Abstract
Dissolving grade pulps are used to manufacture regenerated cellulosic fibres. One promising process for the production of regenerated fibres utilises endoglucanse rich cellulases in the modification of dissolving pulp into alkaline soluble form. The aim of this paper was to characterise cellulases produced by Trichoderma reesei that are available in large quantities and study their effect on the dissolving grade softwood pulp, especially on its alkaline solubility. All the studied cellulases had endoglucanse activity and they decreased the intrinsic viscosity of the pulp. The degradation of cellulose into solubilised sugars increased with the cellulases containing also cellobiohydrolases. The monocomponent endoglucanases enhanced alkaline solubility of the pulp more than the multicomponent cellulases and produced alkaline solutions with higher fluidity. The studies showed that the type of the cellulases in the enzyme mixture has significant effect on the amount of solubilised sugars during the enzyme treatment and on the alkaline solubility of the pulp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many commercial endoglucanse rich cellulase mixtures are produced by Trichoderma reesei due to its efficiency. Trichoderma reesei, a filamentous fungus, secretes a mixture of cellulases capable of degrading cellulose. Generally, the commercial endoglucanse rich cellulases may contain several side activities due to the high costs of purification, and the endoglucanase activity can be derived from many different proteins. The five known endoglucanases of Trichoderma reesei are Cel7B, Cel5A, Cel12A, Cel61A and Cel45A (formerly known as EG I, EG II, EG III, EG IV, EG V, respectively). These endoglucanases attack cellulose chains at random positions and cleave the β-1,4-linkage between the glucose units creating new cellulosic chain ends from which the cellobiohydrolases cleave cellobiose. The known cellobiohydrolases of Trichoderma reesei are Cel7A and Cel6A (formerly known as CBH I and CBH II, respectively). Finally, the β-glucosidases, Cel1A and Cel3B, break cellobiose to glucose. For the efficient overall hydrolysis of cellulose a synergetic action of the cellobiohydrolases and endoglucanases is needed (Bailey et al. 1993; Nidetzky et al. 1994; Kleman-Leyer et al. 1996; Karlsson et al. 1999). Cellulases attack preferentially at the sites that are more easily available such as defects, small external fibrils, loose ends and pores, and they are more likely to cut the cellulose chains at the unstructured amorphous areas compared to the compact crystalline parts (Suurnäkki et al. 2000; Pu et al. 2006; Arantes and Saddler 2011; Orlowski et al. 2015).
Cellulose is soluble in aqueous 8–10 wt% sodium hydroxide (Kamide et al. 1984; Isogai and Atalla 1998), but the complete dissolution is limited to cellulose with low molecular weight or solution with low cellulose content. The pre-treatment of cellulose with cellulases was shown to increase the alkaline solubility of hardwood and softwood dissolving pulps (Rahkamo et al. 1996; Cao and Tan 2002a, b, 2006; Le Moigne et al. 2010; Grönqvist et al. 2014) and cotton linter (Wang et al. 2008). Rahkamo et al. (1996) studied the effect of purified Trichoderma reesei endoglucanases Cel7B and Cel5A (i.e. EG I and EG II) and cellobiohydrolases Cel7A and Cel6A (i.e. CBH I and CBH II) on the alkaline solubility of softwood dissolving pulp. The endoglucanases increased the alkaline solubility more than the cellobiohydrolases and the best solubility was observed after treatments with endoglucanse Cel5A. Similarly, a monocomponent endoglucanase from genetically modified Aspergillus was found to increase the alkaline solubility of softwood and hardwood dissolving pulps more than the monocomponent cellobiohydrolase or multicomponent cellulase from Humicola Insolens (Cao and Tan 2002a, 2006). The increased alkaline solubility was explained by the decreased molecular weight of cellulose by the enzyme treatments (Rahkamo et al. 1996; Cao and Tan 2002a, b, 2006; Wang et al. 2008; Le Moigne et al. 2010). In addition, Le Moigne et al. (2010) claimed that the increased alkaline solubility of the enzyme treated dissolving pulp was due to the decreased crystallinity, while Wang et al. (2008) showed that the enzymes had insignificant effect on the crystallinity of cotton linter even though the alkaline solubility of the treated linter was increased.
