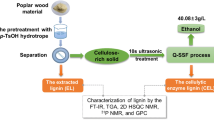
Abstract
Liquid hot water (LHW) pretreatment has become an interesting alternative among the known pretreatment approaches. In this study, an extended combined severity factor (CSF) as LHW pretreatment severity was used to make a balance between the enzymatic hydrolysis of cellulose and the potential application value of lignin. The results, mainly from the degradation of carbohydrates, the enzymatic saccharification of cellulose, and the characterization of lignin by FTIR, TGA, 31P NMR, and 2D HSQC NMR, demonstrated that a CSF of approximately 8.37 was the optimum pretreatment severity to facilitate the cellulose enzymatic hydrolysis and obtain functional lignin. Hemicellulose was removed for > 74.7% with a negligible glucose concentration of 1.19 g/L. The pretreated substrates were subsequently enzymatically hydrolyzed reaching a saccharification yield of 83.92%. The obtained lignin exhibited excellent structural properties, such as a higher hydroxyl (OH) content (2.34 mmol/g of aliphatic hydroxyl and 2.43 mmol/g of phenolic OH) and more β-O-4 aryl ether linkages (27.86%). In a word, this study hence provides useful guidance for the enzymatic saccharification of cellulose and the extraction of functional lignin using LHW pretreatment.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To enhance the exposure of cellulose, functionalize lignin, and finally improve the enzymatic saccharification of cellulose, an appropriate pre-treatment is required. Many pre-treatment techniques are currently developed and assessed, including physical approaches (mechanical comminution and extrusion), chemical methods (using dilute acids, alkalis, organic solvents or ionic liquids), biological techniques, and combination of physico-chemical pretreatment (steam explosion, ammonia fiber explosion (AFEX), and LHW), etc., have been developed (Baeyens et al. 2015; Kang et al. 2015; Zhuang et al. 2016). Among these known technologies, LHW pretreatment, also known as autohydrolysis, which uses just compressed hot water for biomass pretreatment, has become an interesting solution due to its excellent properties with no chemicals addition, less equipment corrosion, avoidance of catalyst-recycling and disposal, relatively low generation of toxic compounds, and limitation of energy consumption (Kim et al. 2013, 2015; Ko et al. 2015). In the process of pretreatment, the hydronium ions are firstly generated in situ by water autoionization (autohydrolysis) at high temperature (160 °C to 240 °C) and lead to the cleavage of acetyl groups releasing acetic acid molecules. The accumulation of the latter in turn improves the depolymerization of hemicellulose by the selective hydrolysis of heterocyclic ether bonds (Carvalheiro et al. 2016). It has already been demonstrated that the contribution of hydronium ions derived from acetic acid on the hydrolysis of hemicellulose is 9400 folds higher than that from water autoionization (Heitz et al. 2010). Indeed, using LHW pretreatment has led to an efficient extraction of hemicellulose and enhanced enzymatic saccharification of cellulose for a wide variety of lignocellulosic feedstocks (Carvalheiro et al. 2016; Ko et al. 2015). However, negative impacts on the changes of lignin structural properties were often ignored. Lignin, an aromatic polymer with three-dimensional network structure containing three phenylpropanolic monomers and functional groups, has shown a broad potential application in bio-jet fuel preparation (Huang et al. 2018; Wang et al. 2017), phenolic resins (Vithanage et al. 2017), concrete additive (Li et al. 2017), wastewater treatment (Ge and Li 2018), etc. High LHW pretreatment severity inevitably results in a structural modification of lignin revealed by the formation of stable C–C, which made it difficult for further upgrading (Upton and Kasko 2016). Although some recent researches investigated the effects of pretreatment temperature on the enzymatic hydrolysis of cellulose and lignin structural properties (Ko 2014; Li et al. 2016; Wang et al. 2016), an effective balance between enzymatic saccharification of cellulose and the structural characteristics of lignin is essentially required to facilitate the enzymatic hydrolysis and obtain functional lignin. By addressing an optimum pretreatment severity, a high enzymatic saccharification yield of cellulose can be obtained while the lignin maintains its high application value.
