Abstract
As a renewable, abundant, and eco-friendly bio-based compound, phytic acid (PA) possesses high phosphorus content, which is a potential flame retardant for cellulosic fibers. Generally, PA is not efficient for cellulosic fibers due to strong acidity that results in greatly reduced strength and lack of soft hand. As proved elsewhere, the compounds with phosphorous and nitrogen was reported to be an efficient flame retardant and exhibited synergistic effect for cellulosic fibers. Therefore, PA was firstly reacted with urea to synthesize a novel green flame retardant containing a high level of phosphorus and nitrogen elements, i.e., phytic acid ammonium, then it was employed for lyocell fibers through pad-dry-cure finishing process. As expected, flame retardancy and durability of finished lyocell fabrics were considerably improved, as evidenced by an increase of limiting oxygen index value up to 39.2% and still 29.7% after 30 laundering cycles. TG–MS and TG–FTIR coupled techniques demonstrate that the formation of carbonaceous residue and non-combustion gases preferably generated during thermal pyrolysis process of finished lyocell fibers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lyocell fibers have been recognized as one of the most popular ‘‘green’’ man-made regenerated cellulosic fibers, and widely used in textiles industry, e.g., carpets, curtains and clothing because they possess numerous advantages, especially high strength, good moisture absorption and soft hand (Hall et al. 1999; Joshi et al. 2010). However, similar to other cellulosic fibers, lyocell fibers are inflammable which would limit their application if not given flame retardant treatment by one of several methods, e.g., chemical grafting (Bai et al. 2014), blending spinning (Seddon et al. 1996) and finishing process (Kim et al. 2016). Chemical grafting method is efficient to prepare durable fire retardant cellulosic fibers.
Alternative methods for obtaining durable fire retardant lyocell fibers are blending and finishing. Typically, the blending strategy introduced flame retardants during spinning process of lyocell fibers. The spinning solution containing flame retardants is extruded through an orifice, drawn into an air gap, and precipitated in a coagulation bath (Loubinoux and Chaunis 1987). Flame retardants should possess excellent dispersibility and compatibility with spinning solution. To achieve high degree of durability and flame reatardancy, a relatively larger amount of flame retardants is required, which not only increase the production cost but also deteriorate the physical properties of fibers. Delhom et al. added organic modified montmorillonite nanoparticles into cellulose/NMMO/water spinning solution (Delhom and White 2010). The char residue of flame retardant lyocell fibers shows a positive dependence on clay amount. The char yield can reach 30% with 15 wt% clay addition. Generally, the blending method is simple, easy to handle, cost-effective, and can impart lyocell fibers better flame retardancy. However, the mechanical properties of lyocell fibers will be affected due to a high amount of flame retardant addition. Besides, flame retardant will be lost in spinning and washing processes, thus inducing a decrease of flame retardant durability of lyocell fibers.
The finishing method can firmly bind flame retardant units to lyocell fibers through covalent bonding, which can significantly enhance flame retardant durability. Pyrovatex CP new (Yang et al. 2005) and Proban (Zhang and Horrocks 2010) are two representative industrialized flame retardant finishing methods for obtaining high durability. For example, Helena et al. utilized Provatex CP and a cross-link agent methylolated melamine to finish lyocell fibers (Seddon et al. 1996). The LOI value of finished lyocell fibers was 40% indicating high flame retardancy. However, the use of methylolated melamine will release high levels of formaldehyde during finishing and using processes. Formaldehyde is now regarded as a carcinogenic compound by World Health Organization (Nielsen and Wolkoff 2010). Therefore, it is an urgent issue to decrease or eliminate the amount of formaldehyde. Moreover, Naveed et al. prepared flame retardant lyocell fibers by replacing methylolated melamine with citric acid as the cross-link agent using similar finishing system (Mengal et al. 2016). Surprisingly, the amount of formaldehyde decreased 25% compared with previous research. However, the flame retardant durability of the treated lyocell fibers became poor. In a word, the main drawback of Pyrovatex CP method is the release of toxic degradation products during the combustion process (Horrocks 2011). Especially, the strict safety laws and regulations concerning fibers and textiles were formulated and reinforced in recent years (Zhang et al. 2016a, b). Thus, it becomes increasingly important to develop halogen-free, formaldehyde release-free, and green fire retardant agents for lyocell fibers in view of human’s life and environmental conservation (Kai et al. 2016; Zhang et al. 2016a, b). Recently, many researchers have paid their attention to using renewable biomacromolecule such as DNA (Alongi et al. 2013a, b; Carosio et al. 2013; Alongi et al. 2013a, b), protein (Bosco et al. 2013; Wang et al. 2014a, b; Alongi et al. 2014), starch (Carosio et al. 2015), green natural materials banana pseudostem sap (Basak et al. 2015) and coconut shell extract (Basak et al. 2016) as flame retardants (Costes et al. 2017).