Monocomponent endoglucanases (Köpcke et al. 2008; Engström et al. 2006; Ibarra et al. 2010) and commercial endoglucanse rich enzyme preparations containing cellobiohydrolase and b-glucosidase activities (Wang et al. 2014; Miao et al. 2014) were found to increase the reactivity of hardwood and softwood pulps when measured as the Fock reactivity. The Fock method gives the amount of cellulose that reacts with carbon disulphide (CS2) when treated with the mixture of sodium hydroxide (NaOH) and carbon disulphide (Fock 1959). The increased Fock reactivity of the enzyme treated pulps was explained by the decreased molecular weight of the pulps (Köpcke et al. 2008; Engström et al. 2006; Ibarra et al. 2010; Wang et al. 2014; Miao et al. 2014). However, Engström et al. (2006) showed that acid hydrolysis and enzymatic treatment decreased the intrinsic viscosity (molecular weight) of the dissolving pulp equally, but the acid hydrolysed pulp had lower reactivity in comparison with the enzyme treated pulp. Miao et al. (2014) suggested that the endoglucanse treatment with gentle kneading opened up the structure of the pulp fibres that enhanced the accessibility of the pulp fibres to the Fock’s solvent. On the other hand, mechanical agitation alone is known to modify the structure of pulp fibres by increasing the pore volume and loosening the structure (Grönqvist et al. 2014; Virtanen et al. 2015).
Enzyme-assisted NaOH-based dissolution process has proven to be suitable for the production of regenerated cellulosic fibres (Vehviläinen et al. 2008, 2015a). In the process, dissolving pulp is treated mechanically and subjected to endoglucanase rich cellulases before the dissolution into sodium zincate. It is necessary to decrease the molecular weight of the pulp by endoglucanases to achieve high solubility for the pulp and high fluidity for the solution, but at the same time keep the molecular weight of the pulp high enough to achieve strong regenerated fibres. The high fluidity of the solution is not only beneficial for the filtration and deaeration efficiency but also indicates the good quality of the solution without gelling. Additionally, it is desirable to keep the amount of solubilised sugars low to achieve the high yield of cellulose after the enzyme treatment and to avoid the accumulation of solubilised sugars in a continuous process. To further study the efficiency of the process, it is necessary to gain more understanding about the cellulases that are available in large quantities. In this study, a selection of commercial Trichoderma reesei based endoglucanase rich enzyme preparations was characterised for their protein content and activity against different substrates. Finally, softwood dissolving pulp was treated with the enzymes and the effect of the treatments on the properties of pulp was studied.
Materials and methods
Enzymes
The cellulases, produced by genetically modified strain of Trichoderma reesei, were obtained from AB Enzymes Oy, Finland and Genencor International, Finland (Table 1).
Protein concentrations of the enzymes were assayed according to Lowry et al. (1951) using bovine albumin as standard after precipitation with trichloroacetic acid.
The activities of the enzymes were assayed by using different substrates at pH5 and 50 °C. Endoglucanase activity was measured using 1 % hyroxyethylcellulose, HEC (Sigma-Aldrich) as substrate (Bailey and Nevalainen 1981). Xylanase activity was measured using 1 % birch wood xylan (Sigma-Aldrich, xylose residues ≥90 % by HPAE) as substrate (Bailey et al. 1992). Activity against filter paper (FPU) was assayed according to Ghose (1987). Specific activity was calculated by dividing the activity with the total amount of protein.
The effect of pH on the enzyme activity was determined using 1 % HEC as substrate at 50 °C. The effect of temperature on the enzyme activity was determined with the same substrate at pH 5. The substrate was hydrolysed for 10 min and the production of glucose was analysed with the DNS method using glucose as standard (Bailey and Nevalainen 1981). The different pH values were obtained with the Britton-Robinson buffer system adjusted to the desired pH with 0.2 M sodium hydroxide solution.
Molecular weights of the enzymes were assayed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 12 % gels (Laemmli 1970). The gels were stained with coomassie blue (Imperial protein stain, Thermo Scientific). The standard molecular weight marker (Pierce Blue, Thermo Scientific) was used to estimate the relative molecular weights of the enzyme proteins.
Pulp
The pulp used was a commercial dissolving grade softwood (TCF) pulp delivered by Domsjö Fabriker AB, Sweden. Prior to the enzyme treatments the pulp was shredded mechanically at pulp consistency of 20 % for 5 h using a Baker Perkins shredding machine (size 6-1 Universal mixer S/N 44777).