In this study, an extended combined severity factor (CSF) as LHW pretreatment severity was used to balance the cellulose saccharification and the lignin structural properties. An optimum pretreatment severity (CSF) will be determined according to the enzymatic hydrolysis yields of pretreated substrates and the results of lignin characterization by FTIR, thermogravimetric analysis TGA, 31P NMR, and 2D HSQC NMR.
Materials and methods
Materials
Wood chips (cellulose = 45.58%; hemicellulose = 27.91%; lignin = 24.15%; other = 2.36%) were kindly provided by Shan Dong Sun Paper Industry Joint Stock Co., Ltd. (Jining, China); The 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane was purchased from Sigma-Aldrich, Inc. (MA, United States); The DMSO-d6 was supplied by Cambridge Isotope Laboratories Inc. (MA, United States); The H2SO4 (95% to 98%, w%), 1,4-dioxane and other organic solvents were obtained from Beijing Chemical Company (Beijing, China). Cellulase with enzyme activity of 217 FPU/mL (Cellic® CTec3) was kindly provided by Novozymes (Beijing, China).
Pretreatment
The composition analysis of wood chips was conducted according to the method from the National Renewable Energy Laboratory (Sluiter et al. 2012). For the pretreatment experiment, a 2 L stainless reactor (GKCF-2, Yingyu high technique instrument factory, Gongyi, China) was filled with 250 g wood chips and 1.5 L deionized water. The reactions were conducted at a temperature range from 100 to 240 °C in 20 °C increment for 60 min with a stirring speed of 150 rpm. After each reaction, the liquor and solid in medium were separated using filter paper (diameter 15 cm, slow, Aoke filter paper factory, Taizhou, China). The monosaccharides in liquor were analyzed by a Dionex HPLC system (ICS-5000, Sunnyvale, United States) equipped with an anion exchange column (CarboPac PA20, Thermo Fisher Scientific, MA, United States). Furfural and acetic acid in the liquor were measured by high performance liquid chromatography (HPLC) equipped with a separated column (Aminex HPX-87H, Bio-Rad, CA, United States) and an ultraviolet detector (VWD-3400RS, Thermo Fisher Scientific, MA, United States) (Ji et al. 2017b; Wang et al. 2018). The solid was dried at 65 °C for further enzymatic hydrolysis. The crystallinity of the dried solid was measured by X-ray diffraction (XRD) on a diffractometer (D8-ADVANCE, BRUKER, Germany) with a diffraction range from 2° to 45° and a scanning speed of 2°/min. The crystallinity index (CrI) was calculated according to the Segal method in a previous publication (Thygesen et al. 2005).
The enzymatic saccharification of cellulose
2 g pretreated substrate and 100 ml a sodium acetate buffer (0.05 mol/L, pH = 4.8) were put into a 250 mL flask. The enzymatic hydrolysis was carried out on a shaker (HZQ-F160, Peiying Experimental Equipment Co., Ltd., Suzhou, China) with a rotation speed of 150 rpm, a temperature of 50 °C, and a cellulase loading of 15 FPU/g glucan. For each experiment, 30 ppm of tetracycline solution was used to prevent infection of bacteria. At given intervals, a 1 mL sample was taken out and centrifuged with 10,000 rpm at a set interval. The glucose in the liquor was analyzed by HPLC with the method above.
Lignin extraction and characterization
To isolate the lignin from pretreated substrate, the cellulose-rich substrate was firstly milled ball mill (XQM-2, Changsha TENCAN PODER Co., Ltd., Changsha, China) with a rotation speed of 450 rpm for 3 h. The obtained powder was subsequently hydrolyzed by cellulase (50 FPU/g glucan) to remove cellulose and hemicellulose. The residual solid was washed with deionized water for two times and freeze-dried. The lignin samples were finally prepared after the extraction with dioxane/water (96:4, v/v) under a nitrogen atmosphere and freeze-dried. The obtained lignin samples with a purity of 93.9% above were characterized using FTIR, TGA, 31P NMR, and 2D HSQC NMR. For FTIR measurement, lignin was scanned on a spectrophotometer (ALPHA, BRUKER, Germany) with a wave number range from 4000 and 800 cm−1 at 4 cm−1 resolution. TGA was conducted on a thermo gravimetric analyzer (TGAQ50, TA Instruments, DE, United States) using a temperature range from 50 to 750 °C with 5 °C/min increments. 31P NMR measurements were performed on a Bruker 600 MHz spectrometer (AVANCE III, BRUKER, Germany) according to a previous publication. (Shen et al. 2016) For 2D-HSQC NMR experiments, a solution containing 40 mg lignin and 0.6 mL DMSO-d6 was measured on a Bruker 600 MHz spectrometer (AVANCE III, BRUKER, Germany).
