Abstract
A novel compound containing silicon, phosphorus and nitrogen, named 1-(9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide)-4-(trimethoxysilylmethyl) piperazine (DOPO-PiP-Si), was synthesized as a reactive flame retardant and applied to prepare flame-retardant cotton fabrics. Its structure was characterized by Fourier transform infrared spectra, 1H and 13C nuclear magnetic resonance (1H NMR and 13C NMR), and flame retardancy of treated cotton fabrics was investigated thoroughly using the limited oxygen index (LOI), vertical burning test, thermogravimetric analysis and cone calorimetry test (CONE), respectively. The LOI value of the cotton fabrics treated with DOPO-PiP-Si increased to 27.6% and the char length decreased to 12.2 cm without afterflame and afterglow. From the results of CONE test, the peak heat release rate and total heat release of treated cotton fabrics decreased from 230.8 to 161.1 kW/m2 and from 6.4 to 4.5 MJ/m2, respectively. Besides, the char residue reached 19.1%, which indicated that the treated cotton fabrics had excellent char formation ability and thermal stability. The morphology of char of the treated cotton fabrics was also characterized by scanning electron microscopy, it can be seen from results that the structure of fiber had no shrinkage and a few bubbles were distributed on the surface of fiber, which suggested that the treated cotton fabrics could display gas-phase flame retardant mechanism.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabrics have been widely used in clothing, decoration, transportation and other industries due to their comfort and breathability (Liu et al. 2019, 2012; Nguyen et al. 2013). However, it has poor thermal stability and flammability, which may cause a potential huge safety hazard in some applications (Grancaric et al. 2017; Rosace et al. 2017). Therefore, it is very necessary to modify the cotton fabrics to obtain flame retardancy and improve thermal stability (Edwards et al. 2015; Wang et al. 2016).
At present, a large number of experiments have been conducted to study how to improve the flame retardancy and thermal stability of cotton fabrics. Numerous studies have shown that the introduction of flame retardants into cotton fibers is a simple and effective way to reduce their flammability (Jiang et al. 2019b). There are many kinds of flame retardants, and different types of flame retardants have different effects on the fire resistance of cotton fabrics. Most of the fire retardant chemicals available in the commercial market are halogen based, but since traditional halogen based flame retardants release toxic and corrosive gases in the combustion process, leading to serious health and environmental problems, the search for efficient, synergistic and environmentally friendly flame retardants has gradually become the focus of research (Li et al. 2019; Wei et al. 2019). To this purpose, some natural biomolecules, including polysaccharides, lignin, deoxyribose nucleic acid (DNA) and proteins have been considered (Alongi et al. 2013, 2014; Basak and Ali 2016). In addition, Alongi et al. tried to reduce the flammability of cotton fabrics by using sol–gel treatments and exploiting the phosphorus-nitrogen synergism to achieve certain effects (Alongi et al. 2011a, b). Phosphorus and nitrogen based synergistic flame retardants (Tetrakis hydroxymethyl phosphonium chloride and N methylol dimethyl phospopropionamide) have appeared on the market, and until now they have been widely used by the textile industry (Shukla et al. 2019). In recent years, a synergistic flame retardant containing silicon, phosphorus and nitrogen has attracted the attention of researchers (Chen et al. 2017b; Przybylak et al. 2016). Chen et al. synthesized a linear piperazine/phosphorus/polysiloxane copolymer (a, x-di[(4-butoxypiperazin-1-yl)-phosphinic acid methyl ether]-terminated linear polysiloxane) and applied it to cotton fabrics. As results, the LOI value and char residue of treated cotton fabrics enhanced to 30% and 22%, respectively. And further research has found that phosphorus in the copolymer will form phosphoric acid and polyphosphate during the burning process, which can promote the dehydration of cellulose into char (Abou-Okeil et al. 2013). Meanwhile, a dense SiO2 film barrier on the surface of cotton fiber will be gotten by oxidation of silicone to inhibit the release of smoke and isolate oxygen (Chang et al. 2014; Mohamed et al. 2014). At the same time, evidence of gas-phase flame retardant mechanism is found by dense blisters (nodules) retained in char layer. Nitrogen can generate incombustible gases such as ammonia and nitrogen oxides during thermal decomposition, which acts to dilute the air (Gaan et al. 2008).
DOPO, named 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide, is a halogen-free, phosphorus-containing, heterocyclic compound, and it can react with a variety of electrophiles, such as C=C double bonds (Bai et al. 2014; Dong et al. 2013), aldehydes (Qian et al. 2014; Wang et al. 2011, 2012; Xie et al. 2014) and ketones (Liu and Tsai 2002), due to the presence of reactive P–H bond in its structure. Its derivatives are mainly used for epoxy resins as excellent flame retardants. For example, Jian et al. introduced 2-aminobenzothiazole into DOPO as a flame retardant for epoxy resins, which was able to improve the thermal stability of epoxy resins and simultaneously prevent the melt-dripping (Jian et al. 2016). Besides, due to the versatile nature of the Atherton–Todd reaction, it is widely exploited in phosphorus chemistry to prepare P-heteroatom bonds (Wagner et al. 2012). For example, Gaan and coworkers synthesized some novel bridged DOPO-derivatives with P-heteroatom bond to improve flame resistance of polymeric materials (Salmeia and Gaan 2015).