The enzyme treatments of the pulp were carried out at pH5 and 50 °C, at pulp consistency of 5 % for 3 h. The dosage was 0.5 and 1 mg protein/g pulp. The enzymes were inactivated by increasing the temperature above 90 °C for 15 min. Thereafter, the pulp was filtrated and washed with cold ion exchanged water. The filtrate was collected for analyses.
The amount of sugars dissolved from the pulp during the enzyme treatment was measured from the filtrate as reducing sugars (Bernfeld 1955). The intrinsic viscosity of the pulp was measured in cupri-ethylene-diamine solution according to the SCAN-CM 15:99 (ISO 5351:2004).
Fourier transform infrared spectra (FTIR) of the untreated and enzyme treated pulp samples were recorded with Bruker Optics Tensor 27 FTIR spectrometer at 4 cm−1 resolution with 32 scans per sample. The samples were air dried and mounted directly in the sample holder. The total crystallinity index (TCI) was calculated from the ratio of absorptions at 1375 and 2900 cm−1 according to Nelson and O’Connor (1964).
Dissolution
The alkaline cellulose solutions (30 g) were prepared as follows: wet enzyme-treated pulp sample (dry weight 15 %) was mixed with sodium zincate to give cellulose concentration of 5.7 % (w/w), NaOH concentration of 6.5 % (w/w) and ZnO concentration of 1.3 % (w/w). The mixture was stirred for 5 min/1000 rpm at ambient temperature to obtain a slurry-like sample. The slurry was frozen in a freezer thereafter thawed in the temperature controlled bath at 15 °C to obtain a solution. Duration of the freezing-thawing cycle was 24 h.
The viscosity of the cellulose solution was measured with the modified ball drop method (ASTM D 1343-86) by using stainless steel balls (1/8″, 130 mg) and a measuring distance of 5 cm. Cellulose content of the solutions was measured by weighting the films casted from the solution into 10 % sulphuric acid.
The solubility of the pulps was measured by preparing alkaline solution equally as explained above, but the sample size was 10 g. After thawing the obtained solution was diluted to cellulose concentration of 1 % by adding 6.5 % NaOH at ambient temperature. The diluted solution was centrifuged (20 min/3000 g) to separate the insoluble fraction. Thereafter, the insoluble fraction was regenerated with 10 % H2SO4, thoroughly washed and dried in an oven (105 °C). The solubility was calculated as a percentage form the original dry weight of the pulp. Before regeneration, the insoluble fraction was studied with an optical microscope (Leitz Laborlux D).
Results and discussion
Characterization of cellulases
The protein content and activities against the different substrates of the studied cellulases are collected in Table 1 and the protein composition measured by SDS electrophoresis in Fig. 1. The cellulase preparation E1 (Biotouch C29) had the main activity against HEC but it showed also activity against crystalline cellulose (FPU activity). The protein composition by SDS gel electrophoresis showed detectable bands for the cellobiohydrolase Cel7A and endoglucanase Cel5A. The molecular mass of the cellobiohydrolase Cel6A and endoglucanse Cel7B are overlapping, and thus the faint band at the area 50–58 kDa is attributed to the both.
The cellulase preparation E2 (IndiAge RFW) had slightly higher activity against xylan than against HEC (Table 1). The bands in the SDS gel after electrophoresis were assigned to the endoglucanases Cel7B and Cel5A. Endoglucanase Cel7B is proved to be active against xylan (Suurnäkki et al. 2000, Nakazawa et al. 2008), thus, we suggest that the high xylan activity in E2 is derived from the Cel7B activity.
The natural complex cellulase from Trichoderma reesei coded as E3 (Primafast 200) had the highest protein content. It showed clearly the highest activity against crystalline cellulose as well as clear activities against other substrates. It showed clear dark bands corresponding Cel7A and Cel6A and faint bands in the area of high molecular weight endoglucanases Cel7B and Cel5A. Cellobiohydrolases comprises 65–78 % of the total secreted protein of Trichoderma reesei while endoglucanases comprises 21–32 % (Tolan 2002), thus the result is in line with the literature.
