 200:14–22
Sukkaew A, Boonsong P, Thongpradistha S, Intan M (2017) Physical and chemical pretreatment of lignocellulosics in pineapple (ananus comosus) peels dried for investment. Int Conf Res 1:090001
Tsubakiac S, Yanagisawa K, Azuma J (2012) Microwave-assisted hydrothermal hydrolysis of maltose with addition of microwave absorbing agents. Procedia Chem 4:288–293
Upton BM, Kasko AM (2015) Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem Rev 116(4):2275–2306
Vadivel D, Speltini A, Zeffiro A, Bellani V, Pezzini S, Buttafava A, Dondi D (2017) Reactive carbons from kraft lignin pyrolysis: stabilization of peroxyl radicals at carbon/silica interface. J Anal Appl Pyrol 128:346–352
Wang F, Lei Z, Wei F, Xuan H, Feng L, Hui C, Chen H, Xing D (2015) Synthesis and characterization of silicon carbide nanowires from lignin-phenolic resin and silicon powder with an in-situ formed molten salt as catalyst. New Carbon Mater 30(3):222–229
Wongsorn C, Kangsadan T, Kongruang S, Burapatana V, Pripanapong P (2010) Ultrasonic pretreatment enhanced the enzymatic hydrolysis of rice straw. In: International Conference on Chemistry & Chemical Engineering pp 20–23
Yong H, Duan Y, Shi Q, Meng W, Chao J, Hui C, Fang Y, Tan T (2017) Lignin-first biorefinery: a reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain Energy Fuels 2(3):637–647
Zhang J, Ma X, Yu J, Zhang X, Tan T (2011) The effects of four different pretreatments on enzymatic hydrolysis of sweet sorghum bagasse. Bioresour Technol 102(6):4585–4589
Zhang J, Gu F, Zhu J, Zalesny RS (2015) Using a combined hydrolysis factor to optimize high titer ethanol production from sulfite-pretreated poplar without detoxification. Bioresour Technol 186:223–231
Zhao X, Wang L, Liu D (2010) Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: a continued work. J Chem Technol Biotechnol 83(6):950–956
Zhu J, Pan X (2010) Woody biomass pretreatment for cellulosic ethanol production: technology and energy consumption evaluation. Bioresour Technol 101(13):4992–5002
Acknowledgments
The authors are grateful for the support of the Project Supported by the Foundation (No. ZZ20190203) of State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Shandong Academy of Sciences, the International Cooperation Funding of Qilu University of Technology (QLUTGJHZ2018030; QLUTGJHZ2018027), the Jiangsu Provincial Key Laboratory of Pulp and Paper Science and Technology (KL201906, KL201905), the Foundation (No. 201704) of Key Laboratory of Pulp and Paper Science and Technology of Ministry of Education/Shandong Province of China
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ji, H., Wang, L., Pang, Z. et al. Using a recyclable acid hydrotrope and subsequent short-term ultrasonic pretreatment to facilitate high-value lignin extraction and high-titer ethanol production. Cellulose 27, 7561–7573 (2020). https://doi.org/10.1007/s10570-020-03285-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03285-5