Owing to the outstanding flame retardant properties of DOPO derivatives, there are further potential applications for textile fibers using chemical modification methods. However, few applications on cotton fabrics have been reported. DOPO-VTS (9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-vinyltrimethoxysilane) is one of the few flame retardants as DOPO derivatives used in cotton fabrics (Vasiljević et al. 2015). The compound is prepared by an addition reaction of a double bond of vinyltrimethoxysilane with a P–H bond of DOPO. Although a certain flame retardant effect (LOI value of 23.5%) is achieved, the flame retardation of the gas phase cannot be fully performed due to the lack of nitrogen in the molecule. Therefore, the focus of this research is to explore a novel molecular form containing DOPO with synergistic flame retardancy of Si/P/N, apply it to cotton fabrics, and search for optimum concentration for excellent flame retardancy without affecting mechanical properties of cotton fabrics. In this study, a new type of reactive flame retardant was synthesized using piperazine as a bridge to link. It was expected that piperazine could act as gas source, and DOPO should act as acid source to promote the char formation, while chloromethyltrimethoxysilane, as a reactive group, can be better combined with fibers and can form a SiO2 film in the process of burning to prevent heat and flammable gas from propagating. And the flammability of treated cotton fabrics was analyzed by the limited oxygen index (LOI), vertical burning test, thermogravimetric analysis (TGA) and cone calorimetry test (CONE).
Experiment
Materials
Scoured and bleached 100% plain woven cotton fabric (14.75 tex × 14.75 tex, 122 g m−2) was supplied by Weifang Qirong Textiles Co., Ltd, Weifang, China. 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd, Shanghai, China. Chloromethyltrimethoxysilane and piperazine were provided by Shanghai for China Macklin Biochemical Co., Ltd. Dichloromethane, chloroform, trimethylamine and carbon tetrachloride were purchased from Sinopharm Chemical Reagent Co., Ltd. Others were commercially available and used without further purification.
Synthesis of N-(9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) piperazine (DOPO-PiP)
DOPO-PiP was synthesized by reacting DOPO with piperazine according to previous researches (Gaan et al. 2014). 0.03 mol of piperazine (2.60 g) and 0.02 mol of DOPO (4.32 g) were dissolved in 40 mL dichloromethane in 250 mL three-necked round bottom flask equipped with N2 inlet tube, a thermometer and a reflux condenser, and then 0.02 mol of Et3N (2.22 g) as an acid-acceptor was added to the solution. After the mixture was stirred and cooled below 10 °C, 0.02 mol of carbon tetrachloride (3.42 g) was slowly added dropwise at a rate that the reaction temperature does not exceed 10 °C. Then the solution was allowed to increase to 20 °C and stirred for 8 h under N2 protection. After completion of the reaction, a white powder product was obtained by evaporation of solvent in vacuum. The obtaining crude product was washed three times with deionized water to remove the other impurities and the DOPO-PiP was obtained by filtration. The reaction process is shown in Scheme 1.
Synthesis of 1-(9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide)-4-(trimethoxysilylmethyl) piperazine (DOPO-PiP-Si)
0.01 mol of white powder DOPO-PiP (3.00 g) and 0.01 mol of Et3N (1.11 g) were dispersed in 50 mL chloroform in the same experimental apparatus as above. Simultaneously, 0.01 mol of chloromethyltrimethoxysilane was slowly added dropwise to the mixture. The admixture was then stirred for 4 h at 80 °C under N2 protection. After the reaction was completed, the precipitates were removed by filtration, and the DOPO-PiP-Si as pale yellow solid was obtained by evaporation of solvent. The reaction process is shown in Scheme 1.
Fabrics treatment
The cotton fabric was soaked in distilled water for 15 min, and dried in oven at 90 °C for 30 min. After that, the weight of pure cotton fabrics was measured with an analytical balance. DOPO-PiP-Si was dissolved in ethanol/chloroform (1:1, v/v) to prepare treating solutions of different concentrations (200 g/L, 300 g/L, 400 g/L), and pH of the solution was adjusted to 3 to promote the hydrolysis of DOPO-PiP-Si. Cotton fabrics were soaked in the treating solutions for 15 min, and about 80% of wet pickup was obtained with two dips and two nips. After treating, the cotton fabrics were dried at 90 °C for 30 min and cured at 110 °C for 10 min. The weight gain (WG) (wt %) of treated cotton fabrics with DOPO-PiP-Si can be calculated according to the following equation:
where w1 and w2 represent the weights of cotton fabrics before and after treatment with DOPO-PiP-Si, respectively.
Characterization
Fourier transform infrared (FT-IR) were recorded by a Nicolet iS 50 FTIR spectrometer (Thermo Fisher Scientific, USA) using the ATR technique in the range of 500–3500 cm−1 to analyze the DOPO-PiP-Si and treated cotton fabrics. 1H nuclear magnetic resonance (1H-NMR) and 13C nuclear magnetic resonance (13C-NMR) of the DOPO-PiP-Si were recorded on a Bruker AVANCE-III 600 NMR spectrometer with CDCl3 as solvent.