According to the producer the enzyme preparations E4 (IndiAge Super L) and E5 (Primafast Luna CL) compose of monocomponent endoglucanase. This was confirmed by the very low activity against crystalline cellulose (FPU) and by the absent bands for the cellobiohydrolases in SDS gel electrophoresis. The E4 and E5 had the main activity towards HEC, but the protein bands showed that the activity of the E4 was derived from Cel12A while the activity of the E5 was derived from the higher molecular weight endoglucanase, Cel5A.
The effect of pH on the HEC activity of the different enzyme preparations was studied at 50 °C (Fig. 2a). The enzyme preparations E3, E4 and E5 showed distinct maximum activity at pH5. Their activity dropped both at lower and higher pH. The enzyme preparations E1 and E2 were stable between pH 4 and 6. Above pH 6, their activity was dropped rapidly. The effect of temperature on the HEC activity of the enzyme preparations was studied at pH5 (Fig. 2b). All the enzymes had higher activity at 50 °C than at lower temperature. The enzyme preparations E1 and E4 had the maximum activity at 50 °C while the enzyme preparations E2 and E5 had the maximum activity at 60 °C. The enzyme preparation E3 retained its activity at the temperature range of 50–70 °C.
Effect of cellulases on pulp
The mechanically shredded dissolving grade pulp was treated with enzymes for 3 h at pH5 and 50 °C. The enzymes were dosed based on their protein content which resulted in varying specific activity profiles for the treatments depending on the enzyme preparation. The dosages were 0.5 and 1.0 mg protein/g of pulp. The loss of the pulp during the hydrolysis measured as dissolved sugars varied between 1 and 4.9 % depending on the enzyme preparation and its dosage. With each studied enzyme preparation, the amount of dissolved sugars increased as the enzyme dosage was increased. The highest amount of sugars was dissolved by the enzyme preparation E3 (loss 2.6 % low dosage and 4.9 % high dosage) that comprised mostly cellobiohydrolases (natural complex cellulases) and had high activity against filter paper (FPU). The endoglucanse rich enzyme preparation that contains also cellobiohydrolases, E1, dissolved more sugars than the preparations E2, E4 and E5 which lack the cellobiohydrolase activity. The monocomponent cellulase composing Cel12A (E4) released least sugars (loss 1 % low dosage and 1.5 % high dosage) (Fig. 3).
The studies with purified enzyme components from Trichoderma reesei (Medve et al. 1998; Kotiranta et al. 1999) have shown that the cellobiohydrolases hydrolysed cellulose more effectively than the endoglucanases, also the synergetic action of cellobiohydrolases and endoglucanases was proven to increase the amount of dissolved sugars (Karlsson et al. 1999). In addition, Karlsson et al. (2002) showed that Trichoderma reesei endoglucanses Cel5A and Cel12A had similar sugar production pattern, but the conversion rate of the substrates (microcrystalline cellulose, Avicel, and carboxymethyl cellulose, CMC) into saccharides was clearly lower in case of Cel12A than Cel5A. Similar trend was observed in our studies although the enzymes were not purified. The enzyme preparations that contain cellobiohydrolases produced more soluble sugars than the preparations with only endoglucanase activity. Among the endoglucanse rich preparations, the lowest amount of sugars was detected with the enzyme preparation E4, composing of Cel12A.
The enzymatic treatment decreased the intrinsic viscosity (SCAN) of the pulp by 33–41 % depending on the type of enzyme preparation. Instead, the increased enzyme dosage did not have significant effect on the intrinsic viscosity (Fig. 4). This is in line with the results reported by Grönqvist et al. (2014) and Köpcke et al. (2008), who suggested that the enzymatic action, when measured as depolymerisation of solid cellulose, is limited by the accessible sites of the pulp fibres rather than the dosage of enzymes. Even though, the enzyme preparations E3 and E4 had the lowest specific activity against HEC, they decreased the intrinsic viscosity of the pulp the most effectively. The enzyme preparation E3 composed of both cellobiohydrolases and endoglucanases, thus the synergistic action of the components enhanced the decrease of the intrinsic viscosity. On the other hand, the enzyme preparation E4 was monocomponent composing of Cel12A. Cel12A has the lowest molecular mass of the studied proteins here, and it lacks the cellulose binding domain (Sandgren et al. 2001). We suggest that these factors enabled the Cel12A enzymes to find more accessible sites than the other enzymes.