Results and discussion
The LHW pretreatment controlled by a CSF
For the hot water pretreatment of lignocellulose, two reaction parameters (both time and temperature) can be grouped in a variable, i.e., empiric variables such as the severity factor, R0, which is generally defined as follows (Gascoigne 1987):
where t is reaction time (min), T is reaction temperature (°C), and 100 °C is a reference temperature. During the process of pretreatment, acetic acid is continuously released from acetylated hemicellulose, its accumulation in turn improves the depolymerization of hemicellulose. When integrating the effects of reaction temperature, time, and acid concentration on the degradation of hemicellulose, a combined severity factor (CSF) as pretreatment severity was developed as follow (Chum et al. 1990):
where pH is the acidity of the liquor after pretreatment. To compare various pretreatment severities, an extended CSF can be calculated as follow: (Pedersen and Meyer 2010)
In this study, this extended CSF is used to control the pretreatment severity and make a balance between the enzymatic saccharification of cellulose and the structural properties of lignin. The specific parameters (pretreatment temperature, time, and pH) and the calculation results of CSFs are listed in Table S1.
Figure 1 displays the effects of variations in conditions (CSF) on the changes of xylan component and generated xylose (Fig. 1a), the formation of degradation products including acetic acid and furfural (Fig. 1b), and the concentrations of glucose and CrIs of substrates in during pretreatment (Fig. 1c). As shown in Fig. 1a, the xylan dissolution is significantly enhanced reaching 74.71% (or 25.29% of remaining xylan) at CSF 8.37. Nearly completely dissolved xylan is found at CSF9.68, then the calculated concentration of xylose from the dissolved xylan is close to that of theoretical maximal xylose from xylan in the substrate. However, the measured xylose concentration is always lower than that of calculated xylose from dissolved xylan, indicating that the hydrolysates from the dissolved xylan was mainly oligomeric xylose and less monomer xylose under such a pretreatment severity. As CSF increases, the concentration of measured xylose increases gradually and subsequently decreases due to its dehydration into furfural (Fig. 1b). Therefore, the concentration of furfural reaches maximum value of 12.77 g/L at CSF9.06. Any increase in CSF directly leads the reduction of furfural since furfural usually undergoes a condensation reaction with itself and other intermediates, such as glucose and HMF, to form resinous products which generally are called humins (Ji et al. 2016). A high pretreatment severity (CSF) not only resulted in the xylan degradation but also improved the formation of acetic acid in the liquor with a maximum concentration of 30.17 g/L at CSF9.06. Moreover, the depolymerization of cellulose occurs during pretreatment but with a negligible concentration of 1.19 g/L at CSF9.06 (Fig. 1c). Figure 1c clearly shows that the CrI of cellulose in the pretreated substrates from different CSF pretreatment conditions increases firstly due to the removal of hemicellulose and subsequently decreases by the destruction of the crystalline region of cellulose, which maybe result in high cellulose digestibility for the following hydrolysis.
