Abstract
Cellulosic fibers are usually finished with flame retardant via repeated pad-dry-cure processes. The present contribution reports a simple, facile, and one-pot chemical treatment process to access durable flame retardant lyocell fibers by directly immersing the fibers into the solution of a flame retardant ester of 2,2-ethanolamine diphosphoric acid (EADP) and urea for a certain time without using catalyst and cross-linker. As demonstrated, the treated lyocell fibers with grafted EADP show excellent flame retardancy and durability, as evidenced by an increase of limiting oxygen index value up to 37.8% and still 25.6% after 40 washing cycles. The key to success is ascribed to the formation of three dimensional flame retardant structures with EADP. Various analytical techniques, including raman spectroscopy, scanning electron microscope, thermogravimetry, and TG-infrared coupled technique prove that the carbonaceous residue and non-combustion gases were preferably generated during thermal decomposition process of treated fibers. Microcombustion calorimetry results revealed a significant reduction in the peak of heat release rate. The results indicate that EADP is potential for using as an efficient durable flame retardant of lyocell fibers.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lyocell fibers, one new generation of ‘‘green’’ man-made regenerated cellulosic fibers, have been widely applied to clothing, bedding, furnishing, and many industrial fields because they possess a variety of advantages, such as softness, comfort, and breathability (Hall et al. 1999; Joshi et al. 2010). However, lyocell fibers are a rich source of hydrocarbons and thus a combustible fuel source during the burning process, leading to a high burning rate and causing serious fires (Xie et al. 2013). This disadvantage greatly limits their applications in many important aspects. Therefore, it is an urgent issue to explore flame retardant (FR) lyocell fibers or fabrics.
To date, there are few reports of FR studies on ‘‘green’’ lyocell fibers. The main methods for preparing flame retardant lyocell fibers or fabrics include blending spinning (Seddon et al. 1996), finishing process (Mengal et al. 2016; Liu et al. 2018), and chemical treatment (Bai et al. 2014; Wang et al. 2016). For the blending spinning strategy, the flame retardants are usually introduced by spinning process, i.e., the spinning solution containing flame retardants and other additives is extruded through an orifice, drawn into an air gap, and precipitated in a coagulation bath (Seddon et al. 1996). Especially, the flame retardants should possess excellent dispersibility and compatibility with mixture solution. Generally, a large amount of flame retardant is required to achieve high flame retardancy, which would deteriorate the physical properties of the obtained fibers (Zheng et al. 2016; Tai et al. 2012; Grancaric et al. 2015). Seddon et al. added an efficient flame retardant N-hydroxymethyl-3-(dimethoxyphosphonyl)propionamide (Pyrovatex CP) to cellulose/NMMO/water spinning solution. The resulting lyocell fibers shows a limiting oxygen index (LOI) value of 40% with 32 wt% flame retardant addition. However, formaldehyde will be released if heat treated during the final heat setting. Formaldehyde has been regarded as a carcinogenic compound by World Health Organization (Alongi et al. 2013; Xie et al. 2017; Vasiljević et al. 2015). Besides, a catalyst and a cross-linker were indispensable. The flame retardant durability of the treated lyocell fibers was also not referred to in the reports.
The finishing method is most commonly used to improve flame retardancy of cellulosic fibers. This method employs a multi-component finishing system consisting of flame retardant, catalyst, and cross-linker, and etc., and is performed via repeated pad-dry-cure processes, i.e., immersing fibers into the FR solution, dipping, drying, and curing at relatively high temperatures (Alongi and Frache 2011; Jiang et al. 2015; Alongi et al. 2014). Mengal et al. adopted Provatex CP to treat lyocell fibers via this strategy (Mengal et al. 2016). The residue char yield of finished lyocell fibers reached 42% with 400 g/L of flame retardant, indicating a high flame retardancy efficiency for Pyrovatex CP-based finishing system (Liu et al. 2012; Ma et al. 2017). Generally, the system need many components (e.g., flame retardant Provatex CP, cross-linker citric acid, and catalyst sodium hypophosphite or phosphoric acid) and complicated processes to ensure high flame retardancy (Qiu et al. 2017). The flame retardant durability of the treated lyocell fibers limited to 10 washing cycles (Zhang et al. 2016). Thus, it is necessary to develop innovative and environmentally friendly flame retardant and a simple, facile, and efficient method for lyocell fibers (Bosco et al. 2013; Li et al. 2017).