The vertical flammability test was evaluated on a LFY-601A apparatus (Shandong Textile Science Research Institute, China) according to GB/T 5455-2014 with an ignition time of 12 s. LOI values were carried out using a LFY-606B digital limiting oxygen index tester (Shandong Textile Science Research Institute, China) according to GB/T 5454-1997.
X-ray diffraction (XRD) analysis was used to analyze the change in the crystal structure of the untreated and treated cotton fibers, and after-burned cotton fibers. The XRD patterns were recorded on a DX-2700 X-Ray Diffractometer (Dandong Haoyuan Instrument Co. Ltd., Dandong, China) at 36 kV and 20 mA. The diffractogram scattering angles ranged from 5° to 90°.
Morphology and elemental composition of the sample surface were investigated by JSM-6010LA SEM apparatus (Japan Electron Optics Laboratory Co., Ltd.).
Thermogravimetric analyses (TGA) of the DOPO-PiP-Si, untreated cotton and treated cotton were conducted using TGA851 thermal analyzer (Mettler-Toledo International Inc.) under air and nitrogen with a heating rate of 10 K/min from 40 to 800 °C.
Flammability of the cotton fabrics was characterized using a cone calorimeter (Fire Testing Technology Ltd) according to ISO 5660 method with a heating flux of 35 kW m−2. Two measurements were performed and averaged for them. A third specimen was measured whenever the first two deviated from each other by more than 20% in any characteristic parameter (Müller and Schartel 2016). The CV% values have been represented in Table 3 (Shukla et al. 2019).
Results and discussion
Characterization of DOPO-PiP-Si
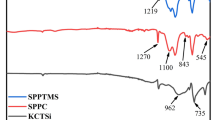
The FTIR spectrum of DOPO, DOPO-PiP and DOPO-PiP-Si are shown in Fig. 1. The characteristic peaks at 2908 and 2848 cm−1 are attributed to stretching vibration of –CH3 and –CH2. The aromatic C=C stretching vibrations appear from 1597 to 1373 cm−1, and the distortion vibrations peak at 749 cm−1 belongs aromatic hydrogen. Besides, compared with the spectra of DOPO, the peak at 2386 cm−1 (P–H) disappears (Vasiljević et al. 2015) and a new peak appears at 776 cm−1 in the spectra of DOPO-PiP, which is corresponding to the formation of P–N bonds by the reaction of DOPO with piperazine (Jiang et al. 2019a). And it can be identified that a monosubstituted piperazine is present in DOPO-PiP due to the presence of a peak at 3279 cm−1 (N–H) observed in the spectra of DOPO-PiP. The successful synthesis of DOPO-PiP-Si by reacting DOPO-PiP with chloromethyltrimethoxysilane can result in the disappearance of N–H bonds, which can be proved by the new absorption peaks appear at 1079 cm−1 (Si–O–C). Other peaks at 1230 and 905 cm−1 stem from P=O and P–O–Ph, respectively.
The structure of DOPO-PiP-Si was further verified by the 1H-NMR and 13C-NMR spectrum, as shown in Fig. 2. 1H NMR (δ in CDCl3) 1.09–1.38 (m, 2H, Si-CH2), 2.70–3.19 (m, 8H, N–CH2CH2–N), 3.30–3.63 (m, 9H, Si–OCH3), 7.25–7.96 (m, 8H, aromatic hydrogens); 13C NMR (δ in CDCl3) 40.66 (Si–CH2), 44.19, 51.98 (N–CH2CH2–N), 50.71 (Si–OCH3), 120.48–137.66 (DOPO).
Flammability of untreated and treated cotton fabrics
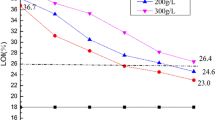
The results of limiting oxygen index (LOI) and photographs vertical burning test of treated cotton fabrics with different DOPO-PiP-Si concentrations were Table 1 and Fig. 3. As can be seen, the introduction of DOPO-PiP-Si can increase the LOI value of cotton fabrics from 18 to 27.6% at 400 g/L of DOPO-PiP-Si concentration and decrease the char length to 12.2 cm without afterflame and afterglow time. In fact, the P/N/Si system in DOPO-PiP-Si can play a synergistic flame retardant effect, which is able to effectively inhibit the combustion. Additionally, it is obvious that the weight gain (WG) of cotton fabrics is relatively high, for example the WG can reach 25.2% when the concentration of DOPO-PiP-Si is only 200 g/L, which may be a factor in the excellent flame retardancy of treated cotton fabrics.
Surface morphologies of untreated and treated cotton fabrics
The crystal structures of untreated cotton fibers, treated cotton fibers and char residues of treated cotton fibers were analyzed using XRD, respectively. As shown in Fig. 4, the XRD spectrum of treated cotton fibers is similar to that of untreated. There are four identical characteristic peaks at 14.83°, 16.83°, 22.95°, 34.31°, which are attributed to the crystal faces (1–10), (110), (200), and (004) of cellulose, respectively (Wang et al. 2018a, b). And XRD results indicate that the overall crystal structures of the cotton fiber are not damaged, although a lower intensity of crystallization of treated cotton fibers is observed. Additionally, a gentle peak can be found in the XRD spectrum of the char residue, which is due to the formation of amorphous carbon after the cotton fabric is burned.