The enzymatic saccharification of cellulose
Hydrolysis of cellulosic biomass to fermentable sugar is a critical step for ethanol production from lignocellulose. The residual cellulose-rich solid substrates after the pretreatment by LHW were enzymatically hydrolyzed to evaluate the pretreatment efficiency. The results of enzymatic saccharification are presented in Fig. 2, revealing some significant differences for the cellulose digestibility among the different substrates. A high pretreatment severity (CSF) significantly promoted the enzymatical hydrolysis of substrate. The cellulose-rich solid substrate obtained from pretreatment condition of CSF9.68 was easily digested. The yield of enzymatic saccharification almost reached 95% after 60 h. Meanwhile, a mild pretreatment severity (CSF8.37) also led a high hydrolysis yield of 83.92% after 72 h. Because a high pretreatment severity (CSF) caused the removal of hemicellulose, the cell wall thus became more porous under this pretreatment condition. The barriers between enzyme and cellulose were removed, which finally resulted in a high yield of enzymatic saccharification (Zhang et al. 2015; Zhou et al. 2013). From the profile of crystallinity of cellulose-rich solid,
we speculated that the increase of CrI for the pretreated substrates obtained from pretreatment conditions of CSF4.66–7.60 attributed to the gradual exposure of the cellulose due to the degradation of hemicellulose, which in turn promoted the contact of cellulase with cellulose. As CSF increased from 7.60 to 9.68, the CrIs of these pretreated substrates decreased, suggesting that the crystalline region of cellulose was destroyed. The pore volume and surface area of residual cellulose-rich solid obviously enlarged, the digestibility of cellulose was enhanced as a result (Zhang et al. 2011). When a lower pretreatment severity (CSF ≤ 7.60) was adopted, the effective hydrolysis of cellulose cannot be achieved (the yield of enzymatic saccharification ≤ 63.57%). Therefore, a pretreatment severity of approximately CSF8.37 was suggested in order to facilitate the enzymatic digestibility of the pretreated substrates when using LHW pretreatment for lignocellulose.
The structural characteristics of lignin in the residual solids
The FT-IR spectra analysis
The structural properties of MWL and the lignin in the residual solids obtained from different CSF pretreatment conditions were measured by FT-IR as shows in Fig. 3. The detailed signals were assigned according to a previous publication (Ji et al. 2017a). A wide absorption band at 3440 cm−1 is ascribed to the O–H stretching vibration in aromatic and aliphatic regions. The absorption bands at 2938 and 2843 cm−1 are originated from the C–H asymmetric and symmetrical vibrations for the methyl (–CH3) and methylene (–CH2–) groups, respectively. The typical characteristic absorption peaks of lignin were detected at 1695 cm−1 (the stretching vibrations of C=O for carboxyl groups and ketone groups), 1595, 1508, and 1419 cm−1 (the aromatic skeletal vibrations), 1458 cm−1 (the C–H deformation combined with the aromatic ring vibrations, 1326 cm−1 (breathing vibration of syringyl and condensed guaiacyl), 1267 cm−1 (guaiacyl units), 1219 cm−1 (C–C plus C=O stretch), 1028 cm−1 (the aromatic C–H in-plane deformation vibrations), and 834 cm−1 (the C–H out-of-plane stretching). a decrease trend of relative intensities of the signals appeared with the increasing CSF in Fig. 3, while all the spectra demonstrated a similar signal profile, suggesting that the lignin under different CSF pretreatment conditions maintained their core structures as that of native lignin (MWL) in the process of LHW pretreatment.
The TGA
The thermal stability of lignin in the residual solids obtained from different CSF pretreatment conditions was measured by TGA. Figure 4 displays all the TGA features which directly reflects the relationship between thermal stability and temperature. Generally, the thermal stability of lignin is closely related to its properties of chemical structure and the amount of functional groups. At beginning of lignin thermo-decomposition (≤ 200 °C), the fractured chemical bonds are mainly the weak ether bonds in the β-O-4 linkages. As the temperature increases to 200–350 °C, the aryl ether linkages begin to decompose. Afterwards, the oxidation of aliphatic hydroxyl group to carbonylation or carboxylation occurs at a temperature range of 350–400 °C. When temperature rises to 400 °C above, the aromatic rings and the C–C bonds, mainly the 5-5 (biphenyl) linkages, are cleaved, their decomposition in turn leads to the release of H2O, CO2, and CO. Finally, the residual carbon forms the biochar revealed by a flatten TGA curves when oxygen is depleted at 500 °C above. Obviously, MWL showed a high thermo-decomposition rate due to its high content of β-O-4 linkages. As pretreatment severity (CSF) increased, the thermo-decomposition rates of lignin obtained from various pretreatment conditions gradually decreased. The most likely reason was that gradually increased pretreatment severity (CSF) significantly enhanced the cleavage of ether bonds and improved the subsequent formation of new stable C–C bonds (lignin recondensation). Consequently, a low pretreatment severity was suggested to obtain functional lignin as near native lignin. These results were in accordance with that of following 31P-NMR and 2D HSQC NMR analysis.