Abundant in plant tissues (soy beans, cereal grains and oil seeds), phytic acid (PA) is one of major storage forms of phosphorus-containing natural compounds (28 wt% phosphorus content based on molecular weight) (Hadi and Zotz 2014). As a biocompatible, environmentally friendly, and nontoxic organic phosphoric acid, PA has been applied in a variety of fields (Wang et al. 2014a, b), particularly as a flame retardant. Zhou et al. successfully used PA as a doping acid or co-dopant to increase flame retardancy of polyaniline deposited paper composite (Zhou et al. 2015). Cheng et al. utilized PA to flame retard poly (lactic acid) non-woven fabric through pad-dry-cure process (Cheng et al. 2016). Additionally, Zhang et al. reported a green and renewable intumescent flame retardant system based on ionic complexation between PA and chitosan for ethylene-vinyl acetate copolymers (Zhang et al. 2014). To the best of our knowledge, there is no report on the use of PA as the flame retardant cellulosic fibers. Considering that PA possesses both a special chemical structure and a high phosphorus content, it is potential for serving as a facile, eco-friendly, and efficient flame retardant finishing agent of cellulosic fibers.
The aim of the present paper is to explore a novel, eco-friendly and durable phosphorus and nitrogen-containing PA-based flame retardant agent (phytic acid ammonium, PAA) to improve the flame retardancy of lyocell fibers through pad-dry-cure process. The structure and surface morphology of the treated lyocell fibers were investigated in detail. Thermogravimetry-mass spectrometry (TG–MS) and thermogravimetry-infrared spectrometry (TG–FTIR) coupled techniques have been employed to evaluate the combustion behaviors and thermal stability of the treated lyocell fibers.
Experimental
Materials
Lyocell fibers (1.33 × 38 mm) were friendly supplied by Shandong Yingli Industrial Co., LTD. (Shandong, China), and were woven into lyocell fabrics for LOI test and vertical burning test. Phytic acid (PA, 70 wt% aqueous solution) was purchased from Nanjing Xiezun Chemical Co. LTD (Nanjing, China). Urea and dicyanodiamide were analytical grade reagents and obtained from Tianjin Guangfu Fine Chemical Research institute (Tianjin, China). All regents were used as received.
Synthesis of phytic acid ammonium (PAA)
PAA was synthesized via the reaction of PA and urea, as shown in Scheme 1. 20.00 g PA (70 wt% aqueous solution) and 12.74 g urea were added into a 250 mL three-necked flask equipped with a magnetic stirrer and a reflux condenser. Then the flask was placed into an oil bath at 100 °C. After 1.5 h, a yellow and transparent liquid was obtained and slowly cooled down to room temperature. The crude product was purified by precipitating in N, N-dimethylformamide (DMF), filtered, and dried in vacuum at 28 °C. The pure product was obtained in 78% yield as a white solid. The number of hydrogen atoms corresponded with that of PAA in Scheme 1. 1H NMR (D2O, 600 MHz) d(ppm): OH (2.7S, 2.8s); NH4 (3.4s, 3.5s, 3.9s, 4.1s); CH (7.7s). The separation between multiple peaks indicated the successful reaction of urea with phytic acid. The elemental analysis results were O (44.5%), C (8.9%), H (5.9%), N (10.8%), P (29.9%), which agreed well with the theoretical values.
Preparation of fire retardant lyocell fibers
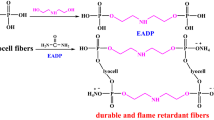
Firstly, lyocell fibers were immersed in the solution containing PAA and dicyanodiamide. Secondly, the mixture was placed in a thermostatically controlled water bath at 50 °C for 0.5 h. Finally, the lyocell fibers were passed through a padder with two dips to reach a wet pickup of 100 wt%, dried at 100 °C for 5 min, and cured at 200 °C for 2.5 min. The reaction between PAA and lyocell fibers is shown in Scheme 2.
Washing procedure
The washing procedure of all samples (fire retardant lyocell fibers after 0, 5, 10, 20 and 30 washing cycles) was carried out referring to AATCC Test Method 61-2003 test NO.1A with 0.37% detergent (Xue et al. 2016). The water temperature was set to 40 °C.