The SEM morphologies before and after burning of treated and untreated cotton fabrics were shown in Fig. 5. As shown in Fig. 5a, e, the surface of untreated cotton fabrics is relatively smooth. For the samples grafted by DOPO-PiP-Si, a rougher fiber surface appears because of the adsorption of DOPO-PiP-Si as shown in Fig. 5b, f. And Fig. 5f shows that the flame retardant was adsorbed on the surface of the fiber bundle and in its gap without falling off, which may be caused by formation of covalent bonds between DOPO-PiP-Si and cotton fibers and increasing the interaction between them. A possible mechanism of the chemical bonding and interactions between cellulosic hydroxyl groups and DOPO-PiP-Si is assumed as shown in Fig. 6. Hydroxyl groups derived from hydrolysis of methoxy groups in flame retardant can self-polymerise into a polysiloxane network via a polycondensation reaction under appropriate conditions and also can simultaneously react with hydroxyl groups on the cellulose to form covalent bonds (Vasiljević et al. 2014a, b). So, the adhesion of DOPO-PiP-Si to cotton fiber is strongly increased due to the formation of covalent bonds, which promotes the durability of the flame retardancy on cotton fabrics.
In addition, in order to evaluate the flame retardant effect of the sample, the char residues were also collected to characterize. The untreated cotton fabrics were completely burned and changed from a complete and tight fiber bundle to a broken fluffy floc structure as shown in Fig. 5c, g. While complete char residues structure without shrinkage and rupture can be observed in Fig. 5d, h, indicating that DOPO-PiP-Si can improve the flame retardancy of cotton fabrics. And some vesicular bulges and cross-linking structures are formed, which can be explained that degradation of the flame retardant will form a dense layer of silicon char in the surface and gaps of the fiber bundle, accompanied by the formation of phosphoric acid and polyphosphoric acid. These phosphorus-containing organic acids will form a crosslinked structure between the fiber bundles, which enhances the stability of the char structure. At the same time, the nitrogen element in flame retardant releases a large amount of non-combustible gases during the combustion process, such as NH3, resulting in expansion of the fiber surface (Wei et al. 2019), and diluting the combustible gas to inhibit combustion. It can be concluded that DOPO-PiP-Si mainly take effect by the synergistic effect of Si/P/N.
EDS test was carried out to investigate the elements composition of the fiber surface, and the results are shown in Fig. 7. It can be seen from Fig. 7b that a few new elements, such as Si, P and N, were detected on the surface of treated cotton fabric compared with Fig. 7a, which further proved that DOPO-PiP-Si was successfully treated to the fiber surface. In addition, Si and P still existed after combustion as shown in Fig. 7c, and the content of them is increased. The content of C was maintained at a high level, which was due to the promotion of the formation of char layer during the degradation process of Si and P.
Thermal decomposition behaviors
The TGA test was carried out to investigate the thermal oxidative stability and flame retardant mechanism of the treated cotton fabrics under air and nitrogen atmosphere from 40 to 800 °C. In an air atmosphere, the corresponding TGA curves of DOPO-PiP-Si, untreated fabric, treated fabric (experimental) and treated fabric (calculated) and DTG curves of untreated and treated fabric are shown in Fig. 8a, b. The detailed data are summarized in Table 2. It is worth noting that there are two evident mass loss stages in treated cotton fabric, which means that DOPO-PiP-Si exhibit flame retardancy at temperatures lower than the pyrolysis temperature of cotton fabrics (Lu et al. 2019). For the treated samples, the initial degradation temperature of the first stage (Tonset) is 220 °C and the peak of degradation rate (Tmax1) occurs at 234 °C, which due to degradation of DOPO-PiP-Si on the surface of the cotton fabric to form phosphoric acid and polyphosphoric acid. The second degradation stage begins at 242 °C and the maximum-rate temperature (Tmax2) is 277 °C, which is assigned to the further degradation of the DOPO-PiP-Si and cotton fabric itself at higher temperatures. Furthermore, in order to demonstrate the synergistic flame retardant effect of DOPO-PiP-Si and cotton fabrics, experimental and theoretical TGA curves of the treated cotton fabrics were analyzed. The theoretical TGA curves is calculated based on the premise that there is no reaction between flame retardant and cotton fibers (Chen et al. 2017a; Liu et al. 2017), and the theoretical residual weight (RW) (wt%) can be calculated according to the following formula:
where RW1 and RW2 represent the char residue of DOPO-PiP-Si and pure cotton fabrics, respectively. However, it turns out that this premise is unreasonable. Before the temperature reaches 340 °C, the treated cotton fabrics exhibit a lower char residue rate than the theoretical one, because the phosphoric acid and polyphosphoric acid formed by the degradation of the flame retardant at a lower temperature promote the dehydration and carbonization of the cotton fiber. It is that intramolecular and intermolecular dehydration causes the loss of cotton fiber weight. However, when the temperature exceeds 340 °C, the experimental curve shows higher char residue, which proves that the chemical reaction between DOPO-PiP-Si and cotton fiber improves thermal stability and char formation ability. The difference between the experimental and theoretical curves demonstrates the synergistic effect between cotton fiber and flame retardant.