31P-NMR analysis
The structural changes and the content of hydroxyl groups in MWL and the lignin obtained from different CSF pretreatment conditions were analyzed by quantitative 31P NMR. Figure S1 shows the 31P NMR spectra. The detailed signals were assigned according to a previous publication (Ji et al. 2017a). The corresponding results are summarized in Table 1. For a comparison, the content of aliphatic OH in MWL was determined to be 6.79 mmol/g, while the lignin extracted from different CSF pretreatment conditions exhibited a decreased aliphatic hydroxyl content from 5.59 to 2.34 mmol/g as the CSF increased from 6.79 to 9.68. The changes of pretreatment severity also significantly affected the content of phenolic OH revealed by an increased quantity from 1.15 to 2.43 mmol/g as CSF elevated from 6.79 to 8.37, which attributed to the cleavage of the β-O-4 aryl ether linkages. After that, a subsequent decrease of the phenolic OH content appeared (from 2.43 to 0.78 mmol/g), suggesting lignin recondensation occurred at high severities. In fact, the changes of hydroxyl content are closely related to the depolymerization at low CSF and the recondensation of lignin at high CSF during LHW pretreatment. When the lignin depolymerization dominated at low pretreatment severity, the aliphatic hydroxyl content thus decreased and phenolic OH content increased. As the pretreatment severity increased, the lignin recondensation appeared and gradually became a leading reaction resulting in an obvious reduction in aliphatic and phenolic OH contents (Shen et al. 2016). The results in this study were in accordance with the trend described above. Furthermore, more COOH groups in lignin obtained at CSF6.79 (0.47 mmol/g) attributed to the oxidation of phenolic OH involving in the LHW pretreatment process. The recondensation of lignin at CSF6.79 above resulted in a decrease of COOH groups. Therefore, the pretreatment should be performed at the condition as mild as possible (approximately CSF8.37) in order to obtain high value lignin.
The 2D HSQC NMR analysis
The MWL and the lignin in the residual solids obtained from different CSF pretreatment conditions were finally characterized with 2D-HSQC NMR to obtain the information about their main chemical structures and the content of β-O-4 bonds. Figure 5 shows the spectra with two independent regions, side-chain (δC/δH 50.0–90.0/2.50–6.00) and aromatic regions (δC/δH 100.0–150.0/5.50–8.50), and the lignin substructures. The main cross-signals were assigned according to the previous literatures (Constant et al. 2016; del Rio et al. 2015; Luterbacher et al. 2015). In the side-chain region, the β-O-4′ substructures were identified by their cross signals at δC/δH 71.9/4.85 (Cα–Hα, A unit), δC/δH 84.4/4.4 (Cβ–Hβ, A unit), and δC/δH 59.4/3.7 (Cγ–Hγ, A unit). Meanwhile, the resinol β–β’ substructures were detected for the appearance of their cross signals at δC/δH 84.9/4.64 (Cα–Hα, B unit) and δC/δH 71.0/3.88 (Cγ–Hγ, B unit). Moreover, phenylcoumaran (β-5′) substructures were found according to the cross signals at δC/δH 85.5/4.6 (Cα–Hα, C unit) and δC/δH 71.2/4.2 and 3.8 (Cγ–Hγ, C unit). Table 2 presents the quantification of side chains and aromatic units in lignin from 2D HSQC NMR. It is obvious that increasing the pretreatment severity (CSF) significantly improved the lignin depolymerization as revealing by a deceased β-O-4 linkages content (Table 2). Only 7.99% of β-O-4 linkages were found in lignin obtain at CSF9.06. The S/G ratios gradually increased from 1.58 (CSF 5.68) to 4.54 (CSF 9.06) and subsequently decreased to 2.95 (CSF9.60), which be ascribed as the quick dissolving of S units at low reaction severity and the occurrence of demethoxylation in S units at high reaction severity. (Costa et al. 2014) As shown in spectra of CSF9.06 (a), most signals, such as that at Aα, Aβ, and Bα, disappeared from the spectra. Therefore, a mild pretreatment severity (approximately CSF8.37) is suggested to obtain near native lignin (MWL).