Characterization
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of PAA and lyocell fibers were recorded with KBr powder using a Nicolet iS50 FTIR infrared spectrophotometer. The resolution factor of FTIR spectrometer was 0.09 cm−1, and the spectral range was from 4000 to 400 cm−1.
Scanning electron microscopy (SEM)
The surface morphology of all samples and their char residue was measured by a scanning electron microscope (Hitachi S4800, Zeuss, Japan). As lyocell fiber and its char residue possess poor electrical conductivity, the electrons will concentrate on their surfaces and form electronic aggregates when scanned by electron beam. This feature causes imaging faults. Therefore, all the fiber samples and their char residues were coated with gold before SEM measurements to increase the electric conductivity of lyocell fiber and its char residue and achieve good imaging ability.
X-ray photoelectron spectroscopy (XPS)
XPS measurements were performed on an AXIS-Ultra DLD XPS spectrometer (K-alpha) using monochromatic Al Kα (1486.6 eV) excitation at a reduced power of 100 W. The residual pressure in the analysis chamber was about 10−9 Pa. The range of kinetic energy for full spectra was between 0 and 1400 eV, and the step size for the high-resolution scan was 0.1 eV. Binding energies were calibrated with respect to the C 1s core level peak at 284.6 eV. Fiber samples were dried for 2 h at 40 °C under vacuum, and then attached to the object stage with double-sided adhesive tape for test.
Thermogravimetric analysis (TG)
The thermal stabilities of all samples were evaluated by TG analysis, using STA449F3 thermogravimetric analyzer from 40 to 800 °C with a heating rate of 10 °C min−1 in nitrogen.
Limiting oxygen index (LOI)
The LOI refers to the minimum concentration of oxygen in a mixture of oxygen and nitrogen, which is often used to study the flammability of materials. The flammability of all fabrics was determined by LOI according to ASTM D6413-08 LOI instrument. The vertical burning tests were performed to observe the flame retardant properties of modified fabrics at room temperature with a related humidity of 63%. The flame length of the burner was 50 ± 2 mm. The sample dimensions were 200 mm × 80 mm and fixed vertically in the chamber. The entire burning process was recorded by a digital video recorder.
Thermogravimetry-infrared spectrometry (TG–IR)
TG–IR of lyocell fibers was performed using the STA 6000 thermogravimetry analyzer that was interfaced to Frontier FTIR spectrophotometer. About 5.0 mg lyocell fibers were put in an alumina crucible and heated from 40 to 900 °C. The heating rate was set as 20 °C min−1 (nitrogen atmosphere, flow rate of 30 mL min−1).
Thermogravimetry–mass spectrometry (TG–MS)
TG–MS analysis of the gases evolved from the samples was conducted simultaneously using the STA-4493 with Skimmer coupled to a quadruple mass spectrometer QMS403C (maximum 300 amu). The TG experiments were operated from room temperature to 800 °C at a heating rate of 10 °C min−1 under continuous flow of N2 (100 mL min−1). A transfer line, specially designed to connect a vacuum pump in order to optimize the amount of evolved gas, transferred from the TG to the MS. The measurement of outlet gas via mass spectrometric intensities was plotted as a function of temperature that showed two well separated regions of devolatilization.
Results and discussion
Surface structure
Preliminarily, phytic acid (PA) was directly used as the finishing agent to treat lyocell fibers under different conditions but the mechanical properties and handing softness of modified fibers lowered greatly. It is mainly caused by the strongly acid medium of PA aqueous solution (pH = 1–2). As proved elsewhere, the compounds bearing phosphorous and nitrogen elements were reported to be efficient flame retardants and exhibited synergistic effect for cellulosic fibers (Zhao et al. 2016; Gaan and Sun 2007; Chen and Wang 2010). Adopting this rule, a facile, novel, eco-friendly flame retardant, phytic acid ammonium (PAA), has been synthesized with a high yield via the reaction of PA and urea at 100 °C, as shown in Scheme 1. Theoretically, PA contains six phosphoric acid members and can react with 1–6 urea equivalents, yielding PAA with varied contents of flame retardant elements (corresponding to 27.5–21.5 wt% of phosphorous and 2–19% nitrogen, respectively). The structure of PAA was confirmed by XPS, FTIR, and 1H NMR. Therefore, the resultant PAA was used to finish lyocell fibers through a pad-dry-cure process, with the purpose to prepare durable flame retardant lyocell fibers. It must be noted that the flame retardant treatment did not considerably change the mechanical properties and handing softness of lyocell fabrics. The fracture strength of treated lyocell fabrics by flame retardant PAA only show a loss by ca. 8% compared with that of control sample.