The results are more pronounced in a nitrogen atmosphere (Fig. 8c, d). The Tonset, Tmax1 and Tmax2 are increased to different extents compared with these in air atmosphere. In particular, the char residue of the treated cotton fabric is 42.0% at 800 °C, which is higher than that in air (19.1%). Additionally, it can be seen that the Tonset, Tmax1 and Tmax2 of the cotton fabrics treated with DOPO-PiP-Si are lower than that of the untreated cotton fabrics. But char residue of treated cotton fabrics at 800 °C is higher than that of untreated cotton fabrics in air or nitrogen atmosphere. The above results indicate that the treated cotton fabrics have a better flame retardant effect. The mechanism of thermal degradation is presumed to be that phosphoric acid and polyphosphate are produced after thermal decomposition of P–O–C bonds in DOPO-PiP-Si, and then the formed phosphoric acid can react with the hydroxyl groups of cellulose to form esters, and the formation of phosphoric esters promotes char formation while reduces the generation of fuel-gas. In addition, SiO2 film formed after siloxane degradation can act as a physical barrier to prevent heat and flammable transfer gases (Wei et al. 2019).
Cone calorimetry test was used to further evaluate the combustion behavior of cotton fabrics. Figure 9a shows the heat release rate (HRR) curves of treated and untreated cotton fabrics. The peak HRR (PHRR) value of cotton fabrics decrease from 230.8 to 161.1 kW/m2 after treating with DOPO-PiP-Si. Similar results can also be observed in the total heat release (THR) curves, as shown in Fig. 9b. The THR value of the treated cotton fabrics is reduced to 4.5 MJ/m2, which is decreased about 29.7% compared to the 6.4 MJ/m2 of untreated samples. The HRR and THR values are important indicators for measuring the thermal stability of cotton fabrics and their reduction can indicate that the treated cotton fabrics have excellent flame retardant properties. After ignition, a carbonaceous shield could be formed, which degrades up to the formation of a stable residue, to prevent heat spreading in cotton fabrics and oxygen exchanging (Duquesne et al. 2004). In that case, the propagation of a fire is restricted because the HRR and THR, which could affect the surrounding materials in a fire and consequently encourage them to ignite, decrease. Furthermore, a decrease of the time to ignition (TTI) and time to PHRR (tPHRR) (Table 3) is noted for treated cotton fabrics, which may be attributed to the surmise that the flame retardant covering the surface of the cotton fabric can change the radiative properties of the fabric itself, or some small organic molecules (such as ethylene) that are combustible evolve at the beginning of the degradation of the DOPO-PiP-Si. Table 3 demonstrate that about 17% of residue can be maintained after cone calorimetry test, and the char shape of the cotton fabric after burning can be observed more intuitively in Fig. 9e, f. The increasing amount of char residue when DOPO-PiP-Si is grafted in cotton fabrics may favour a condensed phase mechanism. The conclusion is consistent with the TG analysis.
In addition, in the cone calorimetry test, the burning efficiency of the cotton fabrics can be evaluated by the smoke production rate (SPR) and total smoke production (TSP). It can be seen from Fig. 9c, d that the SPR and TSP curves show a distinct variation trend with the HRR and THR curves above-described, and the SPR and TSP values of the treated cotton fabrics are generally higher than that of the untreated cotton fabric, and this result can be explained by the fact that the flame retardant on the surface of the cotton fabrics generates a large amount of volatile products (NH3) after combustion, and these products dilute the flammable gas, resulting in insufficient combustion of the cotton fabrics. It is suggested that higher SPR and TSP value indicates the retardant combination take effect through gas phase (Jiang et al. 2019b). This assertion can also be confirmed by the CO2/CO ratio. Indeed, the CO2/CO ratio can be considered as one of the important parameters of combustion efficiency. As shown in Table 3, the CO2/CO ratio of the treated cotton fabric is significantly reduced. The main reason for this result is that the non-combustible gas released from DOPO-PiP-Si can remove the flame-propagating free radicals (·OH and ·H) and inhibit the complete combustion into CO2 (Chen et al. 2017a; Duquesne et al. 2004). It may also be assumed that the gas phase mechanism is due to the formation of phosphorus-containing free radicals during the degradation of DOPO-PiP-Si, which can reduce the overall rate of propagation, and hence flame strength and efficiency of combustion. As a consequence, it can be seen from the above results that a gas phase mechanism is also involved in the fire retardant mode of action of the treated cotton fabrics.