In the aromatic regions/olefinic regions, prominent cross-signals for syringyl (S) and guaiacyl (G) were easily observed at δC/δH 103.8/6.69 (C2,6–H2,6, S unit), δC/δH 110.9/7.00 (C2–H2, G unit), 114.7/6.71 (C5–H5, G unit), and 118.9/6.80 (C6–H6, G unit), respectively. p-coumarate moieties were found according to the cross signals at δC/δH 130.0/7.46 (C2,6–H2,6, PCA unit). Moreover, p-benzoate were detected for the appearance of their cross signals at δC/δH 131.6/7.7 (C2,6–H2,6, PB unit). It is very similar with the structural characteristics in side-chain region that many cross-signals, such as that at PB 2,6 and PCA 2,6, disappeared from the spectra of CSF9.06(b), indicating that a high pretreatment severity (CSF) significantly enhanced the lignin recondensation. Consequently, the pretreatment should be conducted at a mild severity (CSF approximately 8.37). These results are in accordance with that of 31P-NMR analysis.
A balance between the enzymatic saccharification of cellulose and the structural characteristics of lignin
Figure 6 shows a balance between lignin potential revealed by the β-O-4 linkages content and the enzymatic saccharification of pretreated substrates. Although high pretreatment severity (CSF ≥ 9.06) caused a high hemicellulose removal (≥ 84.26%) and excellent enzymatic saccharification of cellulose (≥ 89.20%), the lignin obtained under these pretreatment exhibited a low content of OH groups (≤ 2.15 mmol/g for phenolic OH and ≤ 1.18 mmol/g for aliphatic OH), a low β-O-4 linkages content (≤ 7.99), and high recondensation according to the results analysis from FTIR, 31P-NMR, TGA, and 2D HSQC NMR. Therefore, a mild severity of approximately CSF8.37 using LHW pretreatment for lignocellulose was suggested to facilitate enzymatic saccharification and obtain the lignin with excellent structural characteristics. Then approximately 75% of hemicellulose was hydrolyzed and the enzymatic saccharification reached a high yield of 83.92%. The lignin preserved their original structures as native properties (MWL) with a high hydroxyl content (2.34 mmol/g of aliphatic hydroxyl and 2.43 mmol/g of phenolic OH) and more β-O-4 aryl ether linkages (27.86%). In this respect, it has great potential application as a macromonomer in the preparation of bio-jet fuels and the synthesis of polyurethanes, polyesters, epoxide resins, and phenolic resins (Upton and Kasko 2016).
Conclusion
In this study, an extend combined severity factor (CSF) as LHW pretreatment severity was used to make a balance between the enzymatic saccharification of cellulose and the potential application value of lignin. With the optimum pretreatment severity (approximately CSF 8.37), the removal of hemicellulose reached 74.71%, accompanying by a negligible glucose concentration of 1.19 g/L. The pretreated substrates were subsequently enzymatically hydrolyzed with a saccharification yield of 83.92%. The obtained lignin was characterized by FTIR, TGA, 31P NMR, and 2D HSQC NMR. The results indicated that it exhibited excellent structural properties, such as high hydroxyl content (2.34 mmol/g of aliphatic hydroxyl and 2.43 mmol/g of phenolic OH) and β-O-4 aryl ether linkages (27.86%). In short, this study provided a helpful reference for the enzymatic saccharification of cellulose for bioethanol production and the extraction of functional lignin for bio-jet fuels preparation and bio-resins synthesis using LHW pretreatment.