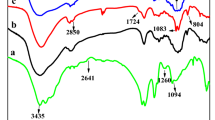
Firstly, the structures of control and modified lyocell fibers are characterized by FTIR technique, as illustrated in Fig. 1. For the control lyocell fibers, the characteristic broad peak at 900–1200 cm−1 is clearly observed. The broad band at around 3340 cm−1 assigns to OH stretching vibration, while the band at 1640 cm−1 assigns to OH bending vibration (Sahito et al. 2015). For the modified lyocell fibers, several new characteristic peaks originated from flame retardant PAA are observed. Obviously, the strong absorption peaks at 1450 and 3210 cm−1 attribute to bending and stretching vibrations of NH4 + units. The peak at 1510–1770 cm−1 attributes to stretching vibration of O–P–O unit. The band at 1230 cm−1 assigns to P=O stretching vibration (Gospodinova et al. 2002). Consequently, the results confirm that –P=O(O–NH4 +)2 group of PAA did react with OH group of cellulose unit of lyocell fibers, forming the P–O–C covalent bonds.
Surface chemical composition
XPS analyses of flame retardant PAA, control lyocell fibers (CLF), modified lyocell fibers (MLF), and modified lyocell fibers after 5 washing cycles (MLFW)) were performed. Their spectra and detailed surface chemical compositions (at.%) are shown in Fig. 2 and Table 1. Besides the peaks at ~ 286 and ~ 533 eV respectively corresponding to C1s and O1s of CLF, two new peaks from flame retardant PAA, N1s (5.9%, ~ 400 eV) and P2p (1.4%, ~ 134 eV) are detected in the XPS spectrum of MLF.
For the fibers after 5 washing cycles (MLFW), the intensities of N1s (2.4%, ~ 400 eV) decreased as ionic bonds between phosphorous acidic anion and ammonium cation have suffered damage to a certain degree when fibers have undergone through hash washing process. However, there is a slight increase for P2p (1.5%, ~ 134 eV) compared with that of MLF (1.4%, ~ 134 eV), indicating the formation of the firm ester linkage between PAA and cellulose unit. Thus the results further proved that flame retardant PAA has been successfully grafted onto the surface of lyocell fibers. Moreover, it is found that the ratio of N/P in PAA (8.7/11.2, entry 1 in Table 1) is less than that in MLF (5.9/1.4, entry 3). The increase of N content may be ascribed to the fact that the finishing agent used for fiber was the reaction solution of PA and urea without any further purification, and the excessive residual urea existed in the surface of fiber after finishing process. This deduction was also confirmed from the decrease of N content for MLFW after only 5 washing cycles (2.4/1.5, entry 4). An additional case for the increase of N content in MLF was the introduction of dicyanodiamide in the finishing process.
Surface morphology
The changes in surface morphology of control and modified fibers were measured by SEM technique (Fig. 3). The surface of control sample is very smooth without obvious defects (A). Contrarily, the surface of modified sample becomes slightly rough, indicating that some new substances adhere to the fiber surfaces (B). Similar phenomenon is also observed for the samples after 5, 10, 20, and 30 washing cycles (C, D, E, and F). The results show that the surface structures of lyocell fibers finished with flame retardant PAA have been changed, and insignificantly influenced by washing processing, demonstrating that PAA successfully reacted with cellulose unit and formed firm covalent bonding.
Furthermore, the surface morphology of char residues of all samples after combusting is also exhibited in Fig. 3. Compared with control sample burning into ash (a), all other burned samples generated compact and continuous char layers even after undergoing various washing cycles (b–f). The carbonaceous residues were produced by dehydration of cellulose catalyzed by phosphorus from flame retardant (Hendrix and Barker 2010). Many small bubbles resulting from volatile gases on the surface of all char residues were visible, forming special swollen fibrous structures. The carbonaceous layer served as a physical barrier and shielded combustible products from oxygen and heat, efficiently protecting the matrix of modified lyocell fibers (Liu et al. 2015). Based on the above results, flame retardant PAA displayed excellent flame retardancy for lyocell fibers.
Flame retardant performance
The vertical burning tests were also utilized to visually observe fire retardant performances of the control and modified lyocell fabrics (Fig. 4). Clearly, when the control sample was exposed to the flame after only 1s, it burned intensely, quickly, and completely without any remained char residue. Conversely, for the modified samples and ones after undergoing various washing cycles, they did not burn obviously when exposed to the flame even after more than 30s. Thus the great contrast suggests that the modified fabrics possess excellent flame retardancy.