FTIR analysis of condensed-phase
In order to get a better understanding of the degradation process of the treated cotton fabrics, the decomposition products in the condensed phase at different temperatures (selected according to TGA curves) have been analyzed by FTIR, as shown in Fig. 10. Compared with the spectrum at the room temperature, the absorption peaks at 1479, 1427 and 752 cm−1, which stem from benzene ring in DOPO-PiP-Si, turn to weak after 200 °C, and disappear at 400 °C, indicating the degradation and releasing of benzene ring. Besides, the intensities of peaks at 1151 and 1116 cm−1 (C–O–C) turn weak and disappear with the increase of temperature, while the increasing intensity for C=C peak at 1601 cm−1 can be observed after 300 °C, which demonstrate that the ring of cellulose starts to open and a carbonous char layer starts to be formed (Chen et al. 2017a; Liu et al. 2017). At 600 °C, the characteristic peaks of P=O at 999 cm−1 and SiO2 at 1070 cm−1 can be found, which confirms that phosphorus and SiO2 are left in the aromatic char residue.
Flame retardant mechanism
As the temperature increases, the benzene ring can be removed from the molecular chain by disrupting the P–N bond and releasing the phosphorus-based fragments (P–O and P–O–Ph). These fragments remove ·OH and ·H radicals in the gas phase and inhibit the combustion process by quenching. The phosphorus-based fragments combined with free radicals will form phosphorus oxides including phosphoric acid and polyphosphoric acid, which will esterify with cellulose to further promote the dehydration of the cotton fabric to char (Jin et al. 2019). The silicon-based fragments forms a SiO2 film on surface of the cellulose after burning, insulating heat and oxygen. In addition, piperazine will release NH3 and ethylene after desorption from the molecular chain, which may result in a cotton fabric that is more ignitable, but the subsequent release of ammonia will dilute the combustible gas and inhibit combustion. The release of a large amount of NH3 causes bubbling of the silicon–phosphorus char layer (Wei et al. 2019). When the amount of released gas exceeds the holding capacity of the char layer, the bubble bursts, and NH3 will carry a part of the silicon–phosphorus fragments into the air, while the nitrogen that does not exert the gas phase will remains in the condensed phase (Fig. 11).
Conclusions
A new type of Si/P/N synergistic flame retardant was prepared and successfully applied to cotton fabrics. The cotton fabric treated with 400 g/L DOPO-PiP-Si solution showed good flame retardancy, which passed vertical flammability test and obtained low char length with 12.2 cm, the LOI values increased to 27.6%, and it had a high tensile strength. The TG results indicated that DOPO-PiP-Si can improve char-formation ability of cotton fabrics, 19.1% of residue remained at 800 °C under air atmosphere. Further investigations have found that cotton fabrics treated with DOPO-PiP-Si has both gas-phase and condensed-phase flame retardant mechanism by releasing incombustible volatiles and forming stable char. And by analyzing its FTIR spectrum at different temperatures, a preliminary hypothesis was proposed for its thermal degradation process.
References
Abou-Okeil A, El-Sawy SM, Abdel-Mohdy FA (2013) Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohydr Polym 92:2293–2298. https://doi.org/10.1016/j.carbpol.2012.12.008
Alongi J, Ciobanu M, Malucelli G (2011a) Novel flame retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol–gel processes. Carbohydr Polym 85:599–608. https://doi.org/10.1016/j.carbpol.2011.03.024
Alongi J, Ciobanu M, Tata J, Carosio F, Malucelli G (2011b) Thermal stability and flame retardancy of polyester, cotton, and relative blend textile fabrics subjected to sol–gel treatments. J Appl Polym Sci 119:1961–1969. https://doi.org/10.1002/app.32954
Alongi J, Carletto RA, Di Blasio A, Carosio F, Bosco F, Malucelli G (2013) DNA: a novel, green, natural flame retardant and suppressant for cotton. J Mater Chem A. https://doi.org/10.1039/c3ta00107e
Alongi J et al (2014) Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym Degrad Stab 99:111–117. https://doi.org/10.1016/j.polymdegradstab.2013.11.016
Bai Z, Jiang S, Tang G, Hu Y, Song L, Yuen RKK (2014) Enhanced thermal properties and flame retardancy of unsaturated polyester-based hybrid materials containing phosphorus and silicon. Polym Adv Technol 25:223–232. https://doi.org/10.1002/pat.3227
Basak S, Ali SW (2016) Sustainable fire retardancy of textiles using bio-macromolecules. Polym Degrad Stab 133:47–64. https://doi.org/10.1016/j.polymdegradstab.2016.07.019