References
Baeyens J, Qian K, Appels L, Dewil R, Lv Y, Tan T (2015) Challenges and opportunities in improving the production of bio-ethanol. Prog Energy Combust Sci 47:60–88
Carvalheiro F, Duarte LC, Gírio F, Moniz P (2016) Hydrothermal/liquid hot water pretreatment (autohydrolysis). In: Mussatto SI (ed) Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier, Amsterdam, pp 315–347. https://doi.org/10.1016/b978-0-12-802323-5.00014-1
Chum HL, Johnson DK, Black SK, Overend RP, Greenbaum E, Wyman CE (1990) Pretreatment-catalyst effects and the combined severity parameter. Appl Biochem Biotechnol 24–25:1
Constant S, Wienk HLJ, Frissen AE, de Peinder P, Boelens R, van Es DS, Grisel RJH, Weckhuysen BM, Huijgen WJJ, Gosselink RJA, Bruijnincx PCA (2016) New insights into the structure and composition of technical lignins: a comparative characterisation study. Green Chem 18:2651–2665. https://doi.org/10.1039/c5gc03043a
Costa CAE, Pinto PCR, Rodrigues AE (2014) Evaluation of chemical processing impact on E. globulus wood lignin and comparison with bark lignin. Ind Crops Prod 61:479–491
del Rio JC, Lino AG, Colodette JL, Lima CF, Gutierrez A, Martinez AT, Lu FC, Ralph J, Rencoret J (2015) Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenerg 81:322–338. https://doi.org/10.1016/j.biombioe.2015.07.006
Gascoigne JA (1987) Fractionation of lignocellulosics by steam-aqueous pretrements: discussion. Philos Trans R Soc A Math Phys Eng Sci 321:523–536
Ge Y, Li Z (2018) Application of lignin and its derivatives in adsorption of heavy metal ions in water: a review. Acs Sustain Chem Eng 6:7181–7192
Heitz M, Carrasco F, Rubio M, Chauvette G, Chornet E, Jaulin L, Overend RP (2010) Generalized correlations for the aqueous liquefaction of lignocellulosics. Can J Chem Eng 64:647–650
Huang Y, Duan Y, Qiu S, Wang M, Ju C, Cao H, Fang Y, Tan T (2018) Lignin-first biorefinery: a reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain Energy Fuels. https://doi.org/10.1039/c7se00535k
Ji H, Chen L, Zhu JY, Gleisner R, Zhang X (2016) Reaction kinetics based optimization of furfural production from corncob using a fully recyclable solid acid. Ind Eng Chem Res 55:11253–11259. https://doi.org/10.1021/acs.iecr.6b03243
Ji H, Song Y, Zhang X, Tan T (2017a) Using a combined hydrolysis factor to balance enzymatic saccharification and the structural characteristics of lignin during pretreatment of hybrid poplar with a fully recyclable solid acid. Bioresour Technol 238:575–581. https://doi.org/10.1016/j.biortech.2017.04.092
Ji H, Zhu JY, Gleisner R (2017b) Integrated production of furfural and levulinic acid from corncob in a one-pot batch reaction incorporating distillation using step temperature profiling. Rsc Adv 7:46208–46214
Kang Q, Baeyens J, Tan T, Dewil R (2015) A novel sintered metal fiber microfiltration of bio-ethanol fermentation broth. Korean J Chem Eng 32:1625–1633. https://doi.org/10.1007/s11814-014-0375-x
Kim DS, Myint AA, Lee HW, Yoon J, Lee YW (2013) Evaluation of hot compressed water pretreatment and enzymatic saccharification of tulip tree sawdust using severity factors. Bioresour Technol 144:460–466. https://doi.org/10.1016/j.biortech.2013.06.071
Kim Y, Kreke T, Ko JK, Ladisch MR (2015) Hydrolysis-determining substrate characteristics in liquid hot water pretreated hardwood. Biotechnol Bioeng 112:677–687. https://doi.org/10.1002/bit.25465
Ko JK (2014) Characterization of lignins isolated from liquid hot water pretreated hardwood. Dissertations and Theses-Gradworks
Ko JK, Kim Y, Ximenes E, Ladisch MR (2015) Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol Bioeng 112:252–262. https://doi.org/10.1002/bit.25349
Li P, Liu Y, Lu J, Yang R, Li H, Wang H (2016) Structural characterization and effect on enzymatic hydrolysis of milled wood lignin isolated from reed straw and corn stover pretreated with liquid hot water. BioResources. https://doi.org/10.15376/biores.11.4.8777-8790