Limiting oxygen index (LOI) refers to the minimal oxygen concentration that supports the combustion of a particular material (Cavdar 2014). LOI is an important criterion to evaluate flame retardant performance of modified lyocell fabrics. Figure 5 shows the evolution of LOI values of all modified lyocell fabrics with washing cycles. The LOI value of control sample was 17.0%, far lower than flame retardant standard of cotton fabrics (LOI = 26.0–28.0%). In contrast, the LOI value of modified sample rose up to 39.2%, significantly higher than that of control sample. After 5 washing cycles, the LOI value decrease down to 36.5%. With the further increase of washing times, the LOI value gradually decreased. However, the LOI value of modified sample after 30 washing cycles is 30.5%, still meeting the flame retardant standard. The introduction of PAA into lyocell fabrics includes chemical bonding or adhering to surface of fabrics through physical interaction. Thus flame retardants adhered to surface of fabrics would be prone to lose during rigorous washing process, leading to the decrease of the LOI values of modified samples. Fortunately, the covalent bonding between PAA and lyocell fabrics was very stable during washing process, and flame retardant synergistic effect between phosphorus and nitrogen did work well during the combustion. As a result, the fire retardant performance and durability of modified lyocell fabrics remained higher levels.
Thermal stability
Thermogravimetric analysis
Thermogravimetric (TG) analysis was firstly used to evaluate thermal stability of flame retardant phytic acid ammonium (PAA), control lyocell fibers (CLF), modified lyocell fibers (MLF), and MLF after 5 washing cycles (MLFW). TG and derivative thermogravimetric (DTG) curves of all samples and the corresponding detailed data are shown in Fig. 6 and Tables 2 and 3 respectively.
PAA show a four-stage weight loss plot, and the detail data are presented in Table 2. The first stage with a weight loss of 1.08% due to moisture occurred at 70–148 °C. The second stage with a 26% weight loss occurred at 148–269 °C, attributed to the removing of OH and NH groups and the producing of numerous non-combustion gases. The third stage with a 16.32% weight loss associated with the carbonization process of phytic acid occurred at 269–590 °C. The last stage with a 35% weight loss was observed when temperature increased to 800 °C, attributed to the thermal pyrolysis of phytate groups and the oxidation of elemental carbon formed in the previous stages. The detailed thermal degradation studies about PAA are beneficial for analyzing the flame retardant mechanism of lyocell fibers modified with PAA. Clearly, the initial decomposition temperature of PAA is much lower than that of control lyocell fibers occurred at 318 °C (Fig. 6), suggesting that PAA is potential for serving as a high efficiency flame retardant for lyocell fibers.
For the control sample, the weight loss with ca. 76.00% occurred at 318–380 °C, and the residue under nitrogen at 700 °C was 16.48% (entry 1 in Table 3). Contrarily, for the modified sample, the weight loss (ca. 30.32%) decreased sharply at 251–290 °C, and the residue at 700 °C was 38.89% (entry 2). Likewise, for the modified sample after 5 washing cycles, the weight loss (ca. 30.32%) decreased sharply at 251–290 °C, and the residue at 700 °C was 39.63% (entry 3). Therefore, PAA-treated lyocell fibers possessed excellent and durable flame retardancy compared with the untreated sample. The results indicate that PAA decomposes firstly and releases a large amount of non-combustion gases during burning, which absorbs heat and dilutes the combustion gases before pyrolysis of cellulose units. Furthermore, the phyosphorylated cellulose undergoes catalytic dehydration and forms carbonaceous residue, which serves as an efficient physical barrier to protect the combustion products from the oxygen and heat (Xiong et al. 2012).
TG–IR coupled analyses
In order to obtain the structure information of degradation products, the evolved gases of lyocell fibers from TG furnace are simultaneously inspected by FTIR analyses. That is, the coupled TG–IR technique can directly provide identification of volatilized products, which significantly contributes to understanding thermal degradation mechanism (Chen and Jiao 2008). FTIR spectra of control lyocell fibers (CLF), modified lyocell fibers (MLF), and MLF after 5 washing cycles (MLFW) for gas products in the temperature range of 160–360 °C were detected (Fig. 7). For all samples, some small molecule gaseous species are easily identified by their characteristic absorbance peaks, such as CO2 at 2360 cm−1, H2O at 1200–1800 and 3400–4000 cm−1. The absorbance bands of other decomposition products are observed due to characteristic bands of functional groups, i.e., alkane at 2974 cm−1, carbonyl at 1748 cm−1, and C–O–C at 1100 cm−1.