Compared with blending and finishing methods, the chemical treatment is simple, and cost-effective, and it only requires one-step process by directly immersing the fibers in the FR solution for an ideal duration (Cheng et al. 2017; Liang et al. 2017). By choosing a suitable treating agent or solution, flame retardant fibers can be easily obtained. Initially, the used flame-retardants are inorganic phosphorus compounds (Kim et al. 2015). For instance, Jeon et al. treated flame retardant lyocell fibers with a mixed aqueous solution of NaCl/H3PO4 (Kim et al. 2014). The LOI value of treated fibers only reaches 26.5%. Moreover, Kim et al. employed a solution of (NH4)2SO4/NH4Cl, and increased LOI value up to 31% (Kim et al. 2016). Nevertheless, the flame retardant durability of the treated lyocell fibers was not mentioned. With this regard, future work on the preparation of durable and flame retardant lyocell fiber via the chemical treatment is in great demand. To date, phosphorus or nitrogen-containing flame retardants have become major substitutes for halogenated ones owing to their high efficiency and low toxicity (Liu et al. 2016; Jia et al. 2017). To the best of our knowledge, there is few report on the utilizing of phosphorus and nitrogen-containing flame retardants to chemically treat lyocell fibers (Wang et al. 2016).
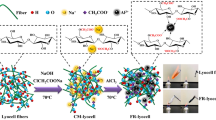
The aim of the present work is to provide a simple and facile chemical treatment method to prepare durable flame retardant lyocell fibers by virtue of a new halogen-free and formaldehyde-free compound (ester of 2,2-ethanolamine diphosphoric acid, EADP) with phosphorus and nitrogen elements (Scheme 1). The structure and surface morphology of the treated fibers were investigated in detail. The thermal decomposition and burning behaviors were also evaluated with numerous analytical techniques.
Experimental
Materials
Lyocell fibers and fabrics were obtained from Shandong Yingli Industrial Co., LTD. (Shandong, China). Diethanolamine was purchased from Aladdin Reagent Co.Ltd. (Shanghai, China). Phosphoric acid (85%), urea, and N, N-dimethylformamide (DMF) were supplied by Tianjin Fengchuan Chemical Reagent Co.Ltd. (Tianjin, China). All chemicals were of the reagent grade and were used without further purification.
Synthesis of ester of 2,2-ethanolamine diphosphoric acid (EADP)
Diethanolamine (0.1 mol) and phosphoric acid (0.2 mol) were added into a 100 mL flask with a magnetic stirring and a reflux condenser. The mixture was then placed into an oil bath at 130 °C. After 2 h, a viscous brown liquid was obtained. The crude flame retardant agent was purified by precipitating using ethanol, filtered and dried in vacuum at 50 °C. FT-IR (KBr) (cm−1): 3198 and 3300 (vs, N–H, O–H), 1353 (vs, P=O), 1280 (vs, P–O–C), and 830 (vs, P–OH). Elemental analysis results: theoretical O (48.31%), C (18.10%), H (4.91%), N (5.28%), P (23.38%); measured O (48.46%), C (18.33%), H (4.95%), N (5.08%), P (23.17%). 1H NMR (D2O, 600 MHz) δ (ppm):3.15 (s, 2CH2, H1, H2) and 3.75 (s, 2CH2, H3, H4), no signal peaks of –OH groups were detected because of the exchange of OH reactive hydrogen by deuterium hydrogen in D2O.
Chemical treatment of lyocell fibers with EADP
The flame retardant solutions were firstly prepared by dissolving EADP and urea in DMF. Lyocell fibers were then impregnated with the solutions for 2 h at 150 °C. Subsequently, the treated fibers were washed two times with deionized water. The fibers were finally dried in vacuum oven at 50 °C for 24 h until to a constant weight.
Characterization
The FT-IR assessment of control and treated lyocell fibers samples for chemical structure was conducted by using Nicolet iS50 FT-IR spectrometer (Thermo Fisher Scientific Inc., China) using the KBr pellet technique in the range of 4000–400 cm−1 with a resolution of 2.0 cm−1.