According to the FTIR spectra, the crystalline form of the samples was cellulose I (Fig. 5). The shape of the board OH stretching peak of the intramolecular hydrogen bonds at 3100–3500 cm−1 and the sharp C-O stretching peak at 1000 cm−1 are typical for cellulose I. In addition, the presence of the peaks at 1430, 1105 and 1055 cm−1 confirmed the crystalline form (Carillo et al. 2004; Gwon et al. 2010). The enzyme treatments did not change the crystalline structure nor the crystallinity of the pulps when measured as the ratio of absorptions at 1375 and 2900 cm−1. The endoglucanse rich enzymes have been found to increase the crystallinity of pulp, however this occurs only with long treatment time and high enzyme dosage (Cao and Tan 2002a, 2006; Pu et al. 2006). Here, the treatment time was short and dosage low, and thus, it was plausible that the crystallinity did not change.
Dissolution studies
The alkaline solubility of the untreated pulp was 49 % and the solubility of the enzyme treated pulps was 88–97 % depending on the type of the enzyme preparation and dosage (Fig. 6). The alkaline solubility of the enzyme treated pulps was clearly higher than of the untreated pulp. This is in line with the other studies and is explained by the decreased intrinsic viscosity (SCAN) of the enzyme treated pulp compared to the untreated pulp (Rahkamo et al. 1996; Cao and Tan 2002a, b, 2006; Wang et al. 2008; Le Moigne and Navard 2010). The highest solubility of the pulp was obtained with the enzyme preparations E4 and E5 (dosage 1 mg/g) with the intrinsic viscosity of 255 and 271 ml/g, respectively. The alkaline solubility of the pulp samples treated with the multicomponent enzymes (E1, E2 and E3) was increased clearly with the increased enzyme dosage, even though the increased enzyme dosage had only slight effect on the SCAN viscosity of the pulp samples. In case of the pulp samples treated with the monocomponent enzymes (E4 and E5), the effect of the enzyme dosage was not that pronounced. Especially, the enzyme preparation E5 with the higher enzyme dosage (1 mg/g) did not decrease the SCAN viscosity at all compared with the lower dosage, but the alkaline solubility was increased. In addition, when comparing the pulp samples treated with the enzyme preparations E3 and E5, who decreased the SCAN viscosity of the pulp equally (by 37 %), the alkaline solubility of the samples varied between 87 and 97 %. Thus, the decreased intrinsic viscosity (i.e. decreased degree of polymerisation) enhanced the alkaline solubility of cellulose, however, it cannot fully explain the phenomenon. Apart from the degradation of cellulose, the endoglucanse rich cellulases have shown to increase the pore volume and to loosen the microfibril bundles of the pulp fibres that can enhance the dissolution (Grönqvist et al. 2014; Virtanen et al. 2015).
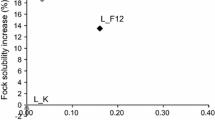
The specific endoglucanase (HEC) activity of the enzyme preparations did not correlate with the alkaline solubility (Fig. 7a). The highest solubility with the both studied enzyme dosages was obtained with the pulp samples treated with the monocomponent enzyme preparations, E4 and E5. It was shown that in multicomponent enzyme system the different enzymes can compete the accessible sites, and especially the adsorption of Cel5A was decreased when Cel7A was present (Medve et al. 1998). Thus, it seems that the solubility of the pulps was related to the ability of the endoglucanases to found accessible sites. Additionally, the degradation of cellulose did not correlate with the alkaline solubility (Fig. 7b). This confirmed that the increased alkaline solubility was achieved by the action of endoglucanses rather than cellobiohydrolases and synergetic action of the components was not necessary to obtain high alkaline solubility.
The insoluble fractions of the samples were studied under the light microscope, Fig. 8. The insoluble fraction of the enzyme treated samples did not differ from each other. They had only highly swollen fragments of the pulp fibres. Contrary, the insoluble fraction of the untreated sample had swollen and ballooned fibres without any fragments. The ballooning is suggested to occur when the outer walls of the fibre act as semi-permeable membrane inside which the dissolved inner part is trapped (Cuissinat and Navard 2006, 2008; Le Moigne and Navard 2010). Ballooning can result in full or incomplete dissolution depending on the solvent. Clearly, the enzyme treatment enhanced the dissolution and changed the dissolution behaviour from ballooning to fragmentation as observed also by Le Moigne and Navard (2010).