One of the main volatilized gaseous products can be identified as CO2, appeared at 180 °C at first. The formation of CO2 may by produced by the scissions of pending –CH2O unit. The intensity of characteristic peak for CO2 at 2360 cm−1 gradually increases with temperature up to 360, 280, and 300 °C for CLF, MLF, and MLFW, respectively, and then turns to a rapid decrease. The volatilized products observed at 260 °C are considered as levoglucosan and furane derivatives, as seen from characteristic groups of C=O and C–O–C. Importantly, the intensities of C=O peak at 1748 cm−1 and C–O–C peak at 1064 cm−1 for modified lyocell fibers (Fig. 7b) are much weaker than those of the control sample (Fig. 7a). This phenomenon originates from the inhibited pyrolysis of cellulose unit by flame retardant PAA. When temperature raised up to 280 °C, a new peak at 2974 cm−1 attributing to alkane compound did appear in all spectra. Contrarily, the peak at 2974 cm−1 disappears for MLF and MLFW (Fig. 7c) as PAA inhibits the generation of combusting products such as alkane and furane derivatives. Besides, two new peaks at 964 and 930 cm−1 are observed for MLF, which is identified as NH3 from ammonium group of PAA. Though MLFW releases a lesser amount of NH3 compare with MLF, it still exhibits higher flame resistance due to the function of phosphorus element. Particularly, it is worth mentioning that the decomposition temperatures of modified fibers (MLF and MLFW) are obviously lower than that of control fibers (CLF), due to the decomposition of PAA. These results are in consistence with the results of TG analyses.
TG–MS coupled analyses
As TG–FTIR analyses only provide qualitative information about pyrolysis products, TG–MS is favorable to investigate the exact composition of decomposed products and the relative pyrolysis mechanism of PAA-treated lyocell fibers during the combusting process (Cavdar 2014). For the control or modified lyocell fibers, the ion signals detected at m/z = 16, 18, 30, 44 and 84 correspond to CH4, H2O, C2H6, CO2 and C6H12, respectively (Fig. 8). This reveals that the cellulose unit proceeds to dehydrate and decarboxylize during burning, releasing much more water, carbon dioxide, and etc.
Initially, a small absorption peak of H2O below 100 °C is observed, indicating of the incorporation of water molecules into lyocell fibers. When temperature rises up to 300 °C, the modified sample (Fig. 8b) shows a reduced abundance of water compared with control sample (Fig. 8a), i.e., a decreased amount of water was generated from the thermal degradation of fire retardant sample. For the modified sample, the generation of CO2, CH4, C2H6, and C6H12 show a same rule, and their peaks significantly decreased compared with those of control sample, even the peak of C6H12 disappeared. It demonstrates that PAA disturbed the thermal decomposition process of fire retardant lyocell fibers and restrained sustaining burning of cellulosic structure. Hence, PAA can be used as a suitable flame retardant for lyocell fibers and inhibits fibers’ pyrolysis. Moreover, a similar phenomenon occurs for MLFW (Fig. 8c), illustrating the excellent durability of fire retardant fibers undergone through repeated washing process. All the results are consistent with TG–IR coupled analysis results.
Mechanism analyses
Based on the coupled analysis results of TG–IR and TG–MS, the possible flame retardant mechanism for PAA-finished lyocell fibers is presented. As shown in Scheme 3, during pyrolysis process of treated sample, PAA grafted onto lyocell fibers firstly decomposes and releases a large amount of non-combustion gases such as H2O, CO2, NH3, and etc. The non-combustion gases are helpful for both diluting the concentration of the combustible gases products including CH4, C2H6, and C6H12, and absorbing heat energy. At the same time, PAA catalyzes cellulose to dehydrate and carbonize, forming intumescent char player. The resultant special swollen carbonaceous fibrous structure efficiently acts as physical barrier for preventing heat and oxygen from the matrix material. Therefore, the flame retardant operates in both condensed phase and gaseous phases during the burning process for modified lyocell fibers, suggesting that the phosphorus and nitrogen elements in PAA exhibits a synergistic effect.