X-ray photoelectron spectrometer (XPS) experiments were performed by a Thermal ESCALAB 250XI X-ray source system (USA).
The surface morphologies of the control and the EADP treated lyocell fibers were observed using a ZEISS Gemini scanning electron microscope (SEM) with a beam voltage of 5 kV. In order to clearly capture their surfaces under SEM, all samples were coated with platinum using a sputter coater.
Thermogravimetry (TG) of the control and modified lyocell fibers (3 mg) was carried out using a Pyris 1 thermogravimetric analyzer (STA449F3, DEU) from room temperature to 800 °C at a heating rate of 10 °C/min under nitrogen and air, respectively.
TG-infrared (TG-IR) was conducted on a Netzsch STA409PC thermal analyzer coupled with a Bruker Tensor 27 FT-IR spectrometer via a polytetrafluoroethylene pipe. Lyocell fabrics samples (8.0 mg) were placed under a nitrogen gas flow of 30 mL/min at a heating rate of 20 K/min.
Pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS) system was employed to separate and identify the pyrolysis volatiles. For this purpose, a Frontier PYR-4A type pyrolyser was directly attached to a gas chromatography/mass spectrometry (SHIMADZU GCMS-QP5050A, Agilent). In the characterization process, the pyrolysis temperature of 600 °C was used and kept for 30 s. The temperature of the capillary column (0.25 mm) of GC hold at 40 °C for 5 min; afterwards, the temperature was increased to 300 °C at a heating rate of 10 °C/min and then kept at 300 °C for 15 min.
The vertical flammability of control and treated lyocell fabrics was examined according to the ASTM D6413-99 standard test method on a YG815B vertical fabric FR tester (Nantong Sansi electromechanical Science & Technology Co., Ltd., China).
The limiting oxygen index (LOI) values of the control and treated lyocell fabrics were measured according to ASTM D2863-2000 standard test method using an M606B digital oxygen index apparatus.
Raman measurements of control and treated lyocell fabrics were performed with a XploRA PLUS Raman spectrometer in the range of 500–2500 cm−1.
The burning performance of the control and treated lyocell fabrics was investigated by microcombustion calorimetry according to ASTM E 7309-13. The fabric samples were dried to a constant weight in vacuum oven at 75 °C before testing. Then the samples were heated from 0 to 750 °C at a heating rate of 1 °C/s following a stream of nitrogen (80 mL/min) and oxygen (20 mL/min).
Results and discussion
Structure analyses
The chemical structures of control and treated lyocell fibers with 180 g/L EADP solution were measured by FT-IR technique. Their FT-IR spectra are shown in Fig. 1. Compared with the control lyocell fibers displaying the broad band at around 1640 and 3340 cm−1 assigns to OH bending and stretching vibrations (Sahito et al. 2015), the chemically treated lyocell fibers display the bands at 1290 and 830 cm−1 assigned to the stretching vibrations of P=O and P–O–H groups, respectively (Gospodinova et al. 2002). Meanwhile, the absorption at 1380 cm−1 ascribed to the P-O-C bonds is clearly observed, which may be an indication of the bond between fibers and EADP. Therefore, the results demonstrate that EADP firstly reacted with urea and formed –P=O(O−NH4+)2 group, which then further reacted with OH group of cellulose unit of lyocell fibers, forming the P–O–C covalent bonds. Additionally, a wet pick-up of the fibers treated with EADP reaches 130%, and the dry pick-up reaches 15%. A high pick-up for the treated fibers indicates that EADP is an efficient flame retardant for lyocell fibers and has been successfully grafted onto its surface after one-pot chemical reaction.
Furthermore, the surface chemical compositions (atom%) of the control and EADP-treated lyocell fibers were characterized by XPS technique, and the spectra and the detailed data are shown in Fig. 2 and Table 1, respectively. For the control lyocell fibers, there are two strong peaks at 286 eV and 533 eV, corresponding to carbon and oxygen atoms, respectively. In contrast, for the treated lyocell fibers, the new atomic peaks corresponding to nitrogen at 400 eV (2.9 wt%) and phosphorus at 134 eV (2.1 wt%) are obviously visible. Therefore, the results proved that flame retardant EADP has been successfully grafted onto the surface of lyocell fibers via a simple one-pot chemical treatment.