The fluidity of the alkaline cellulose solutions was determined as the falling ball time and compared to each other. The untreated pulp did not dissolve properly and its fluidity was not possible to measure. The fluidity of the solutions from the enzyme treated pulps with the both studied enzyme dosages are shown in Fig. 9. The solution from the pulp treated with E3 enzyme preparation with the dosage of 0.5 mg/g became instable due to the increased temperature (higher ball drop time). This was due to the gelling of the sample induced by the large swollen fibre fragments in the solution (shown in Fig. 7b) that impaired the quality of the solution. With the other studied solutions, the fluidity increased as the temperature increased. The fluidity of the solutions was dependent on the type of enzyme treatment of the pulps prior to the dissolution. With each studied enzyme preparation, the fluidity of the alkaline cellulose solution was higher with higher enzyme dosage. This was especially pronounced with the multicomponent enzyme preparations, E1, E2 and E3 whose solubility was under 90 %. When the alkaline solubility of the samples was plotted in the same graph with the lowest ball drop time, it was evident that the alkaline solubility had clear effect on the fluidity of the solution, Fig. 10. This is most likely due to the high amount of swollen gel particles in the poorly dissolved samples that decreased their fluidity. Vehviläinen et al. (2015b) showed that the fluidity of the alkaline cellulose solutions was lower after the filtration during which the swollen gel particles were removed.
Conclusions
In this work endoglucanse rich cellulase preparations were characterized and their effect on dissolving pulp and its alkaline solubility was studied. All cellulases contained endoglucanases, two samples were monocomponet endoglucanases and three cellulases were mixtures of endoglucanses and cellobiohydrolases. The cellulases with cellobiohydrolases produced more soluble sugars in comparison with the monocomponent endoglucanases. All the studied cellulases decreased the intrinsic viscosity (SCAN) of the pulp and thus increased their alkaline solubility compared with the untreated pulp. However, among the enzyme-treated samples the intrinsic viscosity did not correlate with the alkaline solubility. With the same enzyme dosage, monocomponent endoglucanases increased the alkaline solubility more than the multicomponent cellulases. The enzyme treatments did not change the crystallinity of the pulps, this was plausible as the time of the enzyme treatment was short and the enzyme dosages were low. The alkaline solubility of the pulps had clear effect on the fluidity of the cellulose solutions: the higher the solubility of the pulp the higher the fluidity of the solution.
The studies showed that the commercially available endoglucanase rich cellulases are able to modify the dissolving pulp so that the high alkaline solubility was achieved. The characterisation of the commercial cellulases allows the selection among the endoglucanse rich preparations to obtain high solubility with the minimum loss of cellulose during the enzyme treatment. The enzyme mixtures with clear activity against filter paper indicated the presence of cellobiohydrolases which increased the loss during the enzyme treatment without enhanced alkaline solubility. The endoglucanse activity (activity against HEC) in the enzyme preparations did not correlate with the alkaline solubility. However, the SDS-PAGE electrophoresis allowed the identification of the monocomponent endoglucanases that gave the highest alkaline solubility. Thus, one band in SDS gel, activity against HEC and lack of activity against filter paper are indicative for the good alkaline solubility of pulp after the enzyme treatment and low loss of pulp during the enzyme treatment.