Conclusions
A novel renewable and eco-friendly flame retardant (phytic acid ammonia, PAA) containing phosphorus and nitrogen elements was elaborately designed and synthesized, on the basis of the reaction of phytic acid and urea. PAA molecules have been successfully grafted onto the surface or penetrated into the interiors of lyocell fibers, as confirmed by SEM and XPS analysis. The LOI value of PAA-finished lyocell fibers increased up to 39.2% and still 29.7% after 30 washing cycles. The TG analysis results show that the char yield of treated lyocell fibers at 700 °C is 38.89 wt%, obviously higher than that of control sample (16.48%). The combined TG-MS and TG-IR results show that the phosphorus and nitrogen elements in PAAA have a remarkable synergistic effect, and PAA simultaneously operates in both condensed and gaseous phase during the combusting of modified fibers. Furthermore, the results demonstrate that modified lyocell fibers displays excellent durable fire resistant performance. Therefore, PAA containing high contents of phosphorus and nitrogen elements can be used as a new kind of durable, halogen-free, formaldehyde-free, and eco-friendly flame retardant system for lyocell fibers. This novel flame retardant finishing system is also believed to provide a novel strategy for the development of durable and eco-friendly flame-proofing cellulosic fibers.
References
Alongi J, Carletto RA, Blasio AD, Carosio F, Bosco F, Malucelli G (2013a) DNA: a novel, green, natural flame retardant and suppressant for cotton. J Mater Chem A 1(15):4779–4785
Alongi J, Carletto RA, Di BA, Cuttica F, Carosio F, Bosco F, Malucelli G (2013b) Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fibers. Carbohydr Polym 96(1):296–304
Alongi J, Carletto RA, Bosco F, Carosio F, Blasio AD, Cuttica F, Antonucci V, Giordano M, Malucelli G (2014) Caseins and hydrophobins as novel green flame retardants for cotton fibers. Polym Degrad Stab 99(1):111–117
Bai BC, Kim EA, Jeon YP, Lee CW, In SJ, Lee YS, Ji SI (2014) Improved flame-retardant properties of lyocell fiber achieved by phosphorus compound. Mater Lett 135(12):226–228
Basak S, Samanta KK, Saxena S, Chattopadhyay SK, Narkar R, Mahangade R, Hadge GB (2015) Flame resistant cellulosic substrate using banana pseudostem sap. Pol J Chem Technol 17(1):123–133
Basak S, Patil PG, Shaikh AJ, Samanta KK (2016) Green coconut shell extract and boric acid: new formulation for making thermally stable cellulosic paper. J Chem Technol Biotechnol 91(11):2871–2881
Bosco F, Carletto RA, Alongi J, Marmo L, Di BA, Malucelli G (2013) Thermal stability and flame resistance of cotton fibers treated with whey proteins. Carbohydr Polym 94(1):372–377
Carosio F, Blasio AD, Alongi J, Malucelli G (2013) Green DNA-based flame retardant coatings assembled through layer by layer. Polymer 54(19):5148–5153
Carosio F, Fontaine G, Alongi J, Bourbigot S (2015) Starch-based layer by layer assembly: efficient and sustainable approach to cotton fire protection. ACS Appl Mater Interfaces 7(22):12158
Cavdar AD (2014) Effect of various wood preservatives on limiting oxygen index levels of fir wood. Measurement 50(1):279–284
Chen X, Jiao C (2008) Thermal degradation characteristics of a novel flame retardant coating using TG-IR technique. Polym Degrad Stab 93(12):2222–2225
Chen L, Wang YZ (2010) A review on flame retardant technology in China. Part I: development of flame retardants. Polym Adv Technol 21(1):1–26
Cheng XW, Guan JP, Tang RC, Liu KQ (2016) Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J Clean Prod 124:114–119
Costes L, Laoutid F, Brohez S, Dubois P (2017) Bio-based flame retardants: when nature meets fire protection. Mater Sci Eng R Rep 117:1–25
Delhom CD, White LA (2010) Development and characterization of cellulose/clay nanocomposites. Compos B 41(6):475–481
Gaan S, Sun G (2007) Effect of phosphorus and nitrogen on flame retardant cellulose: a study of phosphorus compounds. J Anal Appl Pyrolysis 78(2):371–377
Gospodinova N, Grelard A, Jeannin M, Chitanu GC, Carpov A, Thiéry V, Besson T (2002) Efficient solvent-free microwave phosphorylation of microcrystalline cellulose. Green Chem 4(3):220–222
Hadi AH, Zotz G (2014) Phytic acid in green leaves. Plant Biol 16(4):697–701
Hall ME, Horrocks AR, Seddon H (1999) Flammability of lyocell. Polym Degrad Stab 64:505–510