Surface morphology
SEM technique was employed to observe the surface morphology of the control lyocell fibers, treated lyocell fibers and the char residues after burning (Fig. 3). The control fibers show a smooth rod structure without any defects (a and b). Likewise, the treated lyocell fibers (c and d) show nearly the same morphology compared with the untreated sample. No additional substances or residues appear to be adhered on the surface of the EADP-treated lyocell fibers except only a few small accumulation, indicating that EADP may penetrate into the interior space of the fibers instead of being deposited on the surface.
Compared with the untreated fibers burning into little ash, the EADP-treated one after burning kept an intact fiber-like structure (e and f). The residual carbonized char of the lyocell fibers after burning remains continuous, and many bubbles appear on the surface, while the treated fibers does not show any cracks after burning. Consequently, phosphorus and nitrogen elements in EADP are considered to catalyze the dehydration of cellulose unit, and char is thus resulted (Liu et al. 2015). The carbon layer after burning could act as an efficient physical barrier to retard the transfer of the heat and burnable gas into the surface, preventing further combusting of the fibers (Spontón et al. 2009).
Thermogravimetric analyses
TG and derivative thermogravimetric (DTG) curves as well as the corresponding detailed data of the control and EADP-treated lyocell fibers in nitrogen atmosphere are shown in Fig. 4 and Table 2. The pyrolyzing of lyocell fibers in nitrogen usually includes dehydration and depolymerization pathways. In the case of the control sample, its weight decreases rapidly from 280 to 355 °C with ca. 75% weight loss and 15.7% ash residue at 800 °C (Fig. 4).
However, the EADP-treated sample relatively slowly degraded over a range of 210-280 °C, with ca. 32% weight loss and 41.3% residue at 800 °C (Table 2). The results indicate that EADP largely changes the pyrolytic process of lyocell fibers to efficiently form char, thereby rendering this compound as an efficient chemical treating agent for lyocell fibers. These improvements may be attributed to the fact that phosphorus acid or polyphosphoric acid produced during decomposition of EADP prefers to catalyze the dehydration of fibers and form char on the surface of fibers. Thus the shift in TG response to lower temperature and increase in char are typically shown by any P and N-containing species, as originally noted by Tang and Neill 50 years ago (Tang and Neill 1964).
The resulting char can serve as an efficient physical barrier to shield the substrate and the incomplete combustion products from the oxygen and heat, thus causing a lower decomposition temperature and a higher amount of residue for the EADP-treated sample (Nehra et al. 2014).
TG, DTG curves, and corresponding detailed data of control and EADP-treated samples in air are shown in Fig. 5 and Table 3. For control sample, its degradation in air generally comprise two main steps. The first stage occurred at 300–360 °C involves two competitive pathways: generating aliphatic chars or volatile products (Fig. 5). In the second step occurred at 360–475 °C, some aliphatic chars are converted to aromatic ones, and some chars are further oxidized into CO and CO2 species (Price et al. 1997). In contrast, EADP-treated sample strongly altered the decomposition behaviour and favored the char formation, as evidenced from a significant decrease in initial decomposition temperature (210-280 °C) with ca. 50% mass loss. Importantly, after a critical temperature (ca. 340 °C), the rate of decomposition (Rmax (%/ °C)) of the treated fibers is considerably less than that of the control sample. The final residue (5.8%) of the treated lyocell fibers is higher than that of control sample (3.6%) at 700 °C (Table 3). It is attributed to the further oxidation of unstable char on the surface into CO and CO2 species in the presence of oxygen for the control fibers.
TG-IR coupled analyses
The structure of degradation products of the control and EADP-treated lyocell fabrics were also monitored by TG-IR coupled analyses (Chen and Jiao 2008), as shown in Fig. 6. For the control sample (Fig. 6a), the peak at 3560 cm−1 is ascribed to the vibration absorption of O–H group, suggesting the release of water vapor. The peaks at 2914 and 2974 cm−1 are attributed to the vibration absorption of C-H bond derived from hydrocarbon compounds. The sharp peak at 2360 cm−1 is attributed to the absorbance of CO2. The intense peaks at 1748 cm−1 are attributed to the vibration absorption of C=O derived from carbonyl compounds, and the absorption peak at 1064 cm−1 is ascribed to C–O–C bonds derived from ethers. Thus the main degradation products include inflammable species (e.g., H2O and CO2) and flammable species (e.g., hydrocarbons, carbonyl compounds, and ethers) (Chen et al. 2013).