References
Arantes V, Saddler JN (2011) Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol Biofuels 4:3–18
Bailey MJ, Nevalainen KMH (1981) Induction, isolation and testing of stable Trichoderma reesei mutants with improved production of solubilizing cellulose. Enzyme Microb Technol 3:153–157
Bailey MJ, Biley P, Poutanen K (1992) Interlaboratory testing methods for assay of xylanse activity. J Biotechnol 29:257–270
Bailey MJ, Siika-aho M, Valkeajärvi A, Penttilä ME (1993) Hydrolytic properties of two cellulases Trichoderma reesei expressed in yeast. Biotechnol Appl Biochem 17:65–76
Bernfeld P (1955) Amylases, α and ß. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 1. Academic Press, New York, pp 149–158
Cao Y, Tan H (2002a) Effects of cellulase on the modification of cellulose. Carbohydr Res 337:1291–1296
Cao Y, Tan H (2002b) The properties of enzyme-hydrolyzed cellulose in aqueous sodium hydroxide. Carbohydr Res 337:1453–1457
Cao Y, Tan H (2006) Improvement of alkali solubility of cellulose with enzymatic treatment. Appl Microbiol Biotechnol 70:176–182
Carillo F, Colom X, Sunõl JJ, Saurina J (2004) Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur Polym J 40:22229–22234
Cuissinat C, Navard P (2006) Swelling and dissolution of cellulose part II: free floating cotton and woof fibres in NaOH-water-additives systems. Mocromol Symp 244:19–30. doi:10.1002/masy.200651202
Cuissinat C, Navard P (2008) Swelling and dissolution of cellulose, part III: plant fibres in aqueous systems. Cellulose 15:67–74. doi:10.1007/s10570-007-9158-4
Engström AC, Ek M, Henriksson G (2006) Improved accessibility and reactivity of dissolving pulp for the viscose process: pretreatment with monocomponent endoglucanase. Biomacromolecules 7:2027–2031
Fock W (1959) A modified method for determining the reactivity of viscose-grade dissolving pulps. Papier (Bingen, Germany) 13:92–95
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Grönqvist S, Hakala TK, Kamppuri T, Vehviläinen M, Hänninen T, Liitiä T, Maloney T, Suurnäkki A (2014) Fibre porosity development of dissolving pulp during mechanical and enzymatic processing. Cellulose 21:3667–3676. doi:10.1007/s10570-014-0352-x
Gwon JG, Lee SY, Doh GH, Kim JH (2010) Characterization of chemically modified wood fibers using FTIR spectroscopy for biocomposites. J Appl Polym Sci 116:3212–3219
Ibarra D, Köpcke V, Ek M (2010) Behavior of different monocomponent endoglucanases on the accessibility and reactivity of dissolving-grade pulps for viscose process. Enzyme Microb Technol 47:355–362
Isogai A, Atalla RH (1998) Dissolution of cellulose in aqueous NaOH. Cellulose 5:309–319
Kamide K, Okajima K, Matsui T, Kowsaka K (1984) Study on the solubility of cellulose in aqueous alkali solution by deuteration IR and 13C NMR. Polym J 16:857–866
Karlsson J, Medve J, Tjerneld F (1999) Hydrolysis of steam-pretreated lignocellulose. Appl Biochem Biotechnol 82:243–258
Karlsson J, Momcilovic D, Wittgren B, Schülein M, Tjerneld F, Brinkmalm G (2002) Enzymatic degradation of carboxymethyl celulose hydrolyzed by the endoglucanase Cel5A, Cel7B, and Cel 45A from Humicola Insolens and Cel7B, Cel12A and Cel45A core from Thrichoderma Reesei. Biopolymers 63:32–40
Kleman-Leyer KM, Siika-aho M, Teeri TT, Kirk TK (1996) The cellulases Endoglucanase I and cellobiohydrolase II of Thrichoderma reesei act synergistically to solubilize native cotton cellulose but not to decrease its molecular size. Appl Environ Microbiol 62:2883–2887
Köpcke V, Ibarra D, Ek M (2008) Increasing accessibility and reactivity of paper grade pulp by enzymatic treatment for use as dissolving pulp. Nord Pulp Pap Res J 23:363–368
Kotiranta P, Karlsson J, Siika-aho M, Medve J, Viikari L, Tjerneld F, Tenkanen M (1999) Adsorption and activity of Trichoderma reesei cellobiohydrolase I, endoglucanase II and the corresponding core proteins on steam pretreated willow. Appl Biochem Biotechnol 81:81–90
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Le Moigne N, Navard P (2010) Dissolution mechanisms of wood cellulose fibres in NaOH-water. Cellulose 17:31–45. doi:10.1007/s10570-009-9370-5