Hendrix JE, Barker RH (2010) Pyrolysis and combustion of cellulose. II. Thermal analysis of mixtures of methyl α-D-glucopyranoside and levoglucosan with model phosphate flame retardants. J Appl Polym Sci 16(1):41–59
Horrocks AR (2011) Flame retardant challenges for textiles and fibres: new chemistry versus innovatory solutions. Polym Degrad Stab 96(3):377–392
Joshi HD, Joshi DH, Patel MG (2010) Dyeing and finishing of lyocell union fibers: an industrial study. Color Technol 126(4):194–200
Kai D, Tan MJ, Pei LC, Yun KC, Yong LY, Xian JL (2016) Towards lignin-based functional materials in a sustainable world. Green Chem 18(5):1175–1200
Kim HG, Bai BC, In SJ, Lee YS (2016) Effects of an inorganic ammonium salt treatment on the flame-retardant performance of lyocell fibers. Carbon Lett 17(1):74–78
Liu H, Wang X, Wu D (2015) Preparation, isothermal kinetics, and performance of a novel epoxy thermosetting system based on phosphazene-cyclomatrix network for halogen-free flame retardancy and high thermal stability. Thermochim Acta 607:60–73
Loubinoux D, Chaunis S (1987) An experimental approach to spinning new cellulose fibers with N-methylmorpholine-oxide as a solvent. Text Res J 57(2):61–65
Mengal N, Syed U, Malik SA, Ali SI, Jeong SH (2016) Citric acid based durable and sustainable flame retardant treatment for lyocell fabric. Carbohydr Polym 153:78–88
Nielsen GD, Wolkoff P (2010) Cancer effects of formaldehyde: a proposal for an indoor air guideline value. Arch Toxicol 84(6):423
Sahito IA, Sun KC, Arbab AA, Qadir MB, Jeong SH (2015) Integrating high electrical conductivity and photocatalytic activity in cotton fabric by cationizing for enriched coating of negatively charged graphene oxide. Carbohydr Polym 130:299–306
Seddon H, Hall M, Horrocks AR (1996) The flame retardancy of lyocell fibres. Polym Degrad Stab 54(2):401–402
Wang X, Lu C, Chen C (2014a) Effect of chicken-feather protein-based flame retardant on flame retarding performance of cotton fabric. J Appl Polym Sci. https://doi.org/10.1002/APP.40584
Wang K, Liu P, Ye Y, Li J, Zhao W, Huang X (2014b) Fabrication of a novel laccase biosensor based on silica nanoparticles modified with phytic acid for sensitive detection of dopamine. Sensors Actuators B Chem 197(197):292–299
Xiong WC, Chen L, Zhao B, Wang DY, Wang YZ (2012) Polyamide 6 with a flame retardant encapsulated by polyamide 66: flame retardation, thermo-decomposition and the potential mechanism. Chin J Polym Sci 30(2):297–307
Xue CH, Zhang L, Wei P, Jia ST (2016) Fabrication of superhydrophobic cotton textiles with flame retardancy. Cellulose 23(2):1471–1480
Yang CQ, Wu W, Xu Y (2005) The combination of a hydroxy-functional organophosphorus oligomer and melamine-formaldehyde as a flame retarding finishing system for cotton. Fire Mater 29(2):109–120
Zhang S, Horrocks AR (2010) Substantive intumescence from phosphorylated 1,3-propanediol derivatives substituted on to cellulose. J Appl Polym Sci 90(12):3165–3172
Zhang T, Yan H, Shen L, Fang Z, Zhang X, Wang J, Zhang B (2014) Chitosan/phytic acid polyelectrolyte complex: a green and renewable intumescent flame retardant system for ethylene–vinyl acetate copolymer. Ind Eng Chem Res 53(49):19199–19207
Zhang QH, Gu J, Chen GQ (2016a) Durable flame retardant finish for silk fabric using boron hybrid silica sol. Appl Surf Sci 387:446–453
Zhang J, Kong Q, Yang L et al (2016b) Few layered Co(OH)2 ultrathin nanosheet-based polyurethane nanocomposites with reduced fire hazard: from eco-friendly flame retardance to sustainable recycling. Green Chem 18(10):3066–3074
Zhao B, Hu Z, Chen L, Liu Y, Wang YZ (2016) A phosphorus—containing inorganic compound as an effective flame retardant for glass—fiber—reinforced polyamide 6. J Appl Polym Sci 119(4):2379–2385
Zhou Y, Ding C, Qian X, An X (2015) Further improvement of flame retardancy of polyaniline-deposited paper composite through using phytic acid as dopant or co-dopant. Carbohydr Polym 115:670–676
Acknowledgments
The authors are very thankful for the support provided by the National Key Research and Development Program of China (No. 2017YFB0309000).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Xh., Zhang, Qy., Cheng, Bw. et al. Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 25, 799–811 (2018). https://doi.org/10.1007/s10570-017-1550-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1550-0