For the treated sample (Fig. 6b), no obvious new absorption peak appears. Contrarily, the peak at 2974 cm−1 disappears as EADP inhibits the generation of combusting hydrocarbon products, such as alkane and furane derivatives. The absorption intensity of CO2 and H2O for the treated lyocell fabric appeared at a lower temperature (200 °C) compared with those of control lyocell fabrics (290 °C). The result indicates that the decomposition temperature for the EADP-treated lyocell fabrics decreases greatly, which is in agreement with the TG results. In addition, the maximum absorption intensities of carbonyl compounds at 1748 cm−1 and ethers at 1064 cm−1 for the EADP-treated lyocell fabrics are considerably lower than those of the control lyocell fabrics. All the results demonstrate that EADP favors dehydration to form char instead of flammable species including hydrocarbons, carbonyl compounds, ethers, and etc.
Py-GC/MS analyses
For the sake of confirming the structures of degraded products, the control and EADP-treated lyocell fabrics were also measured by Py-GC/MS technique (Gu et al. 2013). Figure 7 illustrates their Py-GC/MS spectra at the temperature range of final decomposition stage as shown in TG curves in air (Fig. 5), and the main pyrolyzed volatile product data are summarized in Table 4.
Clearly, the peak quantities and intensities of pyrolyzed volatile products of treated sample are less than those of control sample (Table 4). A series of furane, carbonyl, ketone, ester, and alcohol derivatives are found from the pyrolysis of control lyocell fabrics (Alongi et al. 2013). Obviously, CO2 and H2O appear as common degradation products for control and EADP-treated lyocell fabrics. Importantly, new inflammable species NH3 originating from NH4+ group of EADP is observed at 1.9 min for the treated sample. Therefore the results further suggest that the pyrolysis process of the treated lyocell fabrics produced additional nonflammable species, possibly contributing to an increase of flame retardancy (See below).
Flame retardant performance
Vertical flammability tests were performed to determine flame retardancy of lyocell fabrics before and after being treated with EADP. The results of the samples exposed to flame after 60 s are shown in Fig. 8, and the corresponding information (weight gain, after-flame time, after-glow time, and char length) is presented in Table 5. The untreated lyocell fabrics burned fast, intensely, and completely, leaving a very low amount of ash after 40 s burning (Fig. 8a). In contrast, the treated sample did not burn obviously even exposed to flame after a longer time. With different concentrations of EADP, varied char lengths were retained at the end of the lyocell fabrics (Fig. 8b–d), which is also clearly observed from the fabrics treated with 180 g/L after 20 laundering cycles (Fig. 8e).
As shown in Table 5, the char lengths of the lyocell fabrics using 120 g/L, 150 g/L, and 180 g/L EADP were 90, 83, and 67 mm, respectively. Importantly, the fabrics with 180 g/L EADP after undergoing 20 laundering cycles only shows a char length of 83 mm. Based on the vertical flammability test results, the fabrics with 180 g/L EADP shows the best flame retardancy characteristics.
The limiting oxygen index (LOI) values of treated fabrics with laundering cycles are illustrated in Fig. 9. Obviously, the LOI value of treated fabrics with 180 g/L EADP solution reaches 38%, which is higher than that of the control sample (17%). With the increase of laundering cycles, the LOI values of the treated fabrics decreased down to 25.6% after 40 laundering cycles, which still matches common flame retardant standard of textile. The results demonstrate the effectiveness of EADP as a flame retardant, and enhanced lyocell fabric’s durability is attributed to the formed covalent bonding between EADP and cellulose unit and the corresponding three dimensional flame retardant structures. Therefore, the one-pot chemical treatments of lyocell fibers/fabrics with EADP as the flame retardant with urea were further investigated.
Raman spectra of treated Lyocell fibers after burning
Generally, Raman scattering of the excitation radiation takes place only on the surface of treated lyocell fabric after combustion. As Fig. 10 shows, the Raman spectrum of the residue of the EADP-treated lyocell fibers after spontaneous burning is composed of two broad bands at 1583 cm−1 and 1346 cm−1. The shape of the spectra is typical of carbonaceous materials at 1583 cm−1 and 1346 cm−1, which are characterized by so-called “G”(graphitic) and “D”(disordered) bands (Casiraghi et al. 2007; Stejskal et al. 2005). Thus the results confirm that the treated fibers can efficiently form char during burning.