Chang S, Slopek RP, Condon B, Grunlan JC (2014) Surface coating for flame-retardant behavior of cotton fabric using a continuous layer-by-layer process. Ind Eng Chem Res 53:3805–3812. https://doi.org/10.1021/ie403992x
Chen C, Gu X, Jin X, Sun J, Zhang S (2017a) The effect of chitosan on the flammability and thermal stability of polylactic acid/ammonium polyphosphate biocomposites. Carbohydr Polym 157:1586–1593. https://doi.org/10.1016/j.carbpol.2016.11.035
Chen Z, Dong C, Li Q, Bai Y, Lu Z (2017b) Preparation of linear piperazine/phosphorous/polysiloxane copolymer and its application on cotton fabrics. J Therm Anal Calorim 130:1997–2005. https://doi.org/10.1007/s10973-017-6541-8
Dong Q et al (2013) Synergistic effect of DOPO immobilized silica nanoparticles in the intumescent flame retarded polypropylene composites. Polym Adv Technol 24:732–739. https://doi.org/10.1002/pat.3137
Duquesne S, Lefebvre J, Seeley G, Camino G, Delobel R, Le Bras M (2004) Vinyl acetate/butyl acrylate copolymers. Polym Degrad Stab 85:883–892. https://doi.org/10.1016/j.polymdegradstab.2004.04.004
Edwards B, Rudolf S, Hauser P, El-Shafei A (2015) Preparation, polymerization, and performance evaluation of halogen-free radiation curable flame retardant monomers for cotton substrates. Ind Eng Chem Res 54:577–584. https://doi.org/10.1021/ie502915t
Gaan S, Sun G, Hutches K, Engelhard MH (2008) Effect of nitrogen additives on flame retardant action of tributyl phosphate: phosphorus–nitrogen synergism. Polym Degrad Stab 93:99–108. https://doi.org/10.1016/j.polymdegradstab.2007.10.013
Gaan S, Neisius M, Cuchere O, Liang S, Mispreuve H (2014) Flame retardant polyurethanes based on novel phosphonamidate additives. Fire Saf Sci 11:821–831. https://doi.org/10.3801/iafss.Fss.11-821
Grancaric AM, Colleoni C, Guido E, Botteri L, Rosace G (2017) Thermal behaviour and flame retardancy of monoethanolamine-doped sol–gel coatings of cotton fabric. Prog Org Coat 103:174–181. https://doi.org/10.1016/j.porgcoat.2016.10.035
Jian R, Wang P, Duan W, Wang J, Zheng X, Weng J (2016) Synthesis of a novel P/N/S-containing flame retardant and its application in epoxy resin: thermal property, flame retardance, and pyrolysis behavior. Ind Eng Chem Res 55:11520–11527. https://doi.org/10.1021/acs.iecr.6b03416
Jiang Z, Li H, He Y, Liu Y, Dong C, Zhu P (2019a) Flame retardancy and thermal behavior of cotton fabrics based on a novel phosphorus-containing siloxane. Appl Surf Sci 479:765–775. https://doi.org/10.1016/j.apsusc.2019.02.159
Jiang Z, Xu D, Ma X, Liu J, Zhu P (2019b) Facile synthesis of novel reactive phosphoramidate siloxane and application to flame retardant cellulose fabrics. Cellulose 26:5783–5796. https://doi.org/10.1007/s10570-019-02465-2
Jin S, Qian L, Qiu Y, Chen Y, Xin F (2019) High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym Degrad Stab 166:344–352
Li S, Zhong L, Huang S, Wang D, Zhang F, Zhang G (2019) A novel flame retardant with reactive ammonium phosphate groups and polymerizing ability for preparing durable flame retardant and stiff cotton fabric. Polym Degrad Stab 164:145–156. https://doi.org/10.1016/j.polymdegradstab.2019.04.009
Liu Y, Tsai S-H (2002) Synthesis and properties of new organosoluble aromatic polyamides with cyclic bulky groups containing phosphorus. Polymer 43:5757–5762
Liu W, Chen L, Wang Y-Z (2012) A novel phosphorus-containing flame retardant for the formaldehyde-free treatment of cotton fabrics. Polym Degrad Stab 97:2487–2491. https://doi.org/10.1016/j.polymdegradstab.2012.07.016
Liu X, Gu X, Sun J, Zhang S (2017) Preparation and characterization of chitosan derivatives and their application as flame retardants in thermoplastic polyurethane. Carbohydr Polym 167:356–363. https://doi.org/10.1016/j.carbpol.2017.03.011
Liu J, Dong C, Wei D, Zhang Z, Xie W, Li Q, Lu Z (2019) Multifunctional antibacterial and hydrophobic cotton fabrics treated with cyclic polysiloxane quaternary ammonium salt. Fibers Polym 20:1368–1374. https://doi.org/10.1007/s12221-019-1091-2
Lu Z, Liu J, Dong C, Zhang Z, Wei D (2019) Durable multifunctional antibacterial and hydrophobic cotton fabrics modified with linear fluorinated pyridinium polysiloxane. Cellulose 26:7483–7494. https://doi.org/10.1007/s10570-019-02582-y
Mohamed AL, El-Sheikh MA, Waly AI (2014) Enhancement of flame retardancy and water repellency properties of cotton fabrics using silanol based nano composites. Carbohydr Polym 102:727–737. https://doi.org/10.1016/j.carbpol.2013.10.097
Müller P, Schartel B (2016) Melamine poly(metal phosphates) as flame retardant in epoxy resin: performance, modes of action, and synergy. J Appl Polym Sci. https://doi.org/10.1002/app.43549