Li S, Li Z, Zhang Y, Liu C, Yu G, Li B, Mu X, Peng H (2017) Preparation of concrete water-reducer via fractionation and modification of lignin extracted from pine wood by formic acid. Acs Sustain Chem Eng 5:4214–4222
Luterbacher JS, Azarpira A, Motagamwala AH, Lu FC, Ralph J, Dumesic JA (2015) Lignin monomer production integrated into the gamma-valerolactone sugar platform. Energy Environ Sci 8:2657–2663. https://doi.org/10.1039/c5ee01322d
Pedersen M, Meyer AS (2010) Lignocellulose pretreatment severity—relating pH to biomatrix opening. New Biotechnol 27:739–750. https://doi.org/10.1016/j.nbt.2010.05.003
Shen XJ, Wang B, Huang PL, Wen JL, Sun RC (2016) Understanding the structural changes and depolymerization of eucalyptus lignin under mild conditions in aqueous AlCl3. RSC Adv 6:45315–45325
Sluiter A, Hames B, Ruiz R, Scarlate C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass (NREL/TP-510-42618). Natl Renew Energy Lab. https://doi.org/10.4236/jpee.2015.34026
Thygesen A, Oddershede J, Lilholt H, Thomsen AB, Ståhl K (2005) On the determination of crystallinity and cellulose content in plant fibres. Cellulose 12:563
Upton BM, Kasko AM (2016) Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem Rev 116:2275–2306. https://doi.org/10.1021/acs.chemrev.5b00345
Vithanage AE, Chowdhury E, Alejo LD, Pomeroy PC, DeSisto WJ, Frederick BG, Gramlich WM (2017) Renewably sourced phenolic resins from lignin bio-oil. J Appl Polym Sci 134:44827
Wang W, Zhuang X, Yuan Z, Qi W, Yu Q, Wang Q (2016) Structural changes of lignin after liquid hot water pretreatment and its effect on the enzymatic hydrolysis. Biomed Res Int 2016:1–7
Wang M, He M, Fang Y, Baeyens J, Tan T (2017) The Ni-Mo/γ-Al2O3 catalyzed hydrodeoxygenation of FAME to aviation fuel. Catal Commun 100:237–241. https://doi.org/10.1016/j.catcom.2017.07.009
Wang R, Bian H, Ji H, Yang R (2018) Preparation of lignocellulose/graphene composite conductive paper. Cellulose 25:6139–6149. https://doi.org/10.1007/s10570-018-1998-6
Zhang J, Ma X, Yu J, Zhang X, Tan T (2011) The effects of four different pretreatments on enzymatic hydrolysis of sweet sorghum bagasse. Bioresour Technol 102:4585–4589. https://doi.org/10.1016/j.biortech.2010.12.093
Zhang J, Gu F, Zhu JY, Zalesny RS Jr (2015) Using a combined hydrolysis factor to optimize high titer ethanol production from sulfite-pretreated poplar without detoxification. Bioresour Technol 186:223–231. https://doi.org/10.1016/j.biortech.2015.03.080
Zhou H, Zhu JY, Luo X, Leu S-Y, Wu X, Gleisner R, Dien BS, Hector RE, Yang D, Qiu X, Horn E, Negron J (2013) Bioconversion of beetle-killed lodgepole pine using SPORL: process scale-up design, lignin coproduct, and high solids fermentation without detoxification. Ind Eng Chem Res 52:16057–16065. https://doi.org/10.1021/ie402873y
Zhuang X, Wang W, Yu Q, Qi W, Wang Q, Tan X, Zhou G, Yuan Z (2016) Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour Technol 199:68–75. https://doi.org/10.1016/j.biortech.2015.08.051
Acknowledgments
The authors would like to express thanks for the supports from the National Key R&D Program of China (2017YFB0307900), the National Natural Science Foundation of China (Grant Nos. 31670590, 31670595), the Natural Science Foundation of Shandong Province (Grant. No. ZR2018LB29), the Project of Shandong Province Higher Educational Science and Technology Program (KT2018BAC032), the International Cooperation Funding of Qilu University of Technology (QLUTGJHZ2018027), and the Taishan Scholars Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, S., Ji, X., Ji, H. et al. An optimum combined severity factor improves both the enzymatic saccharification yield and the functional lignin structure. Cellulose 26, 4731–4742 (2019). https://doi.org/10.1007/s10570-019-02442-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02442-9