Microcombustion calorimetry
Microcombustion calorimetry test was used to further evaluate flame retardancy of EADP-treated fabrics. The heat release rate (HRR) results and corresponding detailed data of untreated and treated fabrics are shown in Fig. 11 and Table 6. The untreated lyocell fabrics exhibited significantly higher HRR values than treated fabrics. The peak values of HRR (PHRR) were greatly affected by flame retardant EADP (Fig. 11).
The PHRR value for control lyocell fabrics was 168.1 W/g, which was far higher than that of treated sample (32.8 W/g), as shown in Table 6. The time to the PHRR of treated sample is earlier than the control fabrics. The results of time to PHRR are matched with thermogravimetric analysis. A decrease of PHRR and total heat release (THR) values for treated sample demonstrates that the phosphorus and nitrogen-containing EADP is favorable to not only condensed phase flame retardancy by forming a dense char layer on the surface of fabrics to promote the dehydration and carbonization but also vapor phase flame retardancy (Cheema et al. 2013).
Mechanism analyses
TG and microcombustion calorimetry test results confirm that EADP is found to efficiently catalyze the dehydration of lyocell fibers or fabrics to form char. The resultant char prevents unburned fabrics from heat and flame, thus decreasing the decomposition temperature and PHRR during thermal pyrolysis process.
Based on the integrated results of SEM, PY-GC/MS, and TG-IR coupled analyses, the possible flame retardant mechanism for EADP-treated lyocell fibers/fabrics is presented. EADP grafted onto lyocell fibers firstly decomposes and releases a large amount of non-combustion gases such as H2O, CO2, NH3, etc. during pyrolysis process. The non-combustion gases can dilute the concentration of the combustible gaseous products released during pyrolysis process. Simultaneously, EADP catalyzes fibers/fabrics to dehydrate and carbonize, forming compact char layer. The char layer efficiently acts as physical barrier for preventing the transfer of heat and oxygen to the matrix materials. Consequently, the flame retardant operates in both condensed phase and gaseous phases during the burning process.
Conclusions
EADP bearing phosphorus and nitrogen elements, a new, efficient, halogen-free, and formaldehyde-free flame retardant was successfully synthesized and used to chemical treat lyocell fibers via one pot process. The FT-IR and XPS analyses prove the firm covalent binding between EADP and the cellulose of lyocell fibers. Therefore, the treated samples display excellent flame retardancy and durability as confirmed by various techniques. TG analyses in nitrogen and air demonstrate that the decomposition temperature of treated lyocell fabrics decreased by approximately 100 °C and the residues significantly increased compared with control sample. The microcombustion calorimetry test show that treated lyocell fabrics possess lower PHRR and THR values compared with control sample because the release of flammable volatiles was inhibited for the treated sample. SEM and Raman measurements show that treated lyocell fabrics prefers to generate residual carbonized frame. Furthermore. TG-IR results suggest that EADP synchronously operates in both condensed and gaseous phase during the combusting of modified samples. That is, one pot chemical treating fibers was successfully conducted and generated flame retardant samples, as shown in the cross-linking reaction between EADP and lyocell fibers in Scheme 1. This novel flame retardant system is believed to provide a novel and facile strategy for flame retarding numerous cellulosic fibers and fabrics.