Le Moigne N, Jardeby K, Navard P (2010) Structural changes and alkaline solubility of wood cellulose fibers after enzymatic peeling treatment. Carbohydr Polym 79:325–332
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 23:265–275
Medve J, Karlsson J, Lee D, Tjerneld F (1998) Hydrolysis of microcrystalline cellulose by cellobiohydrolase I and endoglunace II from Trichoderma reesei: adsorption, sugar production pattern and synergism of the enzymes. Biotechnol Bioeng 59:621–634
Miao Q, Chen L, Huang L, Tian C, Zheng L, Ni Y (2014) A process for enhancing the accessibility and reactivity of hardwood kraft-based dissolving pulp for viscose rayon production by cellulase treatment. Bioresour Technol 154:109–113
Nakazawa H, Okada K, Kobayashi R, Kubota T, Onodera T, Ochiai N, Omata N, Ogasawara W, Okada H, Morikawa Y (2008) Characterization of the catalytic domains of Trichoderma reesei endoglucanases I, II, and III, expressed in Escherichia coli. Appl Microbiol Biotechnol 81:681–689
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in cellulose I and II. J Appl Polym Sci 8:1325–1341
Nidetzky B, Steiner W, Hayn M, Claeyssens M (1994) Cellulose hydrolysis by the cellulases from Thrichoderma reesei: a new model for synergistic interaction. Biochem J 298:705–710
Orlowski A, Rog T, Paavilainen S, Manna M, Heiskanen I, Backfolk K, Timonen J, Vattulainen I (2015) How endoglucanase enzymes act on cellulose nanofibrils: role of amorphous regions revealed by atomistic simulations. Cellulose 22:2911–2925
Pu Y, Ziemer C, Ragauskas AJ (2006) CP/MAS 13C NMR analysis of cellulase treated bleached softwood kraft pulp. Carbohydr Res 341:591–597
Rahkamo L, Siika-aho M, Vehviläinen M, Dolk M, Viikari L, Nousiainen P, Buchert J (1996) Modification of hardwood dissolving grade pulp with purified Trchoderma reesei cellulases. Cellulose 3:153–163
Sandgren M, Shaw A, Ropp TH, Wu S, Bott R, Cameron AD, Ståhlberg J, Mitchinson C, Jones TA (2001) The X-ray crystal structure of the Trichoderma reesei family 12 endoglucanase 3, Cel12A, at 1.9 Å resolution. J Mol Biol 308:295–310
Suurnäkki A, Tenkanen M, Siika-aho M, Niku-Paavola ML, Viikari L, Buchert J (2000) Trichoderma reesei cellulases and their core domains in the hydrolysis and modification of chemical pulp. Cellulose 7:189–209
Tolan JS (2002) Iogen’s process for producing ethanol from cellulosic biomass. Clean Technol Environ Policy 3:339–345
Vehviläinen M, Kamppuri T, Rom M, Janicki J, Ciechanska D, Grönqvist S, Siika-aho M, Elg Christoffersson K, Nousiainen P (2008) Effect of wet spinning parameters on the properties of novel cellulosic fibres. Cellulose 15:671–680
Vehviläinen M, Kamppuri T, Grönqvist S, Rissanen M, Maloney T, Honkanen M, Nousiainen P (2015a) Dissolution of enzyme-treated cellulose using freezing-thawing method and the properties of fibres regenerated from the solution. Cellulose 22:1653–1674
Vehviläinen M, Kamppuri T, Setälä H, Grönqvist S, Rissanen M, Honkanen M, Nousiainen P (2015b) Regeneration of fibres from alkaline solution containing enzyme-treated 3-allyloxy-2-hydroxypropyl substituted cellulose. Cellulose 22:2271–2281
Virtanen T, Penttilä PA, Maloney TC, Grönqvist S, Kamppuri T, Vehviläinen M, Serimaa R, Maunu SL (2015) Impact of mechanical and enzymatic pretreatments on softwood pulp fibre wall structure studied with NMR spectroscopy and X-ray scattering. Cellulose 22:1565–1576. doi:10.1007/s10570-015-0619-x
Wang Y, Zhao Y, Deng Y (2008) Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr Polym 72:178–184
Wang H, Pang B, Wu K, Kong F, Li B, Mu X (2014) Two stages of treatments for upgrading bleached softwood paper grade pulp to dissolving pulp for viscose production. Biochem Eng J 82:183–187
Acknowledgments
Funding from the Finnish Funding Agency for Technology and Innovation (TEKES) and Stora Enso Oyj are greatly acknowledged. The technical assistance of Maija Järventausta is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamppuri, T., Vehviläinen, M., Backfolk, K. et al. Characterization of endoglucanase rich Trichoderma reesei cellulase mixtures and their effect on alkaline solubility of dissolving pulp. Cellulose 23, 3901–3911 (2016). https://doi.org/10.1007/s10570-016-1055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1055-2