Nguyen T-M, Chang S, Condon B, Slopek R, Graves E, Yoshioka-Tarver M (2013) Structural effect of phosphoramidate derivatives on the thermal and flame retardant behaviors of treated cotton cellulose. Ind Eng Chem Res 52:4715–4724. https://doi.org/10.1021/ie400180f
Przybylak M, Maciejewski H, Dutkiewicz A, Wesołek D, Władyka-Przybylak M (2016) Multifunctional, strongly hydrophobic and flame-retarded cotton fabrics modified with flame retardant agents and silicon compounds. Polym Degrad Stab 128:55–64. https://doi.org/10.1016/j.polymdegradstab.2016.03.003
Qian L, Qiu Y, Liu J, Xin F, Chen Y (2014) The flame retardant group-synergistic-effect of a phosphaphenanthrene and triazine double-group compound in epoxy resin. J Appl Polym Sci. https://doi.org/10.1002/app.39709
Rosace G, Colleoni C, Trovato V, Iacono G, Malucelli GJC (2017) Vinylphosphonic acid/methacrylamide system as a durable intumescent flame retardant for cotton fabric. Cellulose 24:3095–3108. https://doi.org/10.1007/s10570-017-1294-x
Salmeia KA, Gaan S (2015) An overview of some recent advances in DOPO-derivatives: chemistry and flame retardant applications. Polym Degrad Stab 113:119–134. https://doi.org/10.1016/j.polymdegradstab.2014.12.014
Shukla A, Sharma V, Basak S, Ali SW (2019) Sodium lignin sulfonate: a bio-macromolecule for making fire retardant cotton fabric. Cellulose 26:8191–8208. https://doi.org/10.1007/s10570-019-02668-7
Vasiljević J, Tomšič B, Jerman I, Orel B, Jakša G, Kovač J, Simončič B (2014a) Multifunctional superhydrophobic/oleophobic and flame-retardant cellulose fibres with improved ice-releasing properties and passive antibacterial activity prepared via the sol–gel method. J Sol Gel Sci Technol 70:385–399. https://doi.org/10.1007/s10971-014-3294-8
Vasiljević J, Tomšič B, Jerman I, Orel B, Jakša G, Simončič B (2014b) Novel multifunctional water- and oil-repellent, antibacterial, and flame-retardant cellulose fibres created by the sol–gel process. Cellulose 21:2611–2623. https://doi.org/10.1007/s10570-014-0293-4
Vasiljević J et al (2015) Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose 22:1893–1910. https://doi.org/10.1007/s10570-015-0599-x
Wagner S, Rakotomalala M, Bykov Y, Walter O, Döring M (2012) Synthesis of new organophosphorus compounds using the atherton-todd reaction as a versatile tool. Heteroat Chem 23:216–222. https://doi.org/10.1002/hc.21006
Wang X, Hu Y, Song L, Xing W, Lu H (2011) Thermal degradation mechanism of flame retarded epoxy resins with a DOPO-substitued organophosphorus oligomer by TG-FTIR and DP-MS. J Anal Appl Pyrol 92:164–170. https://doi.org/10.1016/j.jaap.2011.05.006
Wang X, Hu Y, Song L, Xing W, Lu H (2012) Preparation, flame retardancy, and thermal degradation of epoxy thermosets modified with phosphorous/nitrogen-containing glycidyl derivative. Polym Adv Technol 23:190–197. https://doi.org/10.1002/pat.1851
Wang L-H, Ren Y-L, Wang X-L, Zhao J-Y, Zhang Y, Zeng Q, Gu Y-T (2016) Fire retardant viscose fiber fabric produced by graft polymerization of phosphorus and nitrogen-containing monomer. Cellulose 23:2689–2700. https://doi.org/10.1007/s10570-016-0970-6
Wang D, Zhong L, Zhang C, Li S, Tian P, Zhang F, Zhang G (2018a) Eco-friendly synthesis of a highly efficient phosphorus flame retardant based on xylitol and application on cotton fabric. Cellulose 26:2123–2138. https://doi.org/10.1007/s10570-018-2193-5
Wang D, Zhong L, Zhang C, Zhang F, Zhang G (2018b) A novel reactive phosphorous flame retardant for cotton fabrics with durable flame retardancy and high whiteness due to self-buffering. Cellulose 25:5479–5497. https://doi.org/10.1007/s10570-018-1964-3
Wei D, Dong C, Chen Z, Liu J, Li Q, Lu Z (2019) A novel cyclic copolymer containing Si/P/N used as flame retardant and water repellent agent on cotton fabrics. J Appl Polym Sci. https://doi.org/10.1002/app.47280
Xie C, Zeng B, Gao H, Xu Y, Luo W, Liu X, Dai L (2014) Improving thermal and flame-retardant properties of epoxy resins by a novel reactive phosphorous-containing curing agent. Polym Eng Sci 54:1192–1200. https://doi.org/10.1002/pen.23642
Acknowledgments
This work was supported by Natural Science Foundation of Shandong Province (Grant No. ZR2018MEM026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Z., Dong, C., Liu, J. et al. Preparation of a synergistic reactive flame retardant based on silicon, phosphorus and nitrogen and its application to cotton fabrics. Cellulose 27, 1799–1815 (2020). https://doi.org/10.1007/s10570-019-02900-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02900-4