References
Alongi J, Frache TA (2011) Hydrotalcite and nanometric silica as finishing additives to enhance the thermal stability and flame retardancy of cotton. Cellulose 18(1):179–190
Alongi J, Carletto RA, Di Blasio A, Carosio F, Bosco F, Malucelli G (2013) DNA: a novel, green, natural flame retardant and suppressant for cotton. J Mater Chem A 1(15):4779–4785
Alongi J, Carosio F, Malucelli G (2014) Current emerging techniques to impart flame retardancy to fabrics: an overview. Polym Degrad Stab 106(8):138–149
Bai BC, Kim EA, Jeon YP, Lee CW, In SJ, Lee YS, Ji SI (2014) Improved flame-retardant properties of lyocell fiber achieved by phosphorus compound. Mater Lett 135(12):226–228
Bosco F, Carletto RA, Alongi J, Marmo L, Di BA, Malucelli G (2013) Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr Polym 94(1):372–377
Casiraghi C, Robertson J, Ferrari AC (2007) Diamond-like carbon for data and beer storage. Mater Today 10(2):44–53
Cheema HA, Elshafei A, Hauser PJ (2013) Conferring flame retardancy on cotton using novel halogen-free flame retardant bifunctional monomers: synthesis, characterizations and applications. Carbohydr Polym 92(1):885–893
Chen X, Jiao C (2008) Thermal degradation characteristics of a novel flame retardant coating using TG-IR technique. Polym Degrad Stab 93(12):2222–2225
Chen X, Huo L, Jiao C, Li S (2013) TG-FTIR characterization of volatile compounds from flame retardant polyurethane foams materials. J Anal Appl Pyrol 100(6):186–191
Cheng Y, He G, Barras A, Coffinier Y, Lu S, Xu W, Szunerits S, Boukherroub R (2017) One-step immersion for fabrication of superhydrophobic/superoleophilic carbon felts with fire resistance: fast separation and removal of oil from water. Chem Eng J 331:372–382
Gospodinova N, Grelard A, Jeannin M, Chitanu GC, Carpov A, Thiéry V, Besson T (2002) Efficient solvent-free microwave phosphorylation of microcrystalline cellulose. Green Chem 4(3):220–222
Grancaric AM, Botteri L, Alongi J, Malucelli G (2015) Synergistic effects occurring between water glasses and urea/ammonium dihydrogen phosphate pair for enhancing the flame retardancy of cotton. Cellulose 22(4):2825–2835
Gu X, Ma X, Li L, Liu C, Cheng K, Li Z (2013) Pyrolysis of poplar wood sawdust by TG-FTIR and Py-GC/MS. J Anal Appl Pyrol 102(7):16–23
Hall ME, Horrocks AR, Seddon H (1999) The flammability of lyocell. Polym Degrad Stab 64(3):505–510
Jia Y, Lu Y, Zhang G, Liang L, Zhang F (2017) Facile synthesis of an eco-friendly nitrogen-phosphorus ammonium salt to enhance durability and flame retardancy of cotton. J Mater Chem A 5(20):9970–9981
Jiang D, Sun C, Zhou Y, Wang H, Yan X, He Q, Guo J, Guo Z (2015) Enhanced flame retardancy of cotton fabrics with a novel intumescent flame-retardant finishing system. Fiber Polym 16(2):388–396
Joshi HD, Joshi DH, Patel MG (2010) Dyeing and finishing of lyocell union fabrics: an industrial study. Color Technol 126(4):194–200
Kim EA, Bai BC, Jeon YP, Lee CW, Lee YS, In SJ, Ji SI (2014) Effects of NaCl/H3PO4 flame retardant treatment on lyocell fiber for thermal stability and anti-oxidation properties. J Korean Ind Eng Chem 25(4):418–424
Kim HG, Kim EA, Lee YS, In SJ (2015) Na3PO4 flame retardant treatment on lyocell fiber for thermal stability and anti-oxidation properties. Fire Sci Eng 29(2):25–32
Kim HG, Bai BC, In SJ, Lee YS (2016) Effects of an inorganic ammonium salt treatment on the flame-retardant performance of lyocell fibers. Carbon Lett 17(1):74–78
Li X, Zhao Z, Wang Y, Yan H, Zhang X, Xu B (2017) Highly efficient flame retardant, flexible, and strong adhesive intumescent coating on polypropylene using hyperbranched polyamide. Chem Eng J 324:237–250
Liang T, Jiang Z, Wang C, Liu J (2017) A facile one-step synthesis of flame-retardant coatings on cotton fabric via ultrasound irradiation. J Appl Polym Sci 134(30):45114–45120
Liu W, Chen L, Wang YZ (2012) A novel phosphorus-containing flame retardant for the formaldehyde-free treatment of cotton fabrics. Polym Degrad Stab 97(12):2487–2491
Liu H, Wang X, Wu D (2015) Preparation, isothermal kinetics, and performance of a novel epoxy thermosetting system based on phosphazene-cyclomatrix network for halogen-free flame retardancy and high thermal stability. Thermochim Acta 607:60–73
Liu Y, Pan YT, Wang X, Acuña P, Zhu P, Wagenknecht U, Heinrich G, Zhang XQ, Wang R, Wang DY (2016) Effect of phosphorus-containing inorganic-organic hybrid coating on the flammability of cotton fabrics: synthesis, characterization and flammability. Chem Eng J 294:167–175
Liu XH, Zhang QY, Cheng BW, Ren YL, Zhang YG, Ding C (2018) Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 25:799–811
Ma C, Qiu S, Yu B, Wang J, Wang C, Zeng W, Hu Y (2017) Economical and environment-friendly synthesis of a novel hyperbranched poly(aminomethylphosphine oxide-amine) as co-curing agent for simultaneous improvement of fire safety, glass transition temperature and toughness of epoxy resins. Chem Eng J 322:618–631
Mengal N, Syed U, Malik SA, Ali SI, Jeong SH (2016) Citric acid based durable and sustainable flame retardant treatment for lyocell fabric. Carbohydr Polym 153:78–88
Nehra S, Hanumansetty S, O’Rear EA, Dahiya JB (2014) Enhancement in flame retardancy of cotton fabric by using surfactant-aided polymerization. Polym Degrad Stab 109(109):137–146
Price D, Horrucks AR, Akalin M, Faroq AA (1997) Influence of flame retardants on the mechanism of pyrolysis of cotton (cellulose) fabrics in air. J Anal Appl Pyrol 40–41:511–524
Qiu X, Li Z, Li X, Zhang Z (2017) Flame retardant coatings prepared by layer by layer assembly: a review. Chem Eng J 334:108–122
Sahito IA, Sun KC, Arbab AA, Qadir MB, Jeong SH (2015) Integrating high electrical conductivity and photocatalytic activity in cotton fabric by cationizing for enriched coating of negatively charged graphene oxide. Carbohydr Polym 130:299–306
Seddon H, Hall M, Horrocks AR (1996) The flame retardancy of lyocell fibres. Polym Degrad Stab 54(2):401–402
Spontón M, Ronda JC, Galià M, Cádiz V (2009) Cone calorimetry studies of benzoxazine-epoxy systems flame retarded by chemically bonded phosphorus or silicon. Polym Degrad Stab 94(1):102–106
Stejskal J, Trchová M, Sapurina I (2005) Flame-retardant effect of polyaniline coating deposited on cellulose fibers. J Appl Polym Sci 98(6):2347–2354
Tai Q, Yuen RKK, Song L, Hu Y (2012) A novel polymeric flame retardant and exfoliated clay nanocomposites: preparation and properties. Chem Eng J 183(8):542–549
Tang WK, Neill WK (1964) Effect of flame retardants on pyrolysis and combustion of α-cellulose. J Polym Sci C 6:65–81
Vasiljević J, Jerman I, Jakša G, Alongi J, Malucelli G, Zorko M, Tomšič B, Simončič B (2015) Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose 22(3):1893–1910
Wang LH, Ren YL, Wang XL, Zhao JY, Zhang Y, Zeng Q, Gu YT (2016) Fire retardant viscose fiber fabric produced by graft polymerization of phosphorus and nitrogen-containing monomer. Cellulose 23:2689–2700
Xie K, Gao A, Zhang Y (2013) Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr Polym 98(1):706–710
Xie H, Yang W, Yuen ACY, Xie C, Xie J, Lu H, Yeoh GH (2017) Study on flame retarded flexible polyurethane foam/alumina aerogel composites with improved fire safety. Chem Eng J 311:310–317
Zhang QH, Gu J, Chen GQ, Xing TL (2016) Durable flame retardant finish for silk fabric using boron hybrid silica sol. Appl Surf Sci 387:446–453
Zheng D, Zhou J, Zhong L, Zhang F, Zhang G (2016) A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 23(3):1–10
Acknowledgments
The authors thank the financial support provided by the National Key Research and Development Program of China (No. 2017YFB0309000).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Xh., Zhang, Yg., Cheng, Bw. et al. Preparation of durable and flame retardant lyocell fibers by a one-pot chemical treatment. Cellulose 25, 6745–6758 (2018). https://doi.org/10.1007/s10570-018-2005-